Abstract
Preterm low-birth-weight infants remain difficult to manage based on adequate laboratory tests. The aim of this study was to establish blood reference intervals (RIs) in those newborns who were admitted to and survived in the neonatal intensive care unit (NICU). A multicenter prospective study was conducted among all infants admitted to 11 affiliated NICUs from 2010 to 2013. The clinical information and laboratory data were registered in a network database designed for this study. The RIs for 26 items were derived using the parametric method after applying the latent abnormal values exclusion method. The influence of birth weight (BW) and gestational age (GA) on the test results was expressed in terms of the standard deviation ratio (SDR), as SDRBW and SDRGA, respectively. A total of 3189 infants were admitted during the study period; 246 were excluded due to a lack of blood sampling data, and 234 were excluded for chromosomal abnormalities (n = 108), congenital anomalies requiring treatment with surgical procedures (n = 76), and death or transfer to another hospital (n = 50). As a result, 2709 infants were enrolled in this study. Both the SDRGA and SDRBW were above 0.4 in the test results for total protein (TP), albumin (ALB), alanine aminotransferase (ALT), and red blood cells (RBC); their values increased in proportion to the BW and GA. We derived 26 blood RIs for infants who were admitted to NICUs. These RIs should help in the performance of proper clinical assessments and research in the field of perinatal-neonatal medicine.
Introduction
The prognosis of preterm low-birth-weight infants has improved dramatically with advances in perinatal medicine. The Neonatal Research Network of Japan revealed that more than 80% of infants delivered at 24 weeks of gestational age (GA) survived in neonatal intensive care units (NICUs) [1], and the survival rates of infants born at GA 22 and 23 weeks were also improved compared with those in previous studies [2]. However, the treatment of these vulnerable newborns and the associated clinical research remain great challenges. It is essential to properly assess these infants based on adequate physiological data [3] as well as laboratory tests.
Due to the nature of the physiological growth and development of infants and children, many efforts have been made to establish pediatric reference intervals (RIs) for routinely-measured laboratory parameters[4] [5]. Christensen et al. published RIs for the complete blood cell (CBC) counts in term and preterm infants using the large database of a health care system [6] [7] [8] [9]. The Canadian Laboratory Initiative on Pediatric Reference Interval Database established RIs for blood chemistry data in healthy and multiethnic child populations [10] [5]. In contrast, few studies have been published regarding the RIs of blood chemistry elements for preterm low-birth-weight infants[11] [12] [13].
The “Kyushu University High-Risk Neonatal Clinical Research Network” Project is a collaborative study conducted among NICUs across the northern part of Kyushu Island in Japan from April 2010 to March 2013. For this project, a prospective observational study was performed in the 11 affiliated hospitals in order to establish blood RIs in preterm low-birth-weight infants within 24 h after birth who were admitted to multiple perinatal care centers and who survived until hospital discharge.
Materials and Methods
Multicenter Prospective Study
All infants admitted to any of the 11 affiliated NICUs on the first day of life were enrolled in this study, with the following exclusion criteria: chromosomal abnormalities, congenital anomalies requiring surgical procedures during the neonatal period, and death or transfer to other hospitals prior to discharge. Data acquisition was carried out using a web-based electronic medical software program that stores laboratory data and clinical information (Hitachi Solutions, Ltd., Tokyo, Japan). The data were classified into three subgroups based on either by BW or GA according to the classification of the World Health Organization (WHO) for neonates. The study protocol was approved by the Institutional Review Board (#22–131; Kyushu University Hospital) at each institution and registered as a prospective observational study with the University Hospital Medical Information Network clinical trial registration system in Japan (UMIN000008763) in April 2010. Written informed consent was obtained from all of the caretakers of the patients prior to their enrollment in this study.
Target Test Items and Standardization of Measurements
The Japan Society of Clinical Chemistry established common RIs for use nationwide in Japan for 40 commonly tested laboratory tests [14]. Annual external quality controls have been done for the major analytes among the affiliated medical facilities in the northern region of Kyushu Island[15], and we confirmed the standardized status of all the assays[16]. The CBC count and differential white blood cell counts were measured using automated Beckman Coulter Hematology Analyzers (Beckman Coulter Inc., FL, USA). The following 16 biochemical and 10 hematological test items were chosen as analytes: total protein (TP), albumin (ALB), blood urea nitrogen (BUN), creatinine (CRE), total bilirubin (T-BIL), direct bilirubin (D-BIL), sodium (Na), potassium (K), chlorine (CL), calcium (Ca), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), creatine kinase (CK), white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), platelets (PLT), neutrophils (NEUT), lymphocytes (LYMP), monocytes (MONO), eosinophils (EOS) and basophils (BASO).
Statistical Analysis
The following items, which may be affected by a pathological state, were excluded depending on the international classification of diseases-10 (ICD-10) code: LDH, AST, and CK in infants with a disease code of P20-29; respiratory and cardiovascular disorders specific to the perinatal period and CRP in infants with P35-39; infections specific to the perinatal period, K, and T-BIL in infants with P50-61; and hemorrhagic and hematological disorders specific to fetuses or newborns. In order to exclude inappropriate infants with multiple abnormal results, a multivariate iterative method called latent abnormal values exclusion (LAVE) [14] [15] [16] was applied for simultaneous derivation of RIs for multiple test items. In this study, the LAVE method was used for the values of WBC, HGB, HCT, TP, BUN, CK, K, LDH, ALT, and CRP with eight iterations and an allowance of up to one result outside the RI and up to one missing result in the reference test items.
The parametric method was used for computing the RIs after transforming the distribution of the reference values into a Gaussian form using a modified Box-Cox transformation [14]. The 90% confidence intervals (CIs) for the upper (UL) and lower limits (LL) of the RIs were estimated by the bootstrap method to avoid any abnormal results in the reference test [17, 18]. The need to partition the reference values by sex, GA, and BW was judged based on the SD ratio (SDR) introduced by Ichihara [14]; the SDR for sex (SDRSEX) is expressed as the SD representing the sex difference divided by the SD of the RI (SDRI, or 1/4th of RI). Similarly, the SDRs for GA and BW (SDRGA, SDRBW) were computed as a ratio of the SD representing the between-GA and between-BW subgroup differences divided by the SDRI, respectively. We adopted an SDR cutoff value of 0.4 by consensus among the collaborators [17, 19, 20].
Results
Outline of the Prospective Study
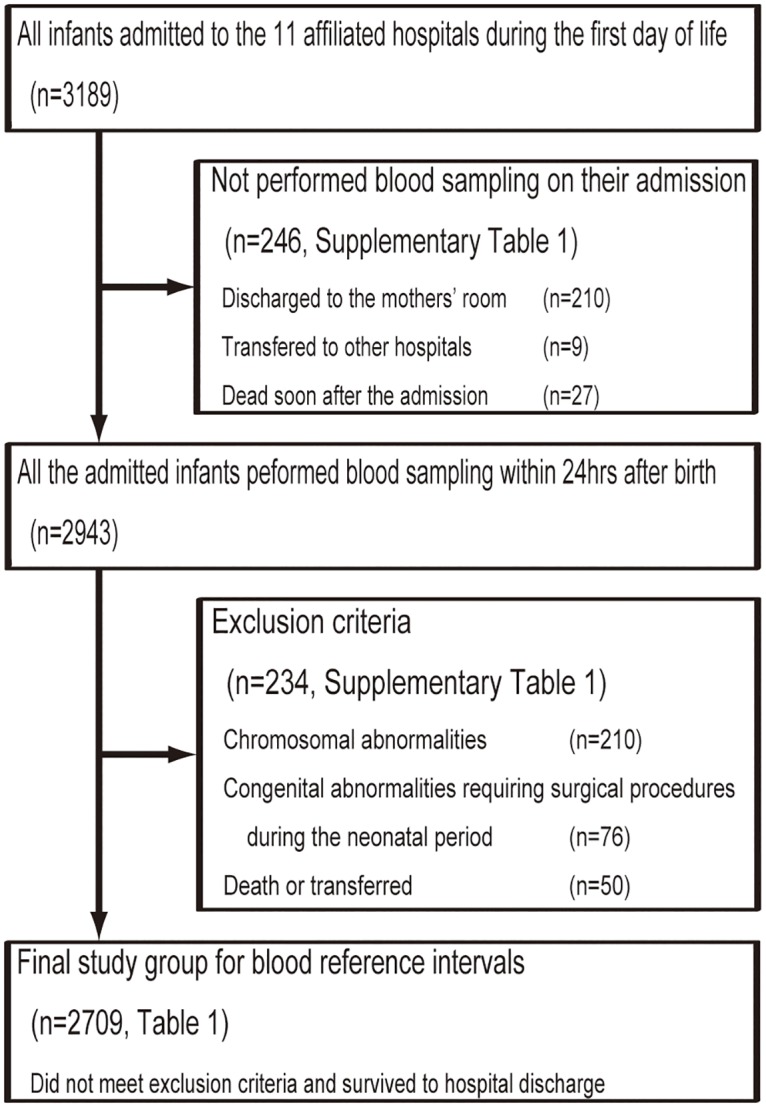
Fig 1 shows an outline for the recruitment of preterm low-birth-weight infants in this prospective study. A total of 3,189 infants were hospitalized on the first day of life at the 11 NICUs between April 2010 and March 2013; 246 did not undergo laboratory testing on admission, 210 were recognized to be healthy and did not require blood testing, 9 were transferred to other hospitals, and 27 died within 24 hours after birth. We also excluded 234 infants who received a diagnosis of congenital abnormalities (n = 210) or congenital abnormalities requiring surgical procedures within 28 days after birth (n = 76) or who died in the hospital or were transferred to other facilities (n = 50). Therefore, the final study group for analyzing the blood RIs comprised 2,709 infants who did not meet the exclusion criteria and survived until hospital discharge.
Fig 1. Overview of the prospective study of blood reference intervals for preterm low-birth-weight infants.
ICD-10
Table 1 shows the number of subjects in the final group classified based on the ICD-10 code. We diagnosed 1,555 low-birth-weight infants (91.6%) (Total <2,500 g) and 1,340 preterm birth infants (89.9%) (total <37 w) with P05-08: disorders related to the length of gestation and fetal growth. S1 Table displays the ICD-10 diagnostic information for the excluded infants.
Table 1. Number of preterm low-birth-weight infants classified in the ICD-10 (n = 2709).
| ICD-10 codes | Total <2500g | Total <37w | ||||||
|---|---|---|---|---|---|---|---|---|
| Classified by BW | Classified by GA | |||||||
| <1000g | 1000-1500g | 1500-2500g | <28w | 28-32w | 32-37w | |||
| P00-P04 Fetus and newborn affected by maternal factors and by complications of pregnancy, labour and delivery | 5 | 0 | 0 | 5 | 9 | 0 | 0 | 9 |
| P05-P08 Disorders related to length of gestation and fetal growth | 1555 | 148 | 225 | 1182 | 1340 | 124 | 215 | 1001 |
| P10-P15 Birth trauma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P20-P29 Respiratory and cardiovascular disorders specific to the perinatal period | 74 | 0 | 2 | 72 | 88 | 0 | 1 | 87 |
| P35-P39 Infections specific to the perinatal period | 8 | 0 | 0 | 8 | 6 | 0 | 0 | 6 |
| P50-P61 Haemorrhagic and haematological disorders of fetus and newborn | 14 | 1 | 0 | 13 | 6 | 1 | 0 | 5 |
| P70-P74 Transitory endocrine and metabolic disorders specific to fetus and newborn | 34 | 0 | 3 | 31 | 31 | 0 | 1 | 30 |
| P75-P78 Digestive system disorders of fetus and newborn | 3 | 0 | 0 | 3 | 5 | 0 | 0 | 5 |
| P80-P83 Conditions involving the integument and temperature regulation of fetus and newborn | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P90-P96 Other disorders originating in the perinatal period | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Q00-Q07 Congenital malformations of the nervous system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q10-Q18 Congenital malformations of eye, ear, face and neck | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Q20-Q28 Congenital malformations of the circulatory system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q30-Q34 Congenital malformations of the respiratory system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q35-Q37 Cleft lip and cleft palate | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Q38-Q45 Other congenital malformations of the digestive system | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 2 |
| Q50-Q56 Congenital malformations of genital organs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q60-Q64 Congenital malformations of the urinary system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q65-Q79 Congenital malformations and deformations of the musculoskeletal system | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q80-Q89 Other congenital malformations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Q90-Q99 Chromosomal abnormalities, not elsewhere classified | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1697 | 150 | 230 | 1317 | 1489 | 126 | 217 | 1146 |
BW; birth weight, GA; gestational age, According with the World Health Organization classification of BW and GA, the data were collected for sub-stratified by six BW and GA groups: low-birth-weight infant as a BW of <2500g, very low-birth-weight infant as <1500g, extremely low-birth-weight infant as <1000g, and moderate to late preterm infant as GA of <37w, very preterm infant as <32w, extremely preterm infant as <28w, respectively.
GA- and BW-specific Blood RIs
Table 2 displays the GA-specific RIs of hematology and blood chemistry (international units). The items with SDRs of more than 0.4 were subgrouped based on the GA classification. The SDRGA values of TP, ALB, CRE, Na, ALT, WBC, RBC, NEUT and MONO were significant. The BW-specific RIs for low-birth-weight infants (international units) are shown in Table 3. The SDRBW values of TP, ALB, ALT, RBCs, and EOS were significant. The values of TP, ALB, ALT and RBC were significant in both SDRGA and SDRBW, with values of more than 0.4. These levels increased in proportion to the BW and GA (Fig 2). S2 and S3 Tables show the RIs converted from international to conventional units.
Table 2. GA-specific RIs of blood chemistry and hematology for preterm infants (International Unit).
| Total <37w | Classified by GA | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <28w | 28-32w | 32-37w | ||||||||||||||||||||||||||||
| LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | |||||||||||||||||||
| Analyte | SI unit | SDRGA | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL |
| TP | g/L | 1.02 | 113 | 25 | 30 | 27 | 48 | 44 | 52 | 194 | 33 | 35 | 34 | 52 | 51 | 54 | 1164 | 40 | 41 | 40 | 63 | 62 | 64 | |||||||
| ALB | g/L | 0.95 | 96 | 19 | 22 | 20 | 32 | 30 | 34 | 161 | 25 | 26 | 25 | 35 | 34 | 36 | 1052 | 28 | 28 | 28 | 40 | 39 | 40 | |||||||
| BUN | mmol/L | 0.14 | 1476 | 1.38 | 1.57 | 1.44 | 5.98 | 5.65 | 6.41 | |||||||||||||||||||||
| CRE | μmol/L | 0.44 | 112 | 24.8 | 29.2 | 27.4 | 79.7 | 72.6 | 86.7 | 192 | 24.8 | 31.0 | 30.1 | 79.7 | 69.9 | 85.0 | 1149 | 35.4 | 37.2 | 36.3 | 85.0 | 81.4 | 87.6 | |||||||
| T-BIL | μmol/L | 0.36 | 1432 | 20.5 | 21.9 | 21.0 | 57.8 | 55.6 | 61.2 | |||||||||||||||||||||
| D-BIL | μmol/L | 0.00 | 868 | 5.6 | 5.8 | 5.8 | 19.2 | 18.3 | 20.2 | |||||||||||||||||||||
| Na | mmol/L | 0.40 | 94 | 128 | 130 | 129 | 142 | 141 | 144 | 162 | 131 | 133 | 131 | 142 | 141 | 143 | 1050 | 133 | 134 | 134 | 142 | 142 | 143 | |||||||
| K | mmol/L | 0.00 | 1272 | 3.6 | 3.7 | 3.7 | 6.1 | 6.0 | 6.2 | |||||||||||||||||||||
| CL | mmol/L | 0.00 | 1314 | 100 | 101 | 101 | 111 | 111 | 112 | |||||||||||||||||||||
| Ca | mmol/L | 0.18 | 1466 | 2.0 | 2.0 | 2.0 | 2.7 | 2.6 | 2.7 | |||||||||||||||||||||
| CRP | μg/L | 0.14 | 1356 | 0.00 | 0.00 | 0.00 | 0.80 | 0.20 | 7.20 | |||||||||||||||||||||
| AST | IU/L | 0.28 | 1281 | 15 | 16 | 16 | 70 | 65 | 78 | |||||||||||||||||||||
| ALT | IU/L | 0.66 | 105 | 1 | 1 | 1 | 6 | 5 | 9 | 193 | 1 | 1 | 1 | 9 | 7 | 11 | 1149 | 2 | 2 | 2 | 12 | 11 | 13 | |||||||
| LDH | IU/L | 0.11 | 1297 | 238 | 263 | 245 | 787 | 742 | 958 | |||||||||||||||||||||
| ALP | IU/L | 0.16 | 462 | 351 | 397 | 372 | 1082 | 1022 | 1152 | |||||||||||||||||||||
| CK | IU/L | 0.44 | 88 | 22 | 60 | 45 | 916 | 583 | 1263 | 173 | 38 | 76 | 53 | 552 | 455 | 694 | 988 | 79 | 113 | 91 | 707 | 644 | 778 | |||||||
| WBC | 109/L | 0.57 | 102 | 2.55 | 3.87 | 3.04 | 31.0 | 22.5 | 39.2 | 179 | 2.58 | 4.07 | 3.40 | 19.9 | 16.4 | 23.5 | 1100 | 5.76 | 6.75 | 6.03 | 21.2 | 20.3 | 22.6 | |||||||
| RBC | 1012/L | 0.59 | 103 | 2.63 | 2.98 | 2.76 | 4.65 | 4.51 | 4.82 | 178 | 3.20 | 3.41 | 3.28 | 5.07 | 4.91 | 5.27 | 1084 | 3.59 | 3.65 | 3.62 | 5.52 | 5.46 | 5.57 | |||||||
| HGB | g/L | 0.33 | 1406 | 125 | 129 | 127 | 203 | 201 | 206 | |||||||||||||||||||||
| HCT | /L | 0.31 | 1408 | 0.37 | 0.38 | 0.38 | 0.60 | 0.60 | 0.61 | |||||||||||||||||||||
| PLT | 109/L | 0.20 | 1344 | 107 | 120 | 114 | 375 | 367 | 382 | |||||||||||||||||||||
| NEUT | 109/L | 0.79 | 46 | 0.17 | 0.81 | 0.64 | 17.0 | 11.7 | 27.6 | 91 | 0.15 | 0.68 | 0.37 | 9.58 | 7.36 | 12.6 | 490 | 0.93 | 1.54 | 1.19 | 12.7 | 10.7 | 14.3 | |||||||
| LYMP | 109/L | 0.30 | 754 | 1.88 | 2.36 | 2.06 | 9.72 | 9.21 | 10.3 | |||||||||||||||||||||
| MONO | 106/L | 0.64 | 52 | 30 | 152 | 57 | 1623 | 1270 | 2185 | 118 | 29 | 110 | 64 | 1147 | 1005 | 1323 | 578 | 150 | 217 | 181 | 1413 | 1286 | 1523 | |||||||
| EOS | 106/L | 0.32 | 745 | 6 | 36 | 8 | 741 | 693 | 797 | |||||||||||||||||||||
| BASO | 106/L | 0.10 | 748 | 1 | 2 | 2 | 270 | 241 | 300 | |||||||||||||||||||||
TP; total protein, ALB; albumin, BUN; blood urea nitrogen, CRE; creatinine, T-BIL; total bilirubin, D-BIL; direct bilirubin, Na; sodium, K; potassium, CL; chlorine, Ca; calcium, CRP; C-reactive protein, AST; aspartate aminotransferase, ALT; alanine aminotransferase, LDH; lactate dehydrogenase, ALP; alkaline phosphatase, CK; creatine kinase, WBC; white blood cell, RBC; red blood cell, HGB; hemoglobin, HCT; hematocrit, PLT; platelet, NEUT; neutrophil, LYMP; lymphocyte, MONO; monocyte, EOS; eosinophil, BASO; basophil, GA; gestational age, RI; reference interval, LL; lower limit of the RI, UL; upper limit of the RI, CI; confidential interval (90%) of LLs and ULs were estimated by the bootstrap method. The results were excluded data of LDH, AST and CK in the infants with P20-29; Respiratory and cardiovascular disorders specific to the perinatal period, CRP with P35-39; Infections specific to the perinatal period, and K and T-Bil with P50-61; Hemorrhagic and homological disorders specific to fetus and newborn, respectively. A multivariate iterative method called latent abnormal value exclusion (LAVE) was applied to nine analytes (TP, BUN, K, LDH, ALT, WBC, CRP, HGB and HCT) which deemed to be adversely affected by hemolysis and inflammation. By use of 3-level nested ANOVA, the influence of GA on test results was expressed in terms of standard deviation (SD) ratio (SDR), as SDRGA. SDR> = 0.4 were used as a criteria for the need of partition by the factor.
Table 3. BW-specific RIs of blood chemistry and hematology for low-birth-weight infants (International Unit).
| Total <2500g | Classified by BW | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1000g | 1000-1500g | 1500-2500g | ||||||||||||||||||||||||||||
| LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | LL90%CI | RI | UL90%CI | |||||||||||||||||||
| Analyte | SI unit | SDRBW | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL | n | LL | UL | LL | UL | LL | UL |
| TP | g/L | 0.94 | 132 | 26 | 31 | 29 | 51 | 48 | 57 | 202 | 33 | 35 | 34 | 60 | 58 | 63 | 1178 | 40 | 41 | 40 | 64 | 63 | 65 | |||||||
| ALB | g/L | 0.89 | 112 | 19 | 22 | 21 | 35 | 33 | 37 | 169 | 25 | 25 | 25 | 38 | 37 | 39 | 1010 | 28 | 28 | 28 | 40 | 40 | 41 | |||||||
| BUN | mmol/L | 0.00 | 1532 | 1.38 | 1.59 | 1.46 | 6.12 | 5.78 | 6.51 | |||||||||||||||||||||
| CRE | μmol/L | 0.32 | 1528 | 31.0 | 35.4 | 31.9 | 85.0 | 81.4 | 92.0 | |||||||||||||||||||||
| T-BIL | μmol/L | 0.11 | 1474 | 20.5 | 22.1 | 21.2 | 60.7 | 58.0 | 64.6 | |||||||||||||||||||||
| D-BIL | μmol/L | 0.00 | 917 | 5.5 | 5.8 | 5.6 | 20.0 | 19.0 | 21.0 | |||||||||||||||||||||
| Na | mmol/L | 0.38 | 1315 | 132 | 133 | 133 | 143 | 142 | 143 | |||||||||||||||||||||
| K | mmol/L | 0.00 | 1287 | 3.6 | 3.7 | 3.7 | 6.1 | 6.0 | 6.2 | |||||||||||||||||||||
| CL | mmol/L | 0.13 | 1315 | 100 | 101 | 101 | 111 | 111 | 112 | |||||||||||||||||||||
| Ca | mmol/L | 0.10 | 1519 | 2.0 | 2.0 | 2.0 | 2.7 | 2.6 | 2.7 | |||||||||||||||||||||
| CRP | μg/L | 0.00 | 1521 | 0.0 | 0.0 | 0.0 | 5.1 | 0.3 | 7.7 | |||||||||||||||||||||
| AST | IU/L | 0.00 | 1268 | 15 | 16 | 15 | 74 | 70 | 82 | |||||||||||||||||||||
| ALT | IU/L | 0.49 | 120 | 1 | 1 | 1 | 7 | 6 | 12 | 202 | 1 | 1 | 1 | 13 | 9 | 17 | 1161 | 2 | 2 | 2 | 12 | 11 | 13 | |||||||
| LDH | IU/L | 0.00 | 1281 | 235 | 261 | 243 | 835 | 767 | 984 | |||||||||||||||||||||
| ALP | IU/L | 0.00 | 463 | 352 | 393 | 372 | 1097 | 1051 | 1181 | |||||||||||||||||||||
| CK | IU/L | 0.34 | 1258 | 58 | 73 | 64 | 685 | 641 | 744 | |||||||||||||||||||||
| WBC | 109/L | 0.22 | 1437 | 4.13 | 4.91 | 4.42 | 24.0 | 23.0 | 25.6 | |||||||||||||||||||||
| RBC | 1012/L | 0.59 | 115 | 2.68 | 3.00 | 2.86 | 4.61 | 4.48 | 4.79 | 189 | 3.23 | 3.47 | 3.29 | 5.38 | 5.22 | 5.65 | 1088 | 3.62 | 3.70 | 3.65 | 5.58 | 5.52 | 5.66 | |||||||
| HGB | g/L | 0.36 | 1450 | 125 | 129 | 127 | 204 | 203 | 206 | |||||||||||||||||||||
| HCT | /L | 0.29 | 1454 | 0.37 | 0.39 | 0.38 | 0.61 | 0.60 | 0.61 | |||||||||||||||||||||
| PLT | 109/L | 0.21 | 1392 | 108 | 121 | 108 | 375 | 365 | 383 | |||||||||||||||||||||
| NEUT | 109/L | 0.27 | 748 | 0.74 | 1.07 | 0.90 | 17.5 | 15.9 | 19.1 | |||||||||||||||||||||
| LYMP | 109/L | 0.00 | 877 | 1.67 | 2.15 | 1.84 | 9.37 | 8.93 | 9.78 | |||||||||||||||||||||
| MONO | 106/L | 0.21 | 875 | 116 | 164 | 143 | 1599 | |||||||||||||||||||||||
| EOS | 106/L | 0.42 | 62 | 1 | 24 | 5 | 411 | 289 | 552 | 112 | 0 | 6 | 0 | 555 | 462 | 755 | 596 | 45 | 73 | 59 | 867 | 778 | 932 | |||||||
| BASO | 106/L | 0.09 | 823 | 1 | 3 | 2 | 284 | 249 | 313 | |||||||||||||||||||||
TP; total protein, ALB; albumin, BUN; blood urea nitrogen, CRE; creatinine, T-BIL; total bilirubin, D-BIL; direct bilirubin, Na; sodium, K; potassium, CL; chlorine, Ca; calcium, CRP; C-reactive protein, AST; aspartate aminotransferase, ALT; alanine aminotransferase, LDH; lactate dehydrogenase, ALP; alkaline phosphatase, CK; creatine kinase, WBC; white blood cell, RBC; red blood cell, HGB; hemoglobin, HCT; hematocrit, PLT; platelet, NEUT; neutrophil, LYMP; lymphocyte, MONO; monocyte, EOS; eosinophil, BASO; basophil, GA; gestational age, RI; reference interval, LL; lower limit of the RI, UL; upper limit of the RI, CI; confidential interval (90%) of LLs and ULs were estimated by the bootstrap method. The results were excluded data of LDH, AST and CK in the infants with P20-29; Respiratory and cardiovascular disorders specific to the perinatal period, CRP with P35-39; Infections specific to the perinatal period, and K and T-Bil with P50-61; Hemorrhagic and homological disorders specific to fetus and newborn, respectively. A multivariate iterative method called latent abnormal value exclusion (LAVE) was applied to nine analytes (TP, BUN, K, LDH, ALT, WBC, CRP, HGB and HCT) which deemed to be adversely affected by hemolysis and inflammation. By use of 3-level nested ANOVA, the influence of BW on test results was expressed in terms of standard deviation (SD) ratio (SDR), as SDRBW. SDR> = 0.4 were used as a criteria for the need of partition by the factor.
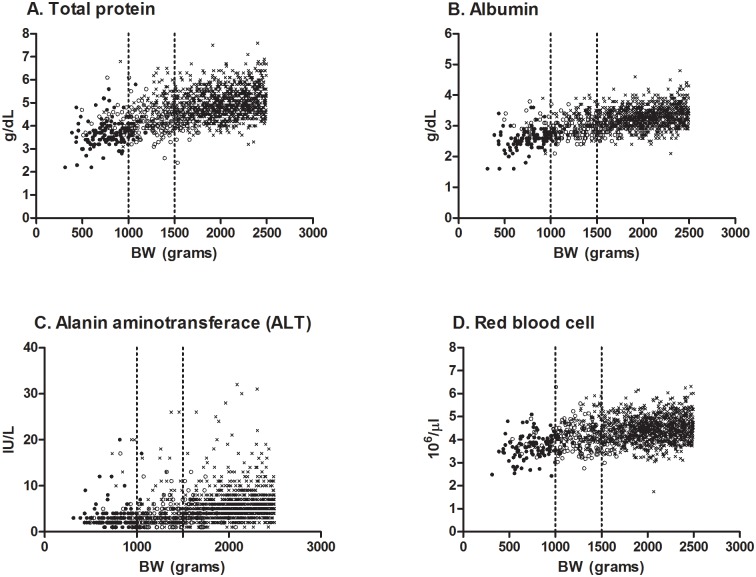
Fig 2. Scatter plots of the items that were significant in both the SDRGA and SDRBW.
The horizontal axis shows the birth weight (BW). The vertical axis shows the measured values of A. Total protein (g/dL), B. Albumin (g/dL), C. Alanine aminotransferace (ALT) (IU/L), and D. Red blood cells (106/μl). The data were classified into three subgroups based on gestational age (GA): GA of 32–37 weeks, cross marks (×); 28–32 weeks, open circles (○); 22–28 weeks, filled circles (●).
Discussion
The Kyushu University High-Risk Neonatal Clinical Research Network Project recently established a university initiative to accumulate information for all infants admitted to the affiliated NICUs using a web-based electronic medical software program. As part of this initiative, our study’s main purpose was to establish RIs for hematology and blood chemistry analytes in preterm low-birth-weight infants who were admitted to perinatal-neonatal care centers in Japan and who survived until discharge.
We were able to obtain a sufficient sample size (2709 infants) during the three-year study period. The practically attainable target sample size for each analyte was set at a minimum of 250 or more, which is greater than twice the minimum number (120 or more) [19]. We excluded outliers based on the clinical diagnosis (ICD-10) and the results of the multivariate iterative method (LAVE) [19]. The LAVE features the simultaneous setting of RIs for multiple test items that are mutually related and the rigid exclusion of individuals with abnormal values for other test items [20]. As a result, the reference individuals were “considered to be normal” preterm low-birth-weight infants based on the ICD-10 code (Table 1).
The Ichihara method utilizes information for the SDR attributable to each source of variation and can be applied in situations in which more than two subgroups are categorized according to the factors [15] [18]. In our study, we presented each RI classified into six subgroups by the BW and GA according to the SDRBW and SDRGA, respectively. The SDRSEX of each item were zero for all analytes for the age groups (data not shown). Therefore, sex-specific RIs were not presented in this study.
In our study, the following tended to increase in proportion to both the GA and BW: TP, Alb, and ALT (Tables 2 and 3 and Fig 2). The TP is made up of Alb and globulin. Alb is synthesized in the liver, and a low serum Alb may result from immaturity of the liver function. ALT is an important transaminase enzyme in various tissue, especially the liver; therefore, the blood ALT level is used clinically as a biomarker for the liver function. In small-for-gestational-age infants, the AST and ALT serum activities were correlated with BW and GA [21]. Our data confirmed the parallel upward trend in these values as the organ function matured. In contrast, extremely premature infants had high plasma enzyme activities compared to babies at a later corrected GA [22], possibly due to suffering more severe illness immediately after birth.
Significant SDRGA and SDRBW values (>0.4) were observed for WBC, RBC, NEUT, and MONO (Table 2); and RBC and EO (Table 3), respectively. When the test results of those analytes were compared among the groups, the RBC count was found to have a tendency to increase in proportion to both the BW and GA (Fig 2). The RIs of HCT and HGB in extremely preterm patients were reported to be lower than those in later preterm and term infants [23]. Several reports have shown the same gradual upward tendency in hematological data [9] [23]. The PLT counts increased for GAs of 22 to 42 weeks using a huge data system [8]. In contrast, an abnormal lymphocyte count at birth is associated with adverse outcomes, including early-onset sepsis, intraventricular hemorrhaging and retinopathy of prematurity [24]. The onset of neutropenia in the first days of life is sometimes noted in SGA infants or those born to mothers with persistent maternal hypertension or early-onset bacterial infection [25]. These reports suggest that the GA and BW, as well as potentially pathogenic maternal and neonatal variables, should be considered when developing RIs.
We recognize various limitations and pitfalls that should be considered when applying these RIs in practice. First, preterm or low-birth-weight babies are considered to be in a clinically pathological or unhealthy state, and many require medical management. Therefore, “normal range” is not a suitable term for the blood chemistry and hematology data for these infants. We therefore used the term “reference interval” in this project and discarded data confirmed to be unacceptable based on the ICD-10 code and LAVE method. A second limitation is that the source of blood specimens (capillary, venous, or arterial) was not taken into account. Some hematological and chemical test values are somewhat higher in capillary samples than in venous or arterial samples. The third limitation is that we did not analyze the trends in the values after birth [23]. The values of analytes may change after several postnatal days depending on the clinical course of the infant.
Conclusions
Our project provides 26 blood RIs in preterm low-birth-weight infants requiring neonatal intensive care in Japan. These RIs should help researchers in the field of perinatal-neonatal medicine perform proper assessments in routine clinical work and research. Further evaluations are needed to determine whether these RIs are representative of the physiological data for those infants.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Institutions and representative physicians enrolled in the database for the Kyushu University High-Risk Neonatal Clinical Research Network Project include: Fukuoka Children’s Hospital and Medical Center for Infectious Diseases: Yasushi Takahata; National Hospital Organization Kyushu Medical Center: Kazuo Sato; Japanese Red Cross Fukuoka Hospital: Shunji Hikino; General Hospital Hamanomachi: Megumi Takemoto; Japan Community Health care Organization Kyushu Hospital: Junko Yamamoto; Kitakyushu Municipal Medical Center: Naoko Matsumoto; National Hospital Organization Kokura Medical Center: Hironori Yamashita; National Hospital Organization Beppu Medical Center: Hiroshi Koga; Oita Prefectural Hospital: Koichi Iida; Yamaguchi Red Cross Hospital: Shinichi Terachi, and Kyushu University Hospital: Shouji Tokunaga, Takeru Abe, Yoko Fukuda. We are indebted a great deal to Mr Shogo Kimura of Yamaguchi University Graduate School of Medicine who devoted to perform the statistical analyses and to produce graphs presented in this paper.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by a grand from the Kyushu University Clinical Research Network Project (MO), The KAKEN #24791113, #15K09717 (MO) and #26860809 (TK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Itabashi K, Horiuchi T, Kusuda S, Kabe K, Itani Y, Nakamura T, et al. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics. 2009;123(2):445–50. Epub 2009/01/28. 123/2/445 [pii] 10.1542/peds.2008-0763 . [DOI] [PubMed] [Google Scholar]
- 2.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics. 2013;132(1):62–71. Epub 2013/06/05. 10.1542/peds.2012-2857 peds.2012-2857 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377(9770):1011–8. Epub 2011/03/18. 10.1016/S0140-6736(10)62226-X S0140-6736(10)62226-X [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung B, Adeli K. Clinical laboratory reference intervals in pediatrics: the CALIPER initiative. Clin Biochem. 2009;42(16–17):1589–95. Epub 2009/07/14. 10.1016/j.clinbiochem.2009.06.025 S0009-9120(09)00293-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 5.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58(5):854–68. Epub 2012/03/01. 10.1373/clinchem.2011.177741 clinchem.2011.177741 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6.Schmutz N, Henry E, Jopling J, Christensen RD. Expected ranges for blood neutrophil concentrations of neonates: the Manroe and Mouzinho charts revisited. J Perinatol. 2008;28(4):275–81. Epub 2008/01/18. 10.1038/sj.jp.7211916 7211916 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7.Christensen RD, Jopling J, Henry E, Wiedmeier SE. The erythrocyte indices of neonates, defined using data from over 12,000 patients in a multihospital health care system. J Perinatol. 2008;28(1):24–8. Epub 2007/11/02. 7211852 [pii] 10.1038/sj.jp.7211852 . [DOI] [PubMed] [Google Scholar]
- 8.Wiedmeier SE, Henry E, Sola-Visner MC, Christensen RD. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol. 2009;29(2):130–6. Epub 2008/09/27. 10.1038/jp.2008.141 jp2008141 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123(2):e333–7. Epub 2009/01/28. 10.1542/peds.2008-2654 peds.2008-2654 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10.Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, et al. Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem. 2009;42(9):885–91. Epub 2009/03/26. 10.1016/j.clinbiochem.2009.01.014 S0009-9120(09)00046-0 [pii]. . [DOI] [PubMed] [Google Scholar]
- 11.Demirel G, Celik IH, Canpolat FE, Erdeve O, Biyikli Z, Dilmen U. Reference values of serum cystatin C in very low-birthweight premature infants. Acta Paediatr. 2013;102(1):e4–7. Epub 2012/09/29. 10.1111/apa.12041 . [DOI] [PubMed] [Google Scholar]
- 12.Vieux R, Hascoet JM, Merdariu D, Fresson J, Guillemin F. Glomerular filtration rate reference values in very preterm infants. Pediatrics. 2010;125(5):e1186–92. Epub 2010/04/07. 10.1542/peds.2009-1426 peds.2009-1426 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Fenton TR, Lyon AW, Rose MS. Cord blood calcium, phosphate, magnesium, and alkaline phosphatase gestational age-specific reference intervals for preterm infants. BMC Pediatr. 2011;11:76 Epub 2011/09/03. 10.1186/1471-2431-11-76 1471-2431-11-76 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichihara K, Yomamoto Y, Hotta T, Hosogaya S, Miyachi H, Itoh Y, et al. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann Clin Biochem. 2015. Epub 2015/09/13. 0004563215608875 [pii] 10.1177/0004563215608875 . [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita S, Toyofuku M, Iida H, Wakiyama M, Kurihara M, Nakahara M, et al. Standardization of laboratory data and establishment of reference intervals in the Fukuoka Prefecture: a Japanese perspective. Clin Chem Lab Med. 2001;39(3):256–62. Epub 2001/05/15. 10.1515/CCLM.2001.040 . [DOI] [PubMed] [Google Scholar]
- 16.Ichihara K, Ozarda Y, Klee G, Straseski J, Baumann N, Ishikura K. Utility of a panel of sera for the alignment of test results in the worldwide multicenter study on reference values. Clin Chem Lab Med. 2013;51(5):1007–25. Epub 2013/05/02. 10.1515/cclm-2013-0248 /j/cclm.2013.51.issue-5/cclm-2013-0248/cclm-2013-0248.xml [pii] /j/cclm.ahead-of-print/cclm-2013-0248/cclm-2013-0248.xml [pii]. . [DOI] [PubMed] [Google Scholar]
- 17.Yamakado M, Ichihara K, Matsumoto Y, Ishikawa Y, Kato K, Komatsubara Y, et al. Derivation of gender and age-specific reference intervals from fully normal Japanese individuals and the implications for health screening. Clin Chim Acta. 2015;447:105–14. Epub 2015/05/20. 10.1016/j.cca.2015.04.037 S0009-8981(15)00236-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18.Ozarda Y, Ichihara K, Aslan D, Aybek H, Ari Z, Taneli F, et al. A multicenter nationwide reference intervals study for common biochemical analytes in Turkey using Abbott analyzers. Clin Chem Lab Med. 2014;52(12):1823–33. Epub 2014/08/26. 10.1515/cclm-2014-0228 /j/cclm.2014.52.issue-12/cclm-2014-0228/cclm-2014-0228.xml [pii] /j/cclm.ahead-of-print/cclm-2014-0228/cclm-2014-0228.xml [pii]. . [DOI] [PubMed] [Google Scholar]
- 19.Ichihara K, Boyd JC. An appraisal of statistical procedures used in derivation of reference intervals. Clin Chem Lab Med. 2010;48(11):1537–51. Epub 2010/11/11. 10.1515/CCLM.2010.319 . [DOI] [PubMed] [Google Scholar]
- 20.Ichihara K. Statistical considerations for harmonization of the global multicenter study on reference values. Clin Chim Acta. 2014;432:108–18. Epub 2014/02/13. 10.1016/j.cca.2014.01.025 S0009-8981(14)00042-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 21.Zanardo V, Perini G. Aspartate aminotransferase and alanine aminotransferase serum activities in small-for-date newborns. Padiatr Padol. 1987;22(4):325–30. Epub 1987/01/01. . [PubMed] [Google Scholar]
- 22.Victor S, Dickinson H, Turner MA. Plasma aminotransferase concentrations in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96(2):F144–5. Epub 2009/07/04. 10.1136/adc.2008.152454 adc.2008.152454 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Christensen RD, Henry E, Jopling J, Wiedmeier SE. The CBC: reference ranges for neonates. Semin Perinatol. 2009;33(1):3–11. Epub 2009/01/27. 10.1053/j.semperi.2008.10.010 S0146-0005(08)00127-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 24.Christensen RD, Baer VL, Gordon PV, Henry E, Whitaker C, Andres RL, et al. Reference ranges for lymphocyte counts of neonates: associations between abnormal counts and outcomes. Pediatrics. 2012;129(5):e1165–72. Epub 2012/04/18. 10.1542/peds.2011-2661 peds.2011-2661 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25.Christensen RD, Henry E, Wiedmeier SE, Stoddard RA, Lambert DK. Low blood neutrophil concentrations among extremely low birth weight neonates: data from a multihospital health-care system. J Perinatol. 2006;26(11):682–7. Epub 2006/10/13. 7211603 [pii] 10.1038/sj.jp.7211603 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.