Abstract
Desmosterolosis, a rare disorder of cholesterol biosynthesis, is caused by mutations in DHCR24, the gene encoding the enzyme 24-dehydrocholesterol reductase (DHCR24). To date, desmosterolosis has been described in only two patients. Here we report on a third patient with desmosterolosis who presented after delivery with relative macrocephaly, mild arthrogryposis, and dysmorphic facial features. Brain MRI revealed hydrocephalus, thickening of the tectum and massa intermedia, mildly effaced gyral pattern, underopercularization, and a thin corpus callosum. The diagnosis of desmosterolosis was established by detection of significant elevation of plasma desmosterol levels and reduced enzyme activity of DHCR24 upon expression of the patient’s DHCR24 cDNA in yeast. The patient was found to be a compound heterozygote for c.281G>A (p.R94H) and c.1438G->A (p.E480K) mutations. Structural and evolutionary analyses showed that residue R94 resides at the flavin adenine dinucleotide (FAD) binding site and is strictly conserved throughout evolution, while residue E480 is less conserved, but the charge shift substitution is accompanied by drastic changes in the local protein environment of that residue. We compare the phenotype of our patient with previously reported cases.
Keywords: desmosterolosis, cholesterol, 24-dehydrocholesterol reductase, desmosterol, brain malformations, arthrogryposis
INTRODUCTION
Sufficient cholesterol supply is essential for proper development of the human brain. All cell membranes require normal or near normal amounts of cholesterol to develop and function properly. Neurons possess distinct cholesterol-based plasma membrane subdomains. Moreover, neuronal differentiation, in particular synaptogenesis, is a cholesterol-dependent process [Mauch et al., 2001; Pfrieger, 2003]. Animal studies have shown that during the first postnatal week desmosterol comprises >30% of the sterol in cerebral hemispheres and brainstem, and that this fraction declines rapidly with age [Jurevics and Morell, 1995]. In addition, during periods of rapid myelination, all cholesterol and desmosterol accumulating in rat brain is synthesized within the brain and not derived from the diet or produced by other organs [Jurevics and Morell, 1995]. Cholesterol is a precursor in the synthesis of steroids, and it influences activity in the sonic hedgehog pathway, an important signaling cascade for the morphogenesis of the brain [Guy, 2000], among many other tissues. Decreased intracellular cholesterol levels impair the activity of smoothened, an essential component of the hedgehog signaling cascades [Cooper et al., 2003]. Several disorders of cholesterol biosynthesis are associated with structural brain abnormalities. Patients with Smith–Lemli–Opitz syndrome (SLOS), which represents the most common inherited defect of cholesterol biosynthesis, frequently manifest with a spectrum of developmental abnormalities of the central nervous system, such as microcephaly, hypoplasia, or agenesis of the corpus callosum, and holoprosencephaly.
Desmosterolosis, a rare disorder of cholesterol biosynthesis, is caused by mutations in the gene encoding the enzyme 24-dehydrocholesterol reductase (DHCR24). To date, desmosterolosis has been described in only two patients. FitzPatrick et al. [1998] reported the first patient with desmosterolosis in 1998; a premature female infant of 34 weeks gestation with multiple congenital malformations, including cleft palate, total anomalous pulmonary venous return, ambiguous genitalia, and rhizomesomelia with generalized osteosclerosis [FitzPatrick et al., 1998]. This patient died at 1 hr of life. A 3-year-old patient described by Andersson et al. [2002] had microcephaly, absent corpus callosum, submucous cleft palate, cutis aplasia, arthrogryposis, and, at age 11 years, exhibits severe to profound intellectual disability (H. Andersson, personal communication).
Here we present detailed phenotypic, molecular, and biochemical characterization of a third patient with desmosterolosis who presented with brain malformations, dysmorphic facial features, and arthrogryposis. We compare the phenotype of our patient with that of the two previously reported cases of desmosterolosis.
CLINICAL SUMMARY
The patient was born at 34 weeks gestation to a 20-year-old G2, P1 mother via spontaneous vaginal delivery. Apgar scores were 9 at 1 and 5 min. Birth weight was 1,722 g (10–25th centile; −1 SD), length 39.5 cm (<10th centile; −2 SD), and FOC 32 cm (50–75th centile; +0.5 SD). The prenatal history was significant for late antenatal care. The mother’s first prenatal sonographic examination 1 day prior to delivery showed hydrocephalus, agenesis of the corpus callosum, arthrogryposis, and retrognathia. Examination at birth showed a female infant in no acute distress with relative macrocephaly, prominent forehead, and metopism. Dysmorphic facial features included telecanthus, a short nose with anteverted nares, low-set ears, and mild arthrogryposis in all four limbs (Fig. 1A). The patient preferred to hold her limbs in flexion, but there was also true limitation of extension of the major joints of both upper and lower extremities. In addition, there was bilateral fifth finger clinodactyly, mild cutaneous 2–4 toe syndactyly, and proximal placement of the great toes (Fig. 1B).
FIG. 1.
Clinical photographs. A: Patient at age 3 weeks. Note flexion of all four extremities, consistent with arthrogryposis. Relative macrocephaly, prominent forehead, and widely open metopic suture were noted. B: AP view of the face highlights the short nose and anteverted nares. C,D: The patient’s feet shows mild cutaneous 2–4 toe syndactyly and proximally placed great toes. E: Patient at age 8 months. Note rhizomesomelic shortening of the extremities. F: Profile at 8 months is significant for progressive relative macrocephaly with frontal bossing. Head circumference was at the 95th percentile for age, while length was 4.5 SD below the mean. Note again the short nose with anteverted nares.
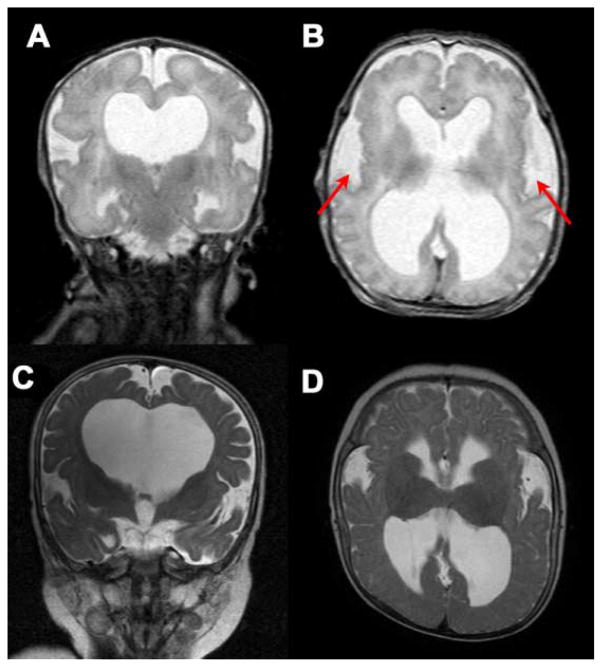
Postnatal chromosome analysis revealed a normal female karyotype, 46,XX. Radiological studies disclosed eventration of the right anterior medial diaphragm, generalized shortening of the extremities, and slight heterogeneity of bone mineralization at the metaphyses of several long bones. A brain MRI at age 3 days showed an immature (~34 weeks) brain with mildly effaced gyral pattern (Fig. 2). There was enlargement of the lateral and third ventricles and significant thickening of the tectum and massa intermedia. The lateral ventricles were also dysmorphic, particularly in the temporal horns, and the septum pellucidum was absent. The fourth ventricle appeared normal in size. The sylvian fissure was notably open (underopercularization), perhaps related to the reduced volume of white matter and ventriculomegaly hydrocephalus. The corpus callosum was markedly thinned but appeared present at the level of the body and genu.
FIG. 2.
T2-weighted MRI images of the brain. A: Coronal view at 3 days of age. Note dilatation of the lateral ventricles and absence of the septum pellucidum. B: Ventriculomegaly, abnormal opercularization, and mildly effaced gyral pattern at age 3 days. C: Progression of hydrocephalus at age 8 months. D: Similar brain abnormalities were still evident at 8 months of age.
The patient exhibited developmental delay. At the age of 8 months, the patient had limited head control, was not able to sit without support, and did not reach for toys, although she cooed and babbled in monosyllables. She was noted to have significant shortening of all four extremities, which was confirmed by radiographs and measurement of the long bones (Table I). Because of progression of a non-communicating hydrocephalus, the patient underwent placement of a ventriculo-peritoneal shunt at the age of 8 months.
TABLE I.
Measurement of Long Bones Based on Radiographs at Age 8 Months
| Bone | Patient (8 months) | Mean (6 months) | Range (6 months) |
|---|---|---|---|
| Humerus | 7.52 | 8.74 | 8.22–9.12 |
| Radius | 5.45 | 6.74 | 6.37–7.14 |
| Ulna | 6.13 | 7.58 | 7.09–7.92 |
| Femur | 10.05 | 11.15 | 10.57–11.58 |
| Tibia | 7.97 | 8.87 | 8.20–9.48 |
| Fibula | 7.40 | 8.52 | 7.74–9.00 |
All values are provided in centimeters. Right and left sides were measured and the mean of both sides is shown. Patient’s measurements (at 8 months of age) are compared to normal female controls at 6 months of age (8-month values not available) [Maresh, 1955].
The combination of congenital anomalies of the central nervous system along with the 2–4 toe syndactyly suggested the diagnosis of SLOS or other cholesterol biosynthesis defects, therefore a plasma sterol panel was ordered.
METHODS
Plasma Sterol Analysis
Plasma sterols were quantified by selected ion-monitoring gas chromatography/mass spectrometry (GC/MS) as previously described [Kelley, 1995].
Mutation Analysis
Exons 1–9 and flanking intron sequences of the DHCR24 gene were PCR amplified from the patient’s genomic DNA using primer pairs tagged with either −21M13 (5′-TGTAAAACGACGGCCAGT-3′) or M13rev (5′-CAGGAAACAGCTATGACC-3′) extensions followed by sequencing as previously described [Waterham et al., 2001].
Predicted Structural Changes on DHCR24
To evaluate the effect of the amino acid substitutions p.R94H and p.E480K, we used the crystal structure of the closest available homolog of DHCR24 and the evolutionary trace (ET) method [Lichtarge et al., 1996; Mihalek et al., 2006; Morgan et al., 2006]. The closest homolog structure found was cytokinin dehydrogenase from Arabidopsis thaliana (PDB ID: 2exr), which shares 20% sequence identity with DHCR24. The root mean square deviation between the model and the real DHCR24 structures was estimated to be around 0.2 nm (2 Å) suggesting that it can be used for structural modeling of the human DHCR24 [Chothia and Lesk, 1986]. The ET method used the structure and an alignment of 140 sequences from 80 different sources (including animals, plants, bacteria, and viruses). To assay the effect of each mutation, we also determined if they belonged to any functional sites and how the local sequence (20 closest residues) and structural environment (residues within 1 nm) of each mutant change to accommodate sequence alterations during evolution [Ward et al., 2008].
Human DHCR24 cDNA Expression in S. cerevisiae
To study the effect of the mutations on enzyme activity, both mutant proteins were expressed in the yeast S. cerevisiae. Total RNA was isolated from the patient’s fibroblasts using Trizol (Invitrogen, Carlsbad, CA) extraction and a total cDNA fraction prepared using a first strand cDNA synthesis kit for RT-PCR (Roche, Indianapolis, IN). The total cDNA fraction was subsequently used to PCR amplify the DHCR24 cDNAs containing the p.R94H and the p.E480K mutations. The DHCR24-R94H and DHCR24-E480K cDNAs were first cloned in the pGEM-T vector (Promega, Madison, WI) followed by sequence analysis for sequence verification and exclusion of PCR-introduced errors. Subsequent cloning of the cDNAs in the yeast-expression plasmid pYES vector followed by expression in S. cerevisiae were done as described previously [Waterham et al., 2001].
DHCR24 Enzyme-Activity Measurements
The activities of wild-type (DHCR24-WT) and both mutant DHCR24 enzymes (DHCR24-R94H and DHCR24-E480K) were determined in yeast homogenates by measuring the production of cholesterol from desmosterol, as previously described [Waterham et al., 2001].
RESULTS
Quantification of plasma sterols by GC/MS was performed on day of life five. A markedly increased level of desmosterol of 738 μmol/L (normal, 2.1 ± 1.2 μmol/L (SD), N = 150) was detected, consistent with the diagnosis of desmosterolosis due to DHCR24 deficiency. The cholesterol level, 2.41 mmol/L, was slightly above upper limit of normal for a 3-day-old infant (1.68 ± 0.31 mmol/L (SD), N = 12). Both 7-dehydrocholesterol [0.10 μmol/L, normal of 0.42 ± 0.23 μmol/L (SD), n = 706] and lathosterol [0.88 μmol/L, normal of 2.51 ± 1.24 μmol/L (SD), n = 177] were in the normal range.
Sequence analysis of all exons and flanking intronic sequences of DHCR24 of the patient and her parents revealed compound heterozygosity for two novel mutations c.281G>A (p.R94H) and c.1438G>A (p.E480K), in the patient, with c.281G>A inherited from the mother and c.1438G>A from the father.
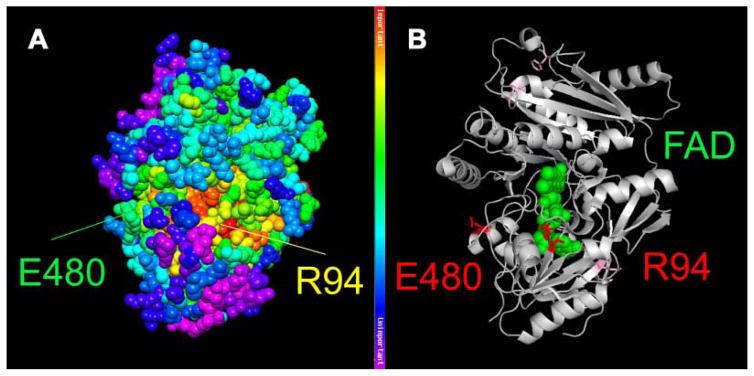
Based on the evolutionary and structural analyses of the DHCR24 protein, both amino acid substitutions (Fig. 3A,B) are predicted to have a severe deleterious effect on enzyme function. The p.R94H mutation is predicted to be located at the FAD binding site, which is almost invariant in DHCR24 orthologs from plantae through animalia. The p.E480K mutation is predicted to be located near a solvent-accessible site of milder evolutionary importance (top 50%). Residue E480 is invariant in animalia sequences, but it is replaced by other hydrophilic amino acids in Plantea sequences. However, the p.E480K substitution is subsequently accompanied by several charge shift changes in the local sequence and structural environment.
FIG. 3.
Structural model of DHCR24. Mapping of the patient’s mutations (p.R94H and p.E480K) onto the closest homologue for which structural data is available (cytokinin oxidase/dehydrogenase; CKX) from Arabidopsis thaliana (PDB id: 2exr). A: Mapping provides information about evolutionary importance of respective amino acids, with red being most highly conserved and purple being least conserved. B: Depiction of the two affected amino acids in red and mutations of previously reported patients are shown as light purple sticks. An FAD molecule is represented as green balls, illustrating that the p.R94H substitution affects the FAD binding domain of the protein. The figure was generated by using PyMOL (DeLano Scientific LLC, Palo Alto, CA) and the Protein Data Bank file: 2exr.
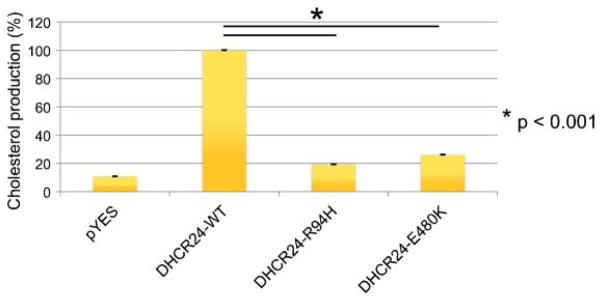
To test the effect of the mutations on DHCR24 enzymatic activity, we expressed the two patient’s DHCR24 cDNAs separately in S. cerevisiae, a eukaryotic organism devoid of endogenous sterol-Δ24-reductase activity and thus normally incapable of cholesterol synthesis. The cDNAs were PCR amplified from cDNA synthesized using RNA isolated from the patient’s fibroblasts, subcloned into a yeast expression vector (pYES) and then expressed in S. cerevisiae. Incubation of yeast homogenates with desmosterol revealed a significant decrease of enzyme activity as compared to wild-type DHCR24, with the effect of the DHCR24-R94H mutation being somewhat more severe than that of the DHCR24-E480K mutation (Fig. 4).
FIG. 4.
Yeast expression studies. cDNAs of the patient’s mutations were cloned into a yeast expression vector (pYES). Production of cholesterol from desmosterol was measured in yeast homogenates. pYES: empty vector control; DHCR24-WT: wild-type DHCR24 activity, set at 100%; DHCR24-R94K and DHCR24-E480K: patient’s mutations.
DISCUSSION
Desmosterolosis is a rare, possibly underdiagnosed disorder of cholesterol biosynthesis. Only two patients have been reported in the literature [FitzPatrick et al., 1998; Andersson et al., 2002]. Here we report on the third case of desmosterolosis in a female neonate with congenital hydrocephalus, dysmorphic features, diaphragmatic eventration, mild arthrogryposis, and rhizomesomelic shortening of all four limbs. Brain MRI examination revealed hydrocephalus, absent septum pellucidum, thin corpus callosum, mildly effaced gyral pattern, and incomplete opercularization. The three cases of desmosterolosis and their clinical features are contrasted in Table II.
TABLE II.
Clinical Features of Desmosterolosis
| FitzPatrick et al. [1998] | Andersson et al. [2002] | This report | ||
|---|---|---|---|---|
| Sex | Female | Male | Female | |
| Karyotype | 46,XX | 46,XY | 46,XX | |
| Viability | No | Yes | Yes | |
| Developmental delay/intellectual disability | N/A | Yes | Yes | |
| Brain malformations | Immature gyration | Agenesis CC | Hydrocephalus | |
| Dilated ventricles | Hypoplastic CC | |||
| Hypoplastic CC | Absent septum pellucidum | |||
| Underopercularization | ||||
| Skeletal anomalies | Osteosclerosis | Talipes equinovarus | Congenital contractures | |
| Rhizomesomelia | Mild contractures of bilateral hands | Rhizomesomelia | ||
| Syndactyly | None | Not reported | Mild, but present 2–4 toe syndactyly | |
| Facial features | Frontal bossing | Downslanting palpebral fissures | Prominent forehead | |
| Depressed nasal bridge | Bilateral epicanthal folds | Short nose | ||
| Microstomia | Micrognathia | Anteverted nares | ||
| Low-set, abnormal ears | Telecanthus | |||
Cholesterol influences the morphogenesis of the human brain and is also known to be an important constituent of the central nervous system. In SLOS, the most common disorder of cholesterol biosynthesis, structural brain anomalies occur in 37% of cases [Hennekam, 2005]. Enlarged ventricles, hypoplastic frontal lobes, hypoplastic or absent corpus callosum, cerebellar hypoplasia, and holoprosencephaly are among the most commonly reported structural defects [Kelley and Hennekam, 2000]. Microcephaly is present in at least 90% of patients. The first patient with desmosterolosis reported in the literature was found to have macrocephaly, premature gyral pattern, poor development of the corpus callosum, and gross dilatation of the ventricles [FitzPatrick et al., 1998], and the second was found to have microcephaly with agenesis of the corpus callosum [Andersson et al., 2002]. While isolated underopercularization can be observed in premature infants, it is rarely observed in full-term neonates, although as noted above, the overall gyral development was equal to 34 weeks gestation. Underopercularization has been reported in glutaric aciduria type I, Aicardi syndrome, and other neuro-developmental disorders [Amir et al., 1987; Hopkins et al., 2008]. There are also several cases in the literature of unknown etiology. For example, Levin et al. [1993] reported a male neonate with unilateral underopercularization, pachygyria, contractures, and genital abnormalities, and compared that case to three other similar cases, one of which was the product of a consanguineous relationship.
Primary defects of cholesterol biosynthesis can present with skeletal abnormalities, of which rhizomesomelia is the most common and prominent. Conradi–Hunermann–Happle and CHILD (congenital hemidysplasia, ichthyosis, and limb defects) syndromes are associated with asymmetric rhizomesomelia or other limb defects. Syndactyly of toes 2–3 is observed in most patients with SLOS. Unilateral or bilateral postaxial polydactyly of the hands and/or feet is also common in these patients. No syndactyly was reported in the two known patients with desmosterolosis. The proposita reported herein displayed mild cutaneous 2–4 toe syndactyly, which is an uncommon finding in SLOS and, when present, is structurally different than seen in this infant. While, chondrodysplasia punctata is a prominent radiological finding in Conradi–Hunermann–Happle syndrome (CDPX2) and CHILD syndrome, the skeletal survey in our patient did not show epiphyseal stippling. Plain radiographs of our patient also did not show the osteosclerosis described in the first reported patient with desmosterolosis [FitzPatrick et al., 1998] but bone mineral density studies were not performed.
Diaphragmatic eventration is s a rare congenital malformation. It typically occurs in infants with diaphragmatic paralysis, as a consequence of birth trauma, such as brachial plexus injury, which was not present in this patient. Diaphragmatic eventration also occurs in Fryns syndrome and spinal muscular atrophy with respiratory distress (SMARD). It has been reported sporadically in individuals with Poland, Wandering spleen, and Matthew–Woods syndromes [Stratton et al., 1993; Chitayat et al., 2007; Golzio et al., 2007; Guenther et al., 2007; Kulkarni et al., 2007].
Importantly, the patient reported in this manuscript had multiple congenital anomalies, including several of the central nervous and skeletal systems. As seen in Table II, the clinical features among patients with desmosterolosis are diverse, and not always suggestive of disorders of cholesterol biosynthesis, per se. Nevertheless, the combination of central nervous system anomalies and peripheral skeletal findings (even atypical and mild as in our patient) should suggest a cholesterol biosynthesis defect. Sequence analysis of DHCR24 in our patient revealed two novel mutations in trans configuration, as confirmed by parental studies. The structural mapping and evolutionary analysis of the respective amino acids strongly suggested that these mutations impair DHCR24 function. This conclusion was further supported by expression of the patient’s cDNAs in yeast to measure enzyme activity, which demonstrated that both mutations decrease DHCR24 activity significantly.
The plasma cholesterol levels in this patient were not decreased, suggesting that the residual activity of the patient’s DHCR24 still allows normal postnatal synthesis of cholesterol, although this was associated with clearly elevated levels of desmosterol. A similar observation was made in the other previously reported and still living patient with desmosterolosis [Andersson et al., 2002], and a normal plasma cholesterol level is common in patients with milder presentations of SLOS and in almost all patients with CDPX2, CHILD syndrome, and sterol-C4-methyl oxidase-like (SC4MOL) deficiency. However, the ability to maintain a normal cholesterol level, which in blood is largely determined by hepatic cholesterol synthesis, might not exit in other tissues, especially brain and cartilage, which must synthesize all cholesterol in situ. Moreover, because the rapid cell growth characteristic of the embryonic period probably demands faster rates of cholesterol synthesis than in the postnatal period, deleterious deficiencies of cholesterol or toxic levels of sterol intermediates might occur in some tissues only during the embryonic period. Indeed, as shown in Anderson et al. [1998], SLOS lymphoblasts in stationary phase with near normal 7DHC levels show striking increases in their 7DHC levels when subcultured in fresh cholesterol-free medium and induced to grow rapidly.
While differences in embryologic rates of cell division and the inability of certain tissues to benefit from exogenous cholesterol from hepatic cholesterol synthesis or maternal transplacental supply could explain both the rhizomelia and hypoplastic corpus callosum seen in our patient, it is difficult to speculate on the cause of many other desmosterolosis malformations, which cannot be explained by decreased hedgehog signaling or other consequences of low cellular cholesterol levels. Moreover, although knockout mice for sterol synthesis enzymes are imperfect models for human sterol teratogenesis because of higher rates of maternal cholesterol transfer to the fetus, the absence of brain, visceral, and skeletal malformations despite the essential absence of cholesterol in DHCR24 knockout mice [Wechsler et al., 2003; Mirza et al., 2006] casts doubt on the possibility that high levels of desmosterol are teratogenic. These embryological considerations and the greatly differing phenotypes of the three known cases of desmosterolosis necessarily suggest the possibility that the biochemical abnormality in desmosterolosis is a bias of ascertainment or a necessary but not sufficient cause of the three different desmosterolosis phenotypes described in Table I. In that case, the patients would be better described as having desmosterolemia.
No definitive treatment for disorders of cholesterol biosynthesis is available to date. For SLOS, treatment strategies to increase bioavailability of cholesterol or to decrease the accumulation of 7-dehydrocholesterol have been studied. Cholesterol supplementation has been tried often, but improvement of neurobehavioral and developmental outcomes remains mostly anecdotal [Elias et al., 1997; Nwokoro and Mulvihill, 1997; Sikora et al., 2004; Tierney et al., 2010]. A combination of cholesterol supplementation and simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, has been tested in children with SLOS and found to lower 7-dehydrocholesterol levels. However, a positive effect on anthropomorphic measurements and behavior has not been observed [Haas et al., 2007; Chan et al., 2009].
DHCR24 is an FAD-dependent oxidoreductase that is strictly dependent on the presence of reduced NADPH [Waterham et al., 2001]. We therefore asked whether or not optimizing FAD and NADPH levels might increase the residual DHCR24 activity in our patient. FAD is synthesized from riboflavin in 2 ATP-dependent catalytic steps by riboflavin kinase and FAD-pyrophosphatase. The second important cofactor of DHCR24, NADPH, is synthesized from NAD through phosphorylation by NAD-kinase. The major source of NADPH in human metabolism is the oxidative pentose phosphate pathway. Transketolase, a key enzyme of the pentose phosphate pathway, is dependent on thiamine (vitamin B1) in the form of thiamine diphosphate as a cofactor of the reaction. We therefore reasoned that supplementation of thiamine might increase NADPH production, with supplemental nicotinamide augmenting the production of NAD. Accordingly, we hypothesized that treatment of desmosterolosis with riboflavin, nicotinamide, and thiamine might augment DHCR24 activity in patients with desmosterolosis. While treatment in our patient was initiated with riboflavin 15, nicotinamide 100, and thiamine 15 mg/kg/day, there were major concerns regarding non-compliance and a follow-up sterol analysis by GC/MS at 13 months of life showed no significant decrease in desmosterol levels and the cholesterol level stayed close to the normal range. The desmosterol/cholesterol ratio had decreased slightly to 0.24 (as compared to 0.30 at the time of diagnosis), a change that was not statistically significant. Additional studies investigating the effect of vitamins and cofactors in desmosterolosis and other sterol biosynthesis defects may be considered, but may not be conclusive due to the small number of patients and the severe congenital malformations associated with these conditions. However, an N-of-1 clinical trial could be evaluated in an attempt to find the best possible treatment for a particular patient.
To conclude, we suggest considering desmosterolosis and other defects of cholesterol biosynthesis in the differential diagnosis of individuals with brain malformations, global developmental delay or intellectual disability, diaphragmatic eventration, limb contractures, or arthrogryposis.
Acknowledgments
The authors thank Liz Bernica and Racquel Young for their tremendous help in this study and their efforts in trying to facilitate follow-up evaluations of this patient in our clinic. Petra Mooijer and Wilma Smit are acknowledged for technical assistance. The authors thank William B. Dobyns (Center for Integrative Brain Research, Seattle Children’s Research Institute) for his valuable input discussing the significance of this patient’s brain phenotype.
References
- Amir N, el-Peleg O, Shalev RS, Christensen E. Glutaric aciduria type I: Clinical heterogeneity and neuroradiologic features. Neurology. 1987;37:1654–1657. doi: 10.1212/wnl.37.10.1654. [DOI] [PubMed] [Google Scholar]
- Andersson HC, Kratz L, Kelley R. Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. Am J Med Genet. 2002;113:315–319. doi: 10.1002/ajmg.b.10873. [DOI] [PubMed] [Google Scholar]
- Chan YM, Merkens LS, Connor WE, Roullet JB, Penfield JA, Jordan JM, Steiner RD, Jones PJ. Effects of dietary cholesterol and simvastatin on cholesterol synthesis in Smith-Lemli-Opitz syndrome. Pediatr Res. 2009;65:681–685. doi: 10.1203/PDR.0b013e31819ea4eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitayat D, Sroka H, Keating S, Colby RS, Ryan G, Toi A, Blaser S, Viero S, Devisme L, Boute-Bénéjean O, Manouvrier-Hanu S, Mortier G, Loeys B, Rauch A, Bitoun P. The PDAC syndrome (pulmonary hypoplasia/agenesis, diaphragmatic hernia/eventration, anophthalmia/micro-phthalmia, and cardiac defect) (Spear syndrome, Matthew-Wood syndrome): Report of eight cases including a living child and further evidence for autosomal recessive inheritance. Am J Med Genet Part A. 2007;143A:1268–1281. doi: 10.1002/ajmg.a.31788. [DOI] [PubMed] [Google Scholar]
- Chothia C, Lesk AM. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986;5:823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Elias ER, Irons MB, Hurley AD, Tint GS, Salen G. Clinical effects of cholesterol supplementation in six patients with the Smith-Lemli-Opitz syndrome (SLOS) Am J Med Genet. 1997;68:305–310. doi: 10.1002/(sici)1096-8628(19970131)68:3<305::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- FitzPatrick DR, Keeling JW, Evans MJ, Kan AE, Bell JE, Porteous ME, Mills K, Winter RM, Clayton PT. Clinical phenotype of desmosterolosis. Am J Med Genet. 1998;75:145–152. [PubMed] [Google Scholar]
- Golzio C, Martinovic-Bouriel J, Thomas S, Mougou-Zrelli S, Grattagliano-Bessieres B, Bonniere M, Delahaye S, Munnich A, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T, Etchevers HC. Matthew–Wood syndrome is caused by truncating mutations in the retinol-binding protein receptor gene STRA6. Am J Hum Genet. 2007;80:1179–1187. doi: 10.1086/518177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther UP, Varon R, Schlicke M, Dutrannoy V, Volk A, Hubner C, von Au K, Schuelke M. Clinical and mutational profile in spinal muscular atrophy with respiratory distress (SMARD): Defining novel phenotypes through hierarchical cluster analysis. Hum Mutat. 2007;28:808–815. doi: 10.1002/humu.20525. [DOI] [PubMed] [Google Scholar]
- Guy RK. Inhibition of sonic hedgehog autoprocessing in cultured mammalian cells by sterol deprivation. Proc Natl Acad Sci USA. 2000;97:7307–7312. doi: 10.1073/pnas.97.13.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D, Garbade SF, Vohwinkel C, Muschol N, Trefz FK, Penzien JM, Zschocke J, Hoffmann GF, Burgard P. Effects of cholesterol and simvastatin treatment in patients with Smith–Lemli–Opitz syndrome (SLOS) J Inherit Metab Dis. 2007;30:375–387. doi: 10.1007/s10545-007-0537-7. [DOI] [PubMed] [Google Scholar]
- Hennekam RC. Congenital brain anomalies in distal cholesterol biosynthesis defects. J Inherit Metab Dis. 2005;28:385–392. doi: 10.1007/s10545-005-7055-2. [DOI] [PubMed] [Google Scholar]
- Hopkins B, Sutton VR, Lewis RA, Van den Veyver I, Clark G. Neuroimaging aspects of Aicardi syndrome. Am J Med Genet Part A. 2008;146A:2871–2878. doi: 10.1002/ajmg.a.32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons M, Elias ER, Abuelo D, Bull MJ, Greene CL, Johnson VP, Keppen L, Schanen C, Tint GS, Salen G. Treatment of Smith–Lemli–Opitz syndrome: Results of a multicenter trial. Am J Med Genet. 1997;68:311–314. [PubMed] [Google Scholar]
- Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- Kelley RI. Diagnosis of Smith–Lemli–Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin Chim Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Hennekam RC. The Smith–Lemli–Opitz syndrome. J Med Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni ML, Sneharoopa B, Vani HN, Nawaz S, Kannan B, Kulkarni PM. Eventration of the diaphragm and associations. Indian J Pediatr. 2007;74:202–205. doi: 10.1007/s12098-007-0018-x. [DOI] [PubMed] [Google Scholar]
- Levin ML, Lupski JR, Carpenter RJ, Jr, Gerson LP, Greenberg F. An additional case of pachygyria, joint contractures and facial abnormalities. Clin Dysmorphol. 1993;2:365–368. [PubMed] [Google Scholar]
- Lichtarge O, Bourne HR, Cohen FE. An evolutionary trace method defines binding surfaces common to protein families. J Mol Biol. 1996;257:342–358. doi: 10.1006/jmbi.1996.0167. [DOI] [PubMed] [Google Scholar]
- Maresh MM. Linear growth of long bones of extremities from infancy through adolescence; continuing studies. AMA Am J Dis Child. 1955;89:725–742. [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mihalek I, Res I, Lichtarge O. Evolutionary trace report-maker: A new type of service for comparative analysis of proteins. Bioinformatics. 2006;22:1656–1657. doi: 10.1093/bioinformatics/btl157. [DOI] [PubMed] [Google Scholar]
- Mirza R, Hayasaka S, Takagishi Y, Kambe F, Ohmori S, Maki K, Yamamoto M, Murakami K, Kaji T, Zadworny D, Murata Y, Seo H. DHCR24 gene knockout mice demonstrate lethal dermopathy with differentiation and maturation defects in the epidermis. J Invest Dermatol. 2006;126:638–647. doi: 10.1038/sj.jid.5700111. [DOI] [PubMed] [Google Scholar]
- Morgan DH, Kristensen DM, Mittelman D, Lichtarge O. ET viewer: An application for predicting and visualizing functional sites in protein structures. Bioinformatics. 2006;22:2049–2050. doi: 10.1093/bioinformatics/btl285. [DOI] [PubMed] [Google Scholar]
- Nwokoro NA, Mulvihill JJ. Cholesterol and bile acid replacement therapy in children and adults with Smith-Lemli-Opitz (SLO/RSH) syndrome. Am J Med Genet. 1997;68:315–321. doi: 10.1002/(sici)1096-8628(19970131)68:3<315::aid-ajmg13>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Sikora DM, Ruggiero M, Petit-Kekel K, Merkens LS, Connor WE, Steiner RD. Cholesterol supplementation does not improve developmental progress in Smith–Lemli–Opitz syndrome. J Pediatr. 2004;144:783–791. doi: 10.1016/j.jpeds.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Stratton RF, Young RS, Heiman HS, Carter JM. Fryns syndrome. Am J Med Genet. 1993;45:562–564. doi: 10.1002/ajmg.1320450507. [DOI] [PubMed] [Google Scholar]
- Tierney E, Conley SK, Goodwin H, Porter FD. Analysis of short-term behavioral effects of dietary cholesterol supplementation in Smith–Lemli–Opitz syndrome. Am J Med Genet Part A. 2010;152A:91–95. doi: 10.1002/ajmg.a.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RM, Erdin S, Tran TA, Kristensen DM, Lisewski AM, Lichtarge O. De-orphaning the structural proteome through reciprocal comparison of evolutionarily important structural features. PLoS One. 2008;3:e2136. doi: 10.1371/journal.pone.0002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Koster J, Romeijn GJ, Hennekam RC, Vreken P, Andersson HC, FitzPatrick DR, Kelley RI, Wanders RJ. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am J Hum Genet. 2001;69:685–694. doi: 10.1086/323473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler A, Brafman A, Shafir M, Heverin M, Gottlieb H, Damari G, Gozlan-Kelner S, Spivak I, Moshkin O, Fridman E, Becker Y, Skaliter R, Einat P, Faerman A, Bjorkhem I, Feinstein E. Generation of viable cholesterol-free mice. Science. 2003;302:2087. doi: 10.1126/science.1090776. [DOI] [PubMed] [Google Scholar]