Abstract
1. Objective
Combine collective clinical experience using oral propranolol therapy in PHACE syndrome infants with cerebrovascular anomalies.
2. Design
Retrospective study of patients evaluated between July 2008 and October 2011.
3. Setting
Seven pediatric dermatology centers.
4. Patients
32 infants with definite PHACE syndrome and cervical and/or intracranial arterial anomalies.
5. Intervention
Oral propranolol: average dose of 1.8 mg/kg/day divided t.i.d. or b.i.d., for an average duration of 12.3 months.
6. Main Outcome Measure
Adverse neurologic events.
7. Results
7/32 (22%) patients were categorized as higher-risk for stroke, defined on MRA as severe, long-segment narrowing or non-visualization of major cerebral or cervical vessels without anatomic evidence for collateral circulation, often in the presence of concomitant cardiovascular comorbidities. Only 1 patient developed a change in neurologic status during propranolol treatment: a mild right hemiparesis that remained static and improved while propranolol was continued. An additional 3 patients had worsening hemangioma ulceration and/or tissue necrosis during therapy.
8. Conclusions
This is the largest report thus far of PHACE patients treated with propranolol. While no catastrophic neurologic events occurred, serious complications, particularly severe ulcerations were seen in a minority of patients, and given the sample size we cannot negate the possibility that propranolol could augment the risk of stroke in this population. We continue to advise caution in using systemic beta-blockers, particularly for children with vascular anomalies at higher risk for stroke. Use of the lowest possible dosage, slow dosage titration, and t.i.d. dosing in order to minimize abrupt changes in blood pressure and close follow-up, including neurologic consultation as needed, are recommended.
Infantile hemangioma (IH) is the most common, benign tumor of childhood.1 A significant subset of children with IH will require systemic therapy for actual or potential medical complications or risk of permanent facial disfigurement and scarring. Propranolol hydrochloride, a nonselective beta-adrenergic blocking agent, is rapidly becoming the standard of care for such patients, due to superior efficacy and an improved side effect profile compared to alternative agents. It has been observed that larger, so-called “segmental” IH of the face, which are at greatest risk for complications, often have the most dramatic response to propranolol.2 However, such patients are also at-risk for PHACE syndrome (OMIM 606519), the cutaneous-neuro-vascular syndrome characterized by IH in conjunction with congenital anomalies of the brain, heart, eyes and chest wall.3
Cerebrovascular anomalies are the predominant extracutaneous features of PHACE; accordingly, neurologic impairments are the greatest source of potential morbidity.4 It is known that a small proportion of PHACE patients with anomalies of major cervical and cerebral vessels develops arterial stenoses and occlusions that may lead to acute arterial ischemic stroke (AIS).5–7 While it is recognized that similar congenital anomalies can also affect the aorta, less is known about the cardiovascular risks in PHACE.8
Propranolol has been well-studied in adults; the most common serious adverse effects include bradycardia and hypotension. While the drug has been used in children for many years for both cardiac and noncardiac disease, pediatric experience has been largely anecdotal and propranolol is not currently approved for pediatric use. Some experts have advised particular caution when using propranolol in PHACE patients with cervical or cerebral arteriopathy, as systemic hypotension carries the theoretical risk of reducing blood flow through narrowed or occluded arteries supplying the brain.9–11 The potential for hypoperfusion in PHACE is underscored by recent reports of transient ischemic attacks in two adult PHACE patients with severe cerebrovascular anomalies.12, 13. Although PHACE is not an absolute contraindication to the use of beta-blockers, benefits versus potential risks must be carefully weighed. It has been recommended that at-risk infants with large facial IH be thoroughly investigated for PHACE syndrome (especially for the presence of arteriopathy) prior to initiating therapy, with consultation with a neurologist and/or cardiologist when appropriate.14
This retrospective review combines the collective clinical experience in 32 PHACE patients from 7 centers with cervical and intracranial arterial anomalies who received oral propranolol therapy.
Methods
This was an IRB-approved, retrospective study of 32 PHACE infants with cerebral and/or cervical arteriopathy who were evaluated between July 2008 and October 2011 and treated with oral propranolol. A minority of these patients has been reported in prior publications.4, 15–22 Data was obtained from a group of pediatric dermatologists, all members of the Hemangioma Investigator Group (HIG) with special expertise in the field of hemangiomas and PHACE syndrome, who attended a multidisciplinary research conference on PHACE syndrome in Milwaukee, Wisconsin from September 15–16, 2010. All enrolled patients underwent complete evaluations for PHACE syndrome, including a thorough skin and ophthalmologic examination, MRI and MRA imaging of the head and neck, and cardiac imaging to include the aortic arch. Inclusion criteria were the diagnosis of definitive PHACE based on recently established consensus criteria23, the presence of cervical or cerebral arteriopathy involving the internal carotid, vertebral or basilar arteries or the anterior, middle or posterior cerebral arteries, and treatment with propranolol for at least 6 months. Patients with persistent embryonic arteries (e.g. persistent trigeminal artery) in isolation were excluded.
Clinical data were collected from medical records using standardized data abstraction forms. Completed forms were centrally reviewed and analyzed at Baylor College of Medicine. IH location on the face was specified based on a published segment map by Haggstrom et al.24. Arterial anomalies were categorized based on published definitions by Hess et al.19, with narrowing defined as severe if a > 75% reduction in luminal cross-sectional diameter compared to normal was observed, as determined by a neuroradiologist.19 A neuroradiologist with special expertise in PHACE syndrome (C.H.) reviewed MRA neuroimaging features of individual cases in order to to categorize patients as standard or higher risk for AIS based on large artery absence or narrowing or and the presence or absence of collateral arterial supply. For patients who experienced changes in neuroimaging or neurologic status during propranolol therapy, additional information requested included baseline and follow-up neurology and neuroimaging reports and neuroimages on DVD.
Results
Table 1.
Propranolol Use in 32 PHACE Syndrome Infants with Cervical and Intracranial Arterial Anomalies: Overall Results
| Category |
| Results: n (%) [case number(s)] |
| IH Location |
| S1 and/or S3: 30 (94%) |
| S4 only: 2 |
| Extensive, unilateral facial involving all 3 segments or bilateral facial: 17 (53%) |
| Scalp: 9 (28%) |
| Visceral: 15 (47%); airway: 10, CNS: 5, g.i.: 3, paraspinous muscle: 1, spleen: 1, liver: 1 |
| Cerebral or Cervical Arteriopathy: 32 (100%) |
| Dysplasia: 20 (63%) |
| Hypoplasia: 17 (53%) |
| Aberrant origin or course: 11 (34%) |
| Narrowing/stenosis: 12 (38%) [cases 2–5, 8, 11, 12 (severe, long-segment), 13, 22, 25, 28, 32] |
| Non-visualization/absence: 6 (19%): [cases 8, 11, 12, 18, 19, 32] |
| Other: persistent trigeminal a: 3, fetal posterior communicating a: 2, aneurysm [case 10], possible aneurysm [case 18], possible dural arteriovenous fistula [case 12] |
| Structural CNS Anomalies: 13 (41%) |
| Cardiovascular Anomalies: 11 (34%) |
| Aberrant rt subclavian artery: 7 (22%) |
| Aortic arch anomalies: 6; mild coarctation with dysplasia [case 2], severe coarctation requiring surgical repair [case 22], coarctation with transverse arch narrowing [case 28], rt aortic arch [case 25], rt aortic arch with dysplasia [case 3], small aneurysm versus pseudoaneurysm [case 32] |
| Other: Patent ductus arteriosus [cases 6, 10, 11], anomalous origin/course of cardiac vessel [cases 10, 15, 30], patent foramen ovale [cases 25, 30], pulmonary stenosis [cases 16, 21], atrial septal defect [case 21], ventricular septal defect [case 30] |
| Indications for Propranolol |
| High-risk for facial scarring/disfigurement: 22 (69%) |
| Visual compromise: 15 (47%) |
| Insufficient response to systemic corticosteroids: 9 (28%) |
| Ulceration (actual or potential): 8 (25%) |
| Other: side effects from systemic corticosteroids: 4 (hypertension in 3, cushingoid appearance in 1), airway compromise: 5, g.i. bleeding: 2, auditory obstruction: 1, persistent bulk/slow involution: 1, persistent intraoral IH: 1 |
| Propranolol Dosing |
| Avg age at initiation: 4.8 mos (range: 7 days to 24 mos) |
| Avg daily dose: 1.8 mg/kg/d |
| Frequency: t.i.d. in 23 (72%), b.i.d. in 9 (28%) |
| Avg duration: 12.3 mos in 19 patients who completed therapy (9 pts still on propranolol, 3 lost to follow-up) |
| Propranolol Side Effects |
| Sleep disturbance/night terrors: 2 |
| Single case each: g.i. upset, asymptomatic hypotension, periodic cold hands & feet, constipation |
| Physician’s Assessment of IH Response to Propranolol |
| Excellent: 23 (72%), excellent to moderate: 1, moderate: 6, moderate to mild: 1, mild: 1 |
| Atypical Events during Propranolol Therapy (see also Discussion) |
| Change in neuroimaging: progressive vessel narrowing [case 12] |
| Change in neurologic status: mild hemiparesis [case 11] |
| Worsening IH ulceration/tissue necrosis [cases 25, 28, 30] |
A = artery; avg = average; g.i. = gastrointestinal; IH = infantile hemangioma; mos = months; rt = right
Table 2.
Propranolol Use in 32 PHACE Syndrome Infants with Cervical and Intracranial Arterial Anomalies: Detailed Summary
| Case | IH Location(s) |
Cerebro- vascular Anomalies |
CNS Anomalies |
Cardiovascular Anomalies |
Other Medical |
Indication(s) for Propranolol |
Prop Initiation |
Propranolol: Age initiated/ Duration |
Propranolol dose |
Repeat Cerebro- vascular imaging |
Atypical Events |
Prop side effects/ IH response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lt S1, S2, Rt S1 |
Mild segmental narrowing of cervical Lt ICA, hypoplasia of intradural Lt VA, hypoplasia of A1 segment of Rt ACA |
N | Aberrant subclavian a |
N | Rapid growth, visual compromise, insufficient response to CS |
CS started 1st (1.6 mg/kg/d from 1–3 mos); hosp admit to ICU w trans- dermal monitor |
2 mos/12 mos | 1.6 mg/kg/d divided t.i.d. |
MRA X 2 (baseline & after 6 mos), stable |
N | Night terrors/ E |
| 2 | Lt S1, lt neck |
Dysplastic segment of Lt ICA, mild hypoplasia of Rt VA, hypoplastic A1 segment of Rt ACA & Rt PCOM |
N | Coarctation of the aorta (mild), dysplastic aorta, aberrant subclavian a |
N | Insufficient response to CS & CS side effects (HTN) |
CS started 1st (2.5 mg/kg/d from 1–6 mos); pt referred to cardiology for cardiac anomalies & HTN, HTN txd w prop |
5 mos/6 mos restarted at 14 months for 3 more months |
2.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & after 6 mos), stable |
N | N/E |
| 3 | Bil S1, Rt scalp, chest |
Aberrant Lt subclavian, dysplastic & narrow Rt ICA, hypoplasia of Lt VA |
N | Aberrant Lt subclavian a, Rt aortic arch w dysplasia |
N | Severe visual compromise |
CS started 1st (1.5 mg/kg/d); hosp admit |
1month/present | 1.3 mg/kg/d divided t.i.d |
Pending | N | Sleep disturbance/E |
| 4 | Bil S3, S4, chest |
Hypoplasia of Rt VA, hypoplasia of P1 segment of Rt PCA |
N | N | Sternal cleft |
High risk of facial disfigurement, ulceration |
Initiated inpt by outside practice at .3/mg/kg/day |
1 mo/present | 1 mg/kg/day divided t.i.d. |
Pending | N | N/E |
| 5 | S1 | Hypoplasia of A1 segment of Rt ACA |
Rt cerebellar hypoplasia & hypoplastic vermis |
N | N | Rapid growth, visual compromise |
Outpt at .5mg/kg/day divided tid and tapered up to 1.2 mg/kg/day |
2 mos/present | 1.2 mg/kg/day divided t.i.d. |
N | N | N/E |
| 6 | Rt S1, CNS, airway (retropharyngeal), Rt post paraspinous muscle |
Dysplastic Lt ICA, low bifurcation of Lt CCA, mild hypoplasia of Rt VA, hypoplastic Lt PCOM |
Hypoplastic Lt cerebellar hemisphere, multiple CNS IH |
PDA | Biliary atresia, anemia |
Rapid growth, visual compromise, high risk of facial disfigurement |
CS started 1st (2 mg/kg/d at 1.5 mos X 3 wks), hosp admit for w/u of biliary atresia; rapid upward taper over 36 hrs to full dose since inpt |
2 mos/13 mos | 2.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & after 6 mos), stable |
N | N/E |
| 7 | S4 | Hypoplastic Lt ACA (Lt A1 segment) |
Agenesis of corpus callosum, bil frontal poly- microgyria |
N | Infantile seizures, med- controlled & d/c by 1 yr, slight motor delay |
Rapid growth, high risk of facial disfigurement |
Slower upward taper |
1 mo/12 mos | 2.5–3.0 mg/kg/d divided t.i.d |
N | N | G.I. upset/E |
| 8 | Rt S1, S3, S4, Rt chest, airway |
Hypoplastic, dysplastic Rt PCA w focal stenosis in P2 segment, absent Rt ICA, hypoplastic vertebrobasilar w aberrant origin/course |
N | N | Sternal defect/pit |
Visual compromise, high risk of facial disfigurement, CS side effects (cushingoid features, rebound IH growth w taper) |
CS started 1st (2 mg/kg/d from 3 wks-11 mos), neurology input, hosp admit for initiation |
13 mos/14.5 mos |
1.5 mg/kg/d divided t.i.d |
MRA X 2 (baseline & a t 1 yr of age), stable |
N | Episodes of asymptomatic hypotension led to dose decrease, f/up vitals WNL; mod-E |
| 9 | Rt S1, Rt scalp, post neck |
Hypoplastic Rt ICA, aberrant origin/course of Rt ACA |
N | N | N | Visual compromise, high risk of facial disfigurement |
Standard | 2 mos/6 mos | 2.0 mg/kg/d divided b.i.d |
MRA X 2 (baseline & after 6 mos), stable |
N | N/E |
| 10 | Bil S3, Rt neck, tongue, gingiva |
Dysplastic Rt ICA & PCA, Rt PCA aneurysm, PTA |
N | Small PDA, bronchial collateral vessel off lesser curvature of aortic arch |
N | Rapid growth, high risk of facial disfigurement , ulceration |
Initiated by outside practice |
1.5 mos/13.5 mos |
2.0 mg/kg/d divided t.i.d |
MRA X 2 ( 2 mos & 10 mos of age), stable |
N | N/E w healed ulceration |
| 11 | S4 | Dysplastic Lt ICA & PCA, absent Lt A1 segment, focal narrowing at origin of Lt MCA |
Hypoplast ic Lt middle cerebral peduncle |
Small PDA | Congenital Hypothyroidism |
Rapid growth, high risk of facial disfigurement |
CS started 1st (2 mg/kg/d at 7 wks for 3 mos), hosp admit for initiation, lower initial & average dose |
4 mos/8 mos | 1.0 mg/kg/d divided t.i.d |
MRA X 3 (baseline, after 5 mos & 9 mos), stable |
Lt hand preference noted by parents at 6 mos of age, mild Rt hemiparesis confirmed by neuro; prop continued & started aspirin 40 mg daily |
N/E |
| 12 | Rt S1, Rt hemi- diaphragm, multiple abdominal : spleen, small bowel mesentery , upper anterior abdomen, anterior abdominal wall, bil hepatic lobes |
Aberrant origin/course of Rt cavernous carotid a, severe dysplasia and/or dural AVF of vessels superomedial & lateral to Rt orbital IH, absent Lt PCA, long- segment stenosis of Rt ICA (initially mild) |
N | N | N | Rapid growth, severe visual compromise, high risk of facial disfigurement , insufficient response to steroids |
CS started 1st (1- 3 mg/kg/d from 1–9 mos), followed by vincristine X 1 dose; neurology input, slower upward taper w more frequent f/up |
7 mos/1st course X 8 mos; restarted at 19 mos X 2 mos; restarted again at 2.5 yrs |
2.0 mg/kg/d divided b.i.d |
MRA X 4 (6 mos, 15 mos, 19 mos & 2.5 yrs of age); MRP at 15 mos, interval vessel changes noted |
Repeat MRA at 15 mos showed progressive vessel stenosis from mild to severe (confirmed with MRP); propranolol was d/c; at 19 mos imaging stable, prop restarte d X 2 mos w/out incident ; repeat MRA at 2.5 yrs showed interval improve ment in degree of stenosis; pt remained neurologically stable through out course |
Mild sleep disturbance/mild |
| 13 | Bil S1, S4, CNS |
Narrowing of bil supraclinoid ICAs (mild, stable, not flow-limiting) |
Hypoplastic Rt cerebellar hemisphere, incomplete, formation of cerebellar vermis, CNS IH |
N | N | Recurrent ulceration after CS d/c, high risk of facial disfigurement |
CS started 1st (2.5 mg/kg/d from 6 wks-8 mos) |
10 mos/8 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 4 (2 mos, 11 mos, 2.5 yrs & 3 yrs of age), stable |
N | N/mild-mod |
| 14 | Rt S1, Lt S1-S3, bil scalp |
Dysplastic bil ICAs & basilar a, aberrant origin of PCA |
N | Aberrant subclavian a |
N | Rapid growth, visual compromise, high risk of facial disfigurement |
Initiation by outside practice, CS started 1st (2 mg/kg/d at 5 wks for 2.5 mos), neurology input |
9 weeks/7 mos |
2.0 mg/kg/d divided t.i.d |
MRA X 3 (baseline & after 6 & 12 mos), stable |
N | Periodic cold hands & feet/E |
| 15 | Bil S3, airway (para- pharyngeal) |
Hypoplastic Lt ICA |
N | Shared origin of innominate & Lt CCA from the arch |
N | Rapid growth, high risk of facial disfigurement |
CS (1 mg/kg/d) & timolol started 1st, slower upward taper |
4 mos/20 mos |
2.0 mg/kg/d divided t.i.d |
N | N | N/mod |
| 16 | Lt S1, S2 | Hypoplastic Lt ICA, ACA, MCA, PCA |
Pial IH, Lt cerebral hemiatrophy, extensive polymicrogyria |
Bil, distal pulmonary a stenosis |
Severe epilepsy |
Rapid & prolonged growth, visual occlusion, high risk of facial disfigurement , ulceration, inadequate response to either CS or vincristine |
CS started 1st (1- 3 mg/kg/d from 3 wks-12 mos), then vincristine |
19 mos/5 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 4 (1 mo, 9 mos, 16 mos, 4 yrs of age), stable |
N | Constipation/mod |
| 17 | Rt S1, S2, S4, CNS (Rt subocciptal), airway (para- pharyngeal) |
Hypoplastic Rt ICA & Rt A1 segment, dysplastic Lt ICA, aberrant origin/course Lt A1 segment to dysplastic bil ACAs, partial fetal configuration of Rt PCA |
Absent corpus callosum, hypoplastic Rt cerebellar hemispher e, anterior midline lipoma, CNS (Rt suboccipital) |
N | Rt sclerocornea |
Rapid growth, insufficient response to CS, auditory obstruction, high risk of facial disfigurement |
CS started 1st (2- 3 mg/kg/d from 2–10 mos) |
5 mos/18 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 4 (6 mos, 1 yr, 2 yrs, 3 yrs of age); MRA at 2 yrs showed possible stenosis but f/up CTA negative, stable |
N | N/E |
| 18 | Rt S1-S3, partial Lt S1 & S2 |
Absent Lt ICA w the Lt ACA supplied by the Rt ACA & the Lt ophthalmic and bil MCAs supplied from a Lt PCOM, dysplastic PTA w possible saccular aneurysm, dysplastic Rt ICA, hypplastic Rt VA, Lt parietal developmenta l venous anomaly, poss old hemorrhage Rt cerebello- pontine angle |
Dandy- Walker malformation |
N | N | Rapid growth, visual compromise, high risk of facial disfigurement |
Neurology input | 10 mos/22 mos |
2.0 mg/kg/d divided t.i.d |
MRA at baseline & every 6 mos until 2 yrs, stable |
N | N/E |
| 19 | Rt S1, S2 | Absent Rt ICA w hypoplastic Rt MCA & hypoplastic or absent A1 segment of Rt ACA, dysplastic Lt intracranial ICA, hypoplastic Rt CCA w aberrant origin/course |
N | N | N | Rapid growth, visual compromise |
Initiation by outside practice, intralesional CS X 1 1st, hosp admit for initiation |
5 wks/present (35 mos) |
2.0 mg/kg/d divided b.i.d |
MRA X 3 (6 wks, 6 mos, 1 yr of age), stable |
N | N/E |
| 20 | Rt S1-S3, partial Lt S1 & S2, CNS (intra- auricular & cavernous sinus) |
Dysplastic Lt hemispheric vessels, aberrant a from basilar a to Lt orbital region |
Hypoplast ic Lt cerebellum, CNS IH (intra- auricular & cavernous sinus) |
Aberrant Rt subclavian |
N | Rapid growth, visual compromise, , high risk of facial disfigurement insuffient response to CS & intralesional bleomycin X 3 for visual occlusion, CS side effects (HTN) |
Initiation by outside practice, intralesional bleomycin X 3 & CS (3 mg/kg/d from 7 wks X 5 mos) started 1st |
4 mos/14 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 3 (baseline, 6 mos, 12 mos), stable |
N | N/E |
| 21 | Rt S1, Rt scalp |
Hypoplastic, dysplastic Rt ICA, hypoplastic A1 segment of Rt ACA, possibly hypoplastic Rt VA |
N | Mild pulmonary stenosis & atrial septal defect |
N | Visual compromise |
CS started 1st (1 mg/kg/d from 8 wks-4.5 mos), neurology input, lower dose, slower upward taper |
2 mos/15 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 2 (6 mos & 12 mos of age), stable |
N | N/E |
| 22 | Bil S3, Lt scalp, G.I. tract, airway (subglottic) |
Dysplastic Lt ICA, MCA narrowing |
N | Coarctation of the aorta, required surgical repair |
Sternal scar |
Airway compromise, insufficient response to CS (2nd course), G.I. bleeding |
Initiation by outside practice; prop d/c at 3 mos & CS started (2 mg/kg/d from 3- 9 mos) b/c of stroke risk, but then restarted at 5 mos due to worsening airway |
2 wks/u/k | 2.0 mg/kg/d divided t.i.d |
N | N | N/E |
| 23 | Bil S3, Rt S1, S4, neck, airway |
Dysplastic ICA |
N | Small patent foramen ovale |
N | High risk of facial disfigurement , potential ulceration, airway compromise |
CS started 1st (3 mg/kg/d from 2 wks to 12 mos) |
11 mos/18 mos at last f/up |
2.0 mg/kg/d divided t.i.d |
N | N | N/E |
| 24 | Rt S3, ear, scalp, intraoral, CNS (lt cavernous sinus and cerebellop ontine angle), midline labia and clitoral hood |
Hypoplastic Lt ICA |
Unilateral cerebellar hypoplasia, gray matter heterotopia (neuronal migrational disorder), CNS IH |
N | N | Persistent intraoral IH |
CS 1st (2 mos-24 mos) |
5 mos/15 mos |
2.0 mg/kg/d divided t.i.d |
N | N | N/mod |
| 25 | Bil S3, Rt chest, back, neck, arm, hand, airway, G.I. tract |
Narrowing of Rt ICA (not severe) |
N | Rt aortic arch, narrow/aberrant Rt subclavian a |
Sternal cleft |
Rapid growth, high risk of facial disfigurement , g.i. bleeding |
Slower upward taper; started in combination w CS (2 mg/kg/d from 2 wks- present) |
2 wks/present (16 mos) |
1.0 mg/kg/d divided t.i.d |
N? | Over RUE affected by IH, also persistent ectatic veins & cold Rt hand w intermittent violaceous color change; due to concerns for impending ulceration/ necrosis of her Rt Fingertip ps, prop dose lowered but later increased to baseline without worsening of skin changes ; at 16 mos, no digit loss, acrocyanosis and nail dystrophy improved |
? worsened peripheral arteriopathy w digital infarcts & severe sleep disturbance led to decease/E |
| 26 | BIl S3, Lt S1, S2, S4 |
Hypoplastic Rt VA, PTA |
N | N | N | Prolonged growth, persistent bulk, slow involution, insufficient response to CS |
CS started 1st (2 mg/kg/d from 2- 18 mos) then interferon |
24 mos/6 mos |
2.0 mg/kg/d divided b.i.d |
MRA X 2 (baseline & 4 yrs of age), stable |
N | N/mod |
| 27 | Rt S1, airway |
Dysplastic & hypoplastic Rt ICA |
N | N | N | Airway compromise, CS side effects (HTN); also needed tracheostomy due to refractory airway disease, prop substituted for other anti- hypertensives to try to decanulate sooner |
CS 1st (3 mg/kg/d from 2- 10 mos) |
7 mos/15 mos |
2.0 mg/kg/d divided t.i.d |
N | N | N/E |
| 28 | Lt S1, S2, S3, scalp, neck, upper back |
Dysplastic & narrowed Lt ICA, narrowed Lt MCA |
Unilateral cerebellar hypoplasia/ dysplasia |
Coarctation of the aorta, Lt transverse arch narrowing |
N | Rapid growth, high risk of facial disfigurement , ulceration |
Lower & t.i.d. dosing in combination w CS (2 mg/kg/d) |
3 wks/present (18 mos) |
1.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & after 4 mos; narrowing improved) |
Very severe scalp & ear ulceration that destroyed upper half of ear |
? worsened tissue necrosis/mod |
| 29 | Lt S1, S4, partial S3, Lt retro- orbital & scalp |
Hypoplastic Lt ICA |
CNS IH (Lt cerebello- pontine angle) |
N | 26 wk prematurity due to maternal anti- phospholipid syndrome |
Rapid growth, visual compromise, high risk of facial disfigurement |
Lower & t.i.d. dosing |
3 mos/present (13 mos) |
1.5 mg/kg/d divided t.i.d |
N | N | N/E |
| 30 | Bil S1, Rt S2, S4, scalp, neck, airway |
Dysplastic & hypoplastic ICA, aberrant origin/course of vertebrobasilar |
Dandy- Walker complex, bil cerebellar hypoplasia/ dysplasia, polymicro gyria, CNS IH (bil internal auditory canals, Meckel's cave) |
Small ventricular septal defect & patent foramen ovale, aberrant Lt superior vena cava that drains to coronary sinus |
Scalp hamartoma NOS |
Rapid growth, visual & airway compromise, high risk of facial disfigurement , impending ulceration, insufficient response to CS |
CS started 1st (2 mg/kg/d from 3 wks- present), lower and t.i.d. dosing |
1.5 mos/present (12 mos) |
1.5 mg/kg/d divided t.i.d |
N | At 6 wks developed severe & destructive ulceration of lip, columella, nasal septum & scalp; attempt ed prop dose increase to improve visual compromise led to rapid IH whitening & worsening of the ulcerate on, thus dose immediately decreased; ulceration healed by 1 yr |
? worsened ulceration/mod |
| 31 | Bil S3, chest |
Hypoplastic Lt ICA, aberrant origin/course of vertebrobasilar |
N | Aberrant origin of Rt subclavian |
N | Rapid growth, high risk of facial disfigurement |
Lower dose | 2 wks/lost to follow-up |
.0 mg/kg/d divided t.i.d |
N | N | N/E |
| 32 | Lt S2, Bil S3, partial S4, intraoral, neck, chest, gluteal cleft/buttocks, airway |
Absent lt A1, narrow & dysplastic lt ICA & MCA, marked dysplasia top of basilar a, dysplastic PCOM |
N | Small focal outpouching at lateral distal aortic arch, differential diagnosis = atypical ductus bump versus aortic aneurysm or pseudoaneurysm |
Hamartom atous growth at chin and Lt tongue, poor oral intake required G-tube placement, hypotonia in trunk & legs, borderline gross motor delay at 6 mos |
Rapid growth, high risk of facial disfigurement , ulceration, airway compromise |
Slow upward taper; increased from 1 mg/kg/d to 2 mg/kg/d for proliferation, then 3 mg/kg/d for airway compromise at which time CS 1 mg/kg/d divided b.i.d. also added |
1 wk/present | 3.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & 4.5 mos of age, stable) |
N | N/E |
A = artery; ACA = anterior cerebral artery; AVF = arteriovenous fistula; Avg = average; b/c = because; bil = bilateral; CCA = common carotid artery; CNS = central nervous system; CS = corticosteroids; CTA = computed tomography angiography; d/c = discontinued; E = excellent; f/up = follow-up; G.I. = gastrointestinal; hosp = hospital; hrs = hours; HTN = hypertension; hosp = hospital; ICA = internal carotid artery; IH = infantile hemangioma; Lt = left; MCA = middle cerebral artery; med = medication; mg/kg/d = milligrams per kilogram per day; mod = moderate; mo(s) = month(s); MRA = magnetic resonance angiography; N = none; PCA = posterior cerebral artery; PCOM = posterior communicating artery; PDA = patent ductus arteriosus; pt = patient, post = posterior; PTA = persistent trigeminal artery; prop = propranolol; Rt = right; S = segment; t.i.d. = three times daily; txd = treated; u/k = unknown; VA = vertebral artery; wks = weeks; w = with; WNL = within normal limits; w/u = work-up; yr = year
Discussion
The serendipitous discovery of propranolol’s effectiveness for IH has forever changed IH management, leading to dramatically improved outcomes for children. To date, most infants have tolerated the medication without severe toxicities; however, prospective studies with uniform toxicity monitoring have not yet been completed. Patients with PHACE syndrome represent a unique treatment challenge in that most affected infants have extensive facial IH with both potential medical morbidities (e.g. periocular disease, airway disease and/or risk of ulceration) and a high risk of facial scarring and disfigurement. As such, they are prime candidates for propranolol therapy. At the same time, there is concern that the drug could increase the hemodynamic risks associated with an otherwise asymptomatic cerebral arteriopathy, in the worst case causing watershed infarct, a rare but potentially devastating complication. This quandary is reflected even among our authorship, which often chose to initiate systemic corticosteroids before moving to propranolol. When using propranolol, we did so with greater caution: obtaining outside neurology and cardiology consultation(s), using lower initial/average dosing and more frequent (t.i.d.) dosing, titrating upward more slowly, and in some cases admitting infants for inpatient monitoring.
While the overall incidence of pediatric stroke is rare, cerebrovascular abnormalities are recognized to be the most important risk factor25, and PHACE syndrome has received increased attention as a potential etiology of AIS in early childhood.7 Fortunately, AIS affects only a very small subset of PHACE children, and recent efforts have focused on identifying which patients are at greatest risk. In a recent study of 22 individuals with PHACE and AIS, the average age of stroke was 14.4 months (range 3–60 months). The majority had severe underlying arteriopathy. Approximately 60% had nonvisualization of a major cerebral artery, and there was a similarly high incidence of co-existent aortic arch anomalies. No patients had arterial dysplasia as the sole class of arteriopathy. The authors concluded that while the mechanism of AIS in PHACE remains unknown, it is likely complex and related to several factors that include severity of arterial stenosis, presence or absence of an intact circle of Willis, degree to which collateral circulation is developed, and co-existing cardiac and aortic arch anomalies that could lead to cardioembolic events.7
Neuroimaging findings most predictive of AIS risk include severe, long-segment narrowing or non-visualization of major cerebral or cervical vessels in the setting of inadequate collateral circulation (Table 3). However, while these are the more common anatomic features that could cause hypoperfusion injury, the lack of these features does not eliminate the risk of AIS. Furthermore, while MRA is the imaging study of choice to assess the head and neck vasculature in PHACE, it has 2 important limitations. First is a tendency to overestimate stenosis on time-of-flight MRA, which led to our definition of severe narrowing as a > 75% reduction in luminal cross-sectional diameter compared to normal.26 Secondly, there are limitations to the ability of MRA to detect collateral vasculature because the imaging is performed without a hemodynamic challenge; e.g. some collaterals may only be dynamically recruited when a stressor to perfusion, such as hypotension, is introduced. Thus, in cases in which MRA features demonstrate severely compromised vessels, but the presence of collateral, compensatory flow is questionable, follow-up perfusion studies can prove helpful to risk assessment.
Table 3.
Comparison of Head and Neck MRA Imaging Features to Stroke Risk in PHACE Syndrome
| Risk Category | Cerebrovascular Anomalies |
|---|---|
| Highera | |
| Standard |
risk further increased if coexistent cardiac or aortic arch anomalies
defined as vessel narrowing >75%
internal carotid artery, middle cerebral artery, anterior cerebral artery, posterior cerebral artery, basilar artery, vertebral artery
there are limitations to the ability of MRA to detect collateral vasculature because the imaging is done without a hemodynamic challenge; e.g. some collaterals may only be dynamically recruited when a stressor to perfusion, such as hypotension, is introduced. For questionable cases, follow-up perfusion studies can prove beneficial.
defined as vessel narrowing <75%, and categorized as standard risk given known tendency to overestimate stenosis on time of flight MRA (Johnson).
any degree of severity
Three recent case reports describe the uneventful use of propranolol in PHACE infants with documented cerebrovascular anomalies: one with an unspecified anomaly of the anterior cerebral artery27, another with a “tortuous tangle” of arteries composed of the left internal carotid, left middle cerebral and left posterior cerebral arteries28, and a third with an absent right vertebral artery and severe coarctation of the aorta requiring surgery29. An additional study by Hernandez-Martin et al describes 7 infants with PHACE syndrome who underwent brain perfusion SPECT (Single Photon Emission Computed Tomography) after 3 or 6 months of propranolol therapy, however no baseline perfusion studies were performed. Three of the seven infants had tortuous, dysplastic vessels within the vertebrobasilar system and the internal carotid artery, and 2 had persistent embryonic vessels; the remainder had structural brain anomalies alone. All patients showed normal uptake and perfusion in all areas of the cerebral cortex.30 While the authors concluded that propranolol “can be considered safe in patients with PHACE and cerebral vasculopathy”, propranolol effects on blood pressure peak approximately 2 hours after an oral dose in children. Since information regarding the timing of SPECT following propranolol intake was not mentioned, the results and conclusions must be interpreted with caution. Furthermore, of the above-noted cases, only the patient described by Manzuna et al might be categorized at higher risk for watershed AIS, though information on the integrity of the collateral circulation was not provided.
New-onset seizures and acute hemiparesis are the most common initial symptoms of AIS.7 None of our 32 patients had documented AIS or other concerning neurologic sequelae while on propranolol, although 7 were considered at higher risk for watershed AIS (cases 8, 12, 18, 19, 22, 28, 32; Table 4). Only 1 patient (case 11) was noted to have a change in neurologic status while on therapy, consisting of a mild right hemiparesis. Of note, this patient’s baseline imaging showed mild, focal vessel stenosis, without cerebral changes or infarction, and repeat neuroimaging showed no change from baseline. Her hemiparesis remained static and improved with physical therapy while the drug was continued. Although the role of propranolol in this patient’s symptoms cannot be completely excluded, her CNS anomaly (hypoplastic left middle cerebral peduncle) is deemed a more likely etiology. Two of our patients had seizures unrelated to propranolol, one with infantile spasms that resolved during infancy (case 7) and another with polymicrogyria/neuronal migrational disorder and intractable epilepsy (case 16). To our knowledge, there has been only one report of AIS in a PHACE patient on a beta-blocker to date. This patient had severe vasculopathy including hypoplasia of the left internal carotid artery, narrowing of the left internal carotid, vertebral, anterior cerebral and middle cerebral arteries, and coarctation of the aorta, and was thus in the higher risk category for AIS based on the absence of anatomic collaterals. At 14 months of age, after approximately 6 months of treatment with nadolol (a beta-1 selective beta-blocker) and aldactozide for uncontrolled hypertension induced by systemic corticosteroids and aortic narrowing, she developed an acute left hemiparesis and was diagnosed with a left parietal infarct. However, given this child’s severe vasculopathy and coexistent medical problems, it is by no means certain whether nadolol was a factor in AIS in this case.7 and personal communication, Elena Pope M.D.
Table 4.
PHACE Patients on Propranolol with Higher Risk Imaging Features for AIS
| Case | IH Location(s) |
Cerebro- vascular Anomalies |
CNS Anomalies |
Cardiovascular Anomalies |
Other Medical |
Indication(s) for Propranolol |
Prop Initiation |
Propranolol: Age initiated/ Duration |
Propranolol dose |
Repeat Cerebro- vascular imaging |
Atypical Events |
Prop side effects/ IH response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rt S1, S3, S4, Rt chest, airway |
Hypoplastic, dysplastic Rt PCA w focal stenosis in P2 segment, absent Rt ICA, hypoplastic vertebrobasilar w aberrant origin/course |
N | N | Sternal defect/pit |
Visual compromise, high risk of facial disfigurement , CS side effects (cushingoid features, rebound IH growth w taper) |
CS started 1st (2 mg/kg/d from 3 wks-11 mos), neurology input, hosp admit for initiation |
13 mos/14.5 mos |
1.5 mg/kg/d divided t.i.d. |
MRA X 2 (baseline & at 1 yr of age), stable |
N | Episodes of asymptomatic hypotension led to dose decrease, f/up vitals WNL; mod-E |
| 2 | Rt S1, Rt hemi- diaphragm , multiple abdominal : spleen, small bowel mesentery , upper anterior abdomen, anterior abdominal wall, bil hepatic lobes |
Aberrant origin/course of Rt cavernous carotid a, severe dysplasia and/or dural AVF of vessels superomedial & lateral to Rt orbital IH, absent Lt PCA, long- segment stenosis of Rt ICA (initially mild) |
N | N | N | Rapid growth, severe visual compromise, high risk of facial disfigurement , insufficient response to steroids |
CS started 1st (1- 3 mg/kg/d from 1–9 mos), followed by vincristine X 1 dose; neurology input, slower upward taper w more frequent f/up |
7 mos/1st course X 8 mos; restarted at 19 mos X 2 mos; restarted again at 2.5 yrs |
2.0 mg/kg/d divided b.i.d |
MRA X 4 (6 mos, 15 mos, 19 mos & 2.5 yrs of age); MRP at 15 mos, interval vessel changes noted |
Repeat MRA at 15 mos showed progressive vessel stenosis from mild to severe (confirmed with MRP); propran olol was d/c; at 19 mos imaging stable, prop restarte d X 2 mos w/out incident ; repeat MRA at 2.5 yrs showed interval improve ment in degree of stenosis ; pt remained neurologically stable through out course |
Mild sleep disturbance/mild |
| 3 | Rt S1-S3, partial Lt S1 & S2 |
Absent Lt ICA w the Lt ACA supplied by the Rt ACA & the Lt ophthalmic and bil MCAs supplied from a Lt PCOM, dysplastic PTA w possible saccular aneurysm, dysplastic Rt ICA, hypoplastic Rt VA, Lt parietal developmental venous anomaly, poss old hemorrhage Rt cerebello- pontine angle |
Dandy- Walker Malformation |
N | N | Rapid growth, visual compromise, high risk of facial disfigurement |
Neurology input | 10 mos/22 mos |
2.0 mg/kg/d divided t.i.d |
MRA at baseline & every 6 mos until 2 yrs, stable |
N | N/E |
| 4 | Rt S1, S2 | Absent Rt ICA w hypoplastic Rt MCA & hypoplastic or absent A1 segment of Rt ACA, dysplastic Lt intracranial ICA, hypoplastic Rt CCA w aberrant origin/course |
N | N | N | Rapid growth, visual compromise |
Initiation by outside practice, intralesional CS X 1 1st, hosp admit for initiation |
5 wks/present (35 mos) |
2.0 mg/kg/d divided b.i.d |
MRA X 3 (6 wks, 6 mos, 1 yr of age), stable |
N | N/E |
| 5 | Bil S3, Lt scalp, G.I. tract, airway (subglottic) |
Dysplastic Lt ICA, MCA narrowing |
N | Coarctation of the aorta, required surgical repair |
Sternal scar |
Airway compromise, insufficient response to CS (2nd course), G.I. bleeding |
Initiation by outside practice; prop d/c at 3 mos & CS started (2 mg/kg/d from 3- 9 mos) b/c of stroke risk, but then restarted at 5 mos due to worsening airway |
2 wks/u/k | 2.0 mg/kg/d divided t.i.d |
N | N | N/E |
| 6 | Lt S1, S2, S3, scalp, neck, upper back |
Dysplastic & narrowed Lt ICA, narrowed Lt MCA |
Unilateral cerebellar hypoplasia/ dysplasia |
Coarctation of the aorta, Lt transverse arch narrowing |
N | Rapid growth, high risk of facial disfigurement , ulceration |
Lower & t.i.d. dosing in combination w CS (2 mg/kg/d) |
3 wks/present (18 mos) |
1.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & after 4 mos; narrowing improved) |
Very severe scalp & ear ulceration that destroyed upper half of ear |
? worsened tissue necrosis/mod |
| 7 | Lt S2, Bil S3, partial S4, intraoral, neck, chest, gluteal cleft/buttocks, airway |
Absent lt A1, narrow & dysplastic lt ICA & MCA, marked dysplasia top of basilar a, dysplastic PCOM |
N | Small focal outpouching at lateral distal aortic arch, differential diagnosis = atypical ductus bump versus aortic aneurysm or pseudoaneurysm |
Hamartom atous growth at chin and Lt tongue, poor oral intake required G-tube placement, hypotonia in trunk & legs, borderline gross motor delay at 6 mos |
Rapid growth, high risk of facial disfigurement , ulceration, airway compromise |
Slow upward taper; increased from 1 mg/kg/d to 2 mg/kg/d for proliferation, then 3 mg/kg/d for airway compromise at which time CS 1 mg/kg/d divided b.i.d. also added |
1 wk/present | 3.0 mg/kg/d divided t.i.d |
MRA X 2 (baseline & 4.5 mos of age, stable) |
N | N/E |
A = artery; ACA = anterior cerebral artery; AVF = arteriovenous fistula; Avg = average; b/c = because; bil = bilateral; CCA = common carotid artery; CNS = central nervous system; CS = corticosteroids; CTA = computed tomography angiography; d/c = discontinued; E = excellent; f/up = follow-up; G.I. = gastrointestinal; hosp = hospital; hrs = hours; HTN = hypertension; hosp = hospital; ICA = internal carotid artery; IH = infantile hemangioma; Lt = left; MCA = middle cerebral artery; med = medication; mg/kg/d = milligrams per kilogram per day; mod = moderate; mo(s) = month(s); MRA = magnetic resonance angiography; N = none; PCA = posterior cerebral artery; PCOM = posterior communicating artery; PDA = patent ductus arteriosus; pt = patient, post = posterior; PTA = persistent trigeminal artery; prop = propranolol; Rt = right; S = segment; t.i.d. = three times daily; txd = treated; u/k = unknown; VA = vertebral artery; wks = weeks; w = with; WNL = within normal limits; w/u = work-up; yr = year
Two of our patients had effects on soft tissue in areas supplied by abnormal narrowed arteries, with unusually severe and relentless ulceration and loss of most of the ear in one (case 30) and marked acrocyanosis, nail dystrophy and small digital infarcts in the other (case 25, Fig. 1 a–c). While the role of propranolol in these peripheral soft-tissue findings cannot be certain, propranolol-induced effects on peripheral vasculature, including decreased cardiac output and unopposed alpha-adrenergic drive, result in reduced extremity blood flow leading to the commonly observed side effects of cold extremities and Raynaud’s phenomenon.31 As such, beta-blockers are contraindicated in severe peripheral arterial disease given rare reports of digital necrosis and gangrene.32–34 In another case, an infant with extensive bilateral IH and severe CNS arterial disease had severe, relentless scalp and facial ulcerations (case 28, Fig. 2). When an increase in propranolol dosage was attempted to treat the periocular disease, an immediate worsening of ulceration and surrounding “whitening” of the IH, a known clinical indicator of impending ulceration35, was observed. Though a number of reports have described the benefits of propranolol for IH ulceration36, and several patients in this study noted similar benefit, to our knowledge potential worsening of ulceration and/or tissue necrosis with propranolol has not been described previously. Notably, all three of our patients experienced these complications while on stable and conservative doses of combined oral corticosteroids and t.i.d. propranolol (Table 2).
Fig. 1.
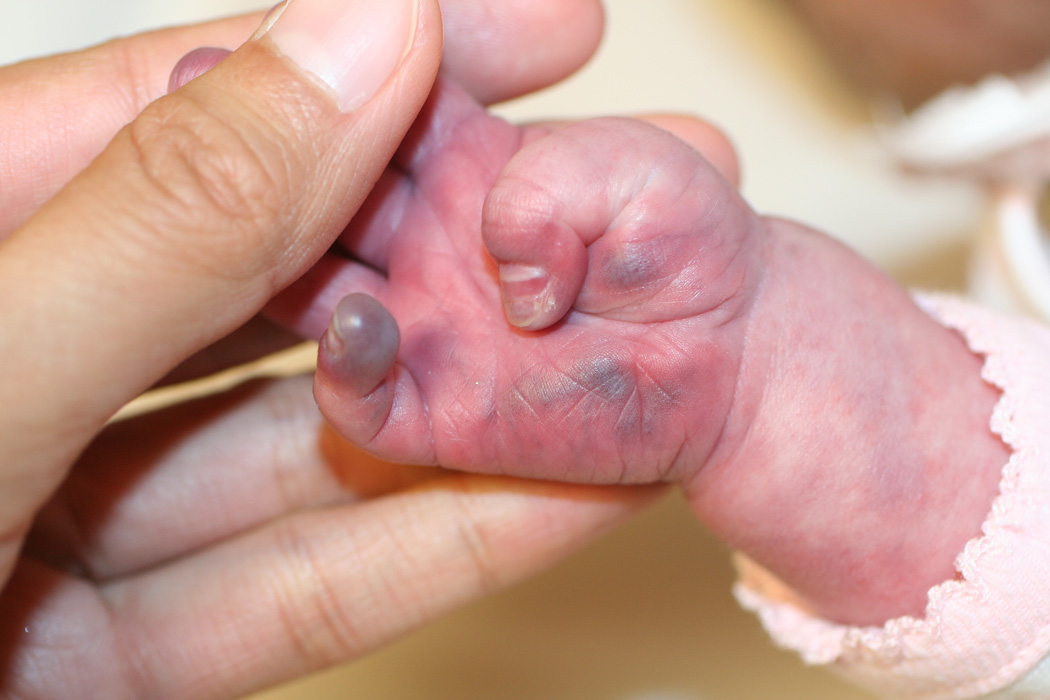
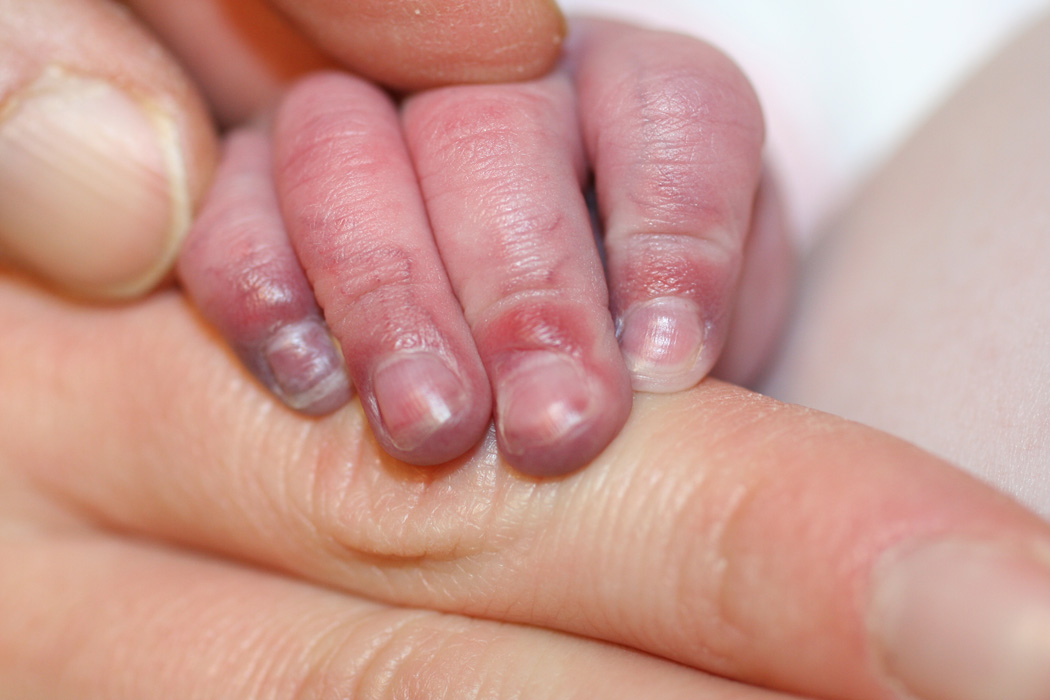
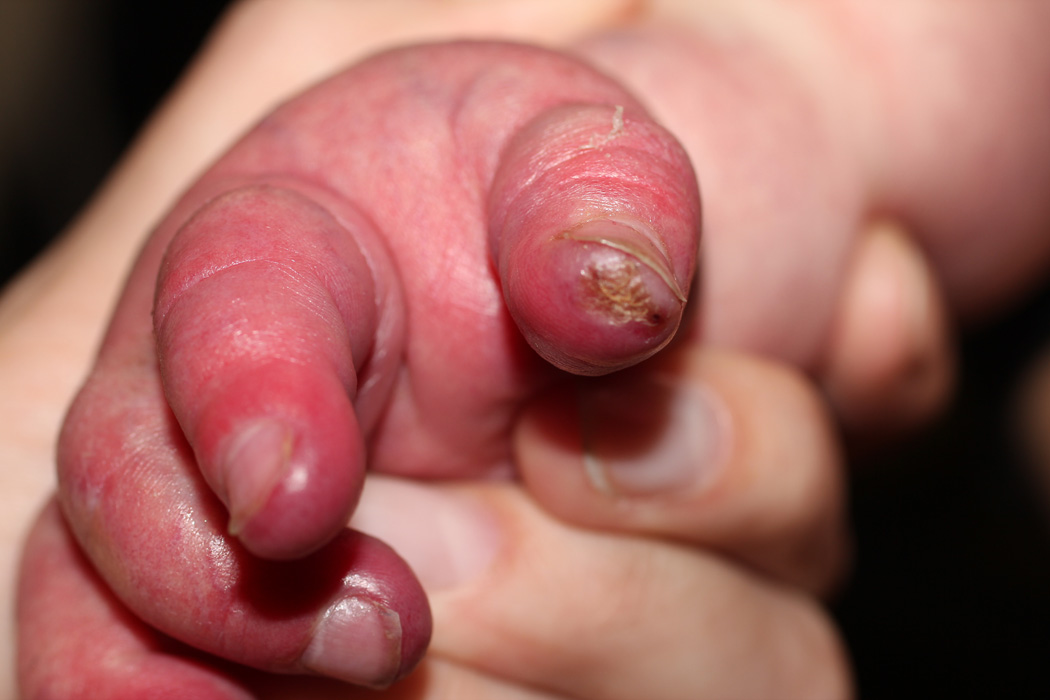
Marked acrocyanosis, nail dystrophy and small digital ulcerations in case #25, on both propranolol and low-dose prednisolone for a large IH involving the face, chest, and affected limb.
Fig. 2.
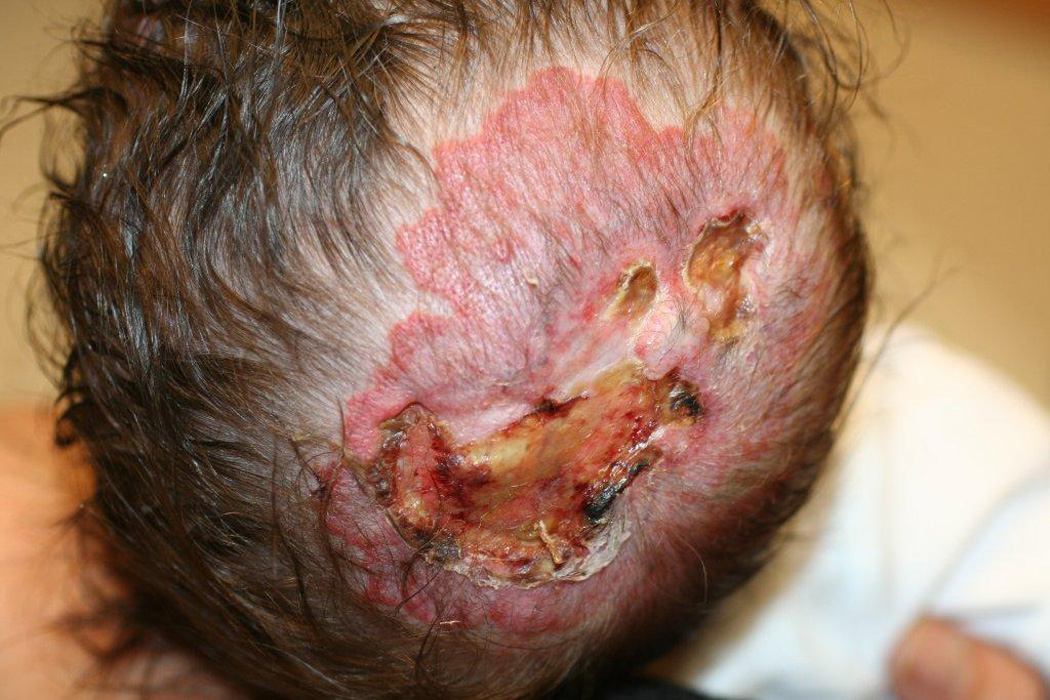
Worsening scalp ulceration in Case # 28; extensive deep facial and neck ulcerations also developed rapidly after institution of oral therapy with both propranolol and prednisolone and took several months to heal.
While the prescribing information for propranolol does not list stroke or a history of cerebrovascular disease as contraindications in adult populations, its use in this age group with known cerebral and cervical arterial anomalies has recently been challenged.37 As a whole, beta-blockers are known to have multiple physiologic effects, with different degrees of B1 and B2 activity, as well as nonadrenergic effects.38 Nonselective β-blockers such as propranolol have been shown to increase variability in systolic blood pressure to a greater extent than β1-selective agents. Labile blood pressure is a known risk factor for stroke, independent of mean blood pressure, which probably accounts for the relative lack of effectiveness of beta-blockers in preventing stroke.39 Despite this, propranolol remains widely used for migraine with aura, a common neurologic disorder associated with at least a doubling in stroke risk.40 Although the absolute risk in young patients is probably very low, a potential link between propranolol use and migrainous stroke has been suggested by case reports showing a temporal association between the introduction of propranolol in patients with migraine and the occurrence of stroke.41–43 A recent study, based on a systematic review of randomized controlled trials comparing different types of B-blockers with placebo or other agents, advised preferential use of B1-selective agents over propranolol for conditions when the risk of stroke is increased.37 Notably, migraine headaches are also a common neurologic complication of PHACE syndrome, affecting nearly one-third of patients in the PHACE Patient Registry, and in a recent study such patients were noted to have similar higher-risk anomalies on neuroimaging to those who had experienced AIS.13
Limitations of our study include its retrospective nature, lack of standardized dosing and monitoring regimens, and potential selection bias since a small number of patients with very severe vascular anomalies were excluded from propranolol treatment. However, we are able to draw the following conclusions:
Infants with large, facial IH should be thoroughly investigated for potential arteriopathy associated with PHACE syndrome, with MRA imaging of the head and neck and cardiac imaging to include the aortic arch, prior to considering propranolol therapy.
Physicians should be aware of the imaging features that may be associated with a higher risk for developing AIS, while at the same time acknowledging that the presence of “standard” imaging features does not eliminate risk of hypoperfusion injury. In cases in which MRA features demonstrate severely compromised vessels, but the presence of collateral, compensatory flow is questionable, follow-up perfusion studies may prove helpful in assessing risk.
We are left with more questions than answers. There has been only one report of AIS in a PHACE patient on a beta-blocker (nadolol) to date, though it is by no means certain whether nadolol was a factor in AIS in this case. While no serious neurologic events occurred in this, the largest study to date of PHACE patients treated with propranolol, the possibility that propranolol could augment the potential risk of stroke in this population cannot be negated.
We continue to advocate use of the most conservative approach that will help control the target signs and symptoms of the IH being treated. We advise consultation with neurology and/or cardiology when appropriate, and use of the lowest possible dose of propranolol, slow upward dosage titration, and three times daily dosing in order to minimize abrupt changes in systolic blood pressure. The potential use of B-1 selective agents in the PHACE population should also be further examined.
Physicians should be aware of the potential for progressive ulceration and/or tissue necrosis with propranolol in the most severe IH patients with PHACE and associated vasculopathy, an observation that warrants further study.
Acknowledgments
Funding/Support: This study was supported in part by: 1R34AR060881-01 Safety Monitoring for Use of Propranolol for Hemangiomas; NIH, NIAMS
Role of the Sponsors: N/A
We acknowledge Deborah Goddard, M.D. for her assistance with data collection.
Footnotes
Author Contributions:
Dr. Metry had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drs. Metry, Frieden and Drolet
Acquisition of data: Drs. Metry, Frieden, Siegel, Baselga, Chamlin, Garzon, Mancini, Powell and Drolet
Analysis and interpretation of data: Drs. Metry, Frieden, Hess and Drolet
Drafting of the manuscript: Dr. Metry
Critical revision of the manuscript for important intellectual content: Drs. Metry, Frieden, Hess, Siegel, Baselga, Chamlin, Garzon, Mancini, Powell and Drolet
Statistical analysis: Dr. Metry
Obtained funding: N/A
Administrative, technical, or material support: N/A
Study supervision: N/A
Financial Disclosure: Dr. Frieden serves as a consultant to Pierre Fabre. Dr. Powell serves as an investigator for Pierre Fabre.
- Employment: Dr. Powell serves as an investigator for Pierre Fabre.
- Consultancies: Dr. Frieden serves as a consultant to Pierre Fabre.
- Honoraria
- Speakers bureau
- Stock ownership or options
- Expert testimony
- Grants
- Patents filed, received, pending, or in preparation
- Royalties
- Donation of medical equipment: N/A
Contributor Information
Denise Metry, Email: dmetry@bcm.edu, Baylor College of Medicine, Dermatology and Pediatrics.
Ilona J. Frieden, Email: FriedenI@derm.ucsf.edu, University of California San Francisco, Dermatology and Pediatrics.
Christopher Hess, Email: christopher.hess@ucsf.edu, University of California San Francisco, Radiology & Biomedical Imaging.
Dawn Siegel, Email: dsiegel@mcw.edu, Medical College of Wisconsin, Dermatology and Pediatrics.
Mohit Maheshwari, Email: mmahesh@mcw.edu, Medical College of Wisconsin, Radiology.
Eulalia Baselga, Email: drabaselga@gmail.com, Hospital de la Santa Creu I Sant Pau, Dermatology.
Sarah Chamlin, Email: Schamlin@childrensmemorial.org, Children’s Memorial Hospital/Northwestern University Feinberg School of Medicine, Dermatology and Pediatrics.
Maria Garzon, Email: mcg2@columbia.edu, Columbia University, Dermatology and Pediatrics.
Anthony J. Mancini, Email: amancini@northwestern.edu, Children’s Memorial Hospital/Northwestern University Feinberg School of Medicine, Dermatology and Pediatrics.
Julie Powell, Email: julie_powell@ssss.gouv.qc.ca, CHU Sainte Justine, University of Montreal, Dermatology and Pediatrics.
Beth A. Drolet, Email: bdrolet@mcw.edu, Medical College of Wisconsin, Dermatology and Pediatrics.
References
- 1.Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the literature. Pediatr Dermatol. 2008;25(2):168–173. doi: 10.1111/j.1525-1470.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 2.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 3.Frieden IJ, Reese V, Cohen D. PHACE syndrome: the association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects and eye abnormalities. Arch Dermatol. 1996;132(3):307–311. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 4.Haggstrom AN, Garzon MC, Baselga E, et al. Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics. 2010;126(2):418–426. doi: 10.1542/peds.2009-3166. [DOI] [PubMed] [Google Scholar]
- 5.Burrows PE, Robertson RL, Mulliken JB, et al. Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: report of eight patients. Radiology. 1998;207(3):601–607. doi: 10.1148/radiology.207.3.9609880. [DOI] [PubMed] [Google Scholar]
- 6.Drolet BA, Dohil M, Golomb MR, et al. Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics. 2006;117(3):959–964. doi: 10.1542/peds.2005-1683. [DOI] [PubMed] [Google Scholar]
- 7.Siegel DH, Tefft KA, Johnson C, et al. Stroke in Children with PHACE Syndrome: a Systematic Review of the Literature. Stroke. 2012 in press. [Google Scholar]
- 8.Rao RP, Drolet BA, Holland KE, Frommelt PC. PHACES association: a vasculocutaneous syndrome. Pediatr Cardiol. 2008;29(4):793–799. doi: 10.1007/s00246-008-9204-5. [DOI] [PubMed] [Google Scholar]
- 9.Dobkin BH. Orthostatic hypotension as a risk factor for symptomatic occlusive cerebrovascular disease. Neurology. 1989;39(1):30–34. doi: 10.1212/wnl.39.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Iwama T, Hashimoto N, Yonekawa Y. The relevance of hemodynamic factors to perioperative ischemic complications in childhood moyamoya disease. Neurosurgery. 1996;38(6):1120–1125. doi: 10.1097/00006123-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Novak V, Hu K, Desrochers L, et al. Cerebral flow velocities during daily activities depend on blood pressure in patients with chronic ischemic infarctions. Stroke. 2010;41(1):61–66. doi: 10.1161/STROKEAHA.109.565556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou PS, Guo YC. Limb-shaking transient ischemic attacks in an adult PHACE syndrome: a case report and review of the literature. Neurol Sci. 2011 Jun 28;:13. doi: 10.1007/s10072-011-0671-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Metry D, O’Conner S, Drolet BA. Migraine-like headaches in PHACE syndrome. In press [Google Scholar]
- 14.Metry DW, Garzon MC, Drolet BA, et al. PHACE Syndrome: Current Knowledge, Future Directions. Pediatric Dermatology. 2009;26(4):381–398. doi: 10.1111/j.1525-1470.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandling-Bennett HA, Metry DW, Baselga E, et al. Infantile hemangiomas with unusually prolonged growth phase: a case series. Arch Dermatol. 2008;144(12):1632–1637. doi: 10.1001/archderm.144.12.1632. [DOI] [PubMed] [Google Scholar]
- 16.Drolet BA, Pope E, Juern AM, et al. Gastrointestinal Bleeding in Infantile Hemangioma: A Complication of Segmental, Rather than Multifocal, Infantile Hemangiomas. J Pediatr. 2012 Jan 10; doi: 10.1016/j.jpeds.2011.12.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Durr ML, Meyer AK, Huoh KC, Frieden IJ, Rosbe KW. Airway Hemangiomas in PHACE Syndrome. Laryngoscope. 2012 doi: 10.1002/lary.23475. (in press) [DOI] [PubMed] [Google Scholar]
- 18.Haggstrom AN, Skillman S, Garzon MC, et al. Clinical spectrum and risk of PHACE syndrome in cutaneous and airway hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137(7):680–687. doi: 10.1001/archoto.2011.113. [DOI] [PubMed] [Google Scholar]
- 19.Hess CP, Fullerton HJ, Metry DW, et al. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. Am J Neuroradiol. 2010;31(10):1980–1986. doi: 10.3174/ajnr.A2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poindexter G, Metry DW, Barkovich AJ, Frieden IJ. PHACE syndrome with intracerebral hemangiomas, heterotopia, and endocrine dysfunction. Pediatr. Neurol. 2007;36(6):402–406. doi: 10.1016/j.pediatrneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Rosbe KW, Suh KY, Meyer AK, Maguiness SM, Frieden IJ. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg. 2010;136(7):658–665. doi: 10.1001/archoto.2010.92. [DOI] [PubMed] [Google Scholar]
- 22.Suh KY, Rosbe KW, Meyer AK, Frieden IJ. Extensive airway hemangiomas in two patients without beard hemangiomas. Pediatr Dermatol. 2011;28(3):347–348. doi: 10.1111/j.1525-1470.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 23.Metry D, Heyer G, Hess C, et al. Consensus statement on diagnostic criteria for PHACE sys syndrome. Pediatrics. 2009;124(5):1447–1456. doi: 10.1542/peds.2009-0082. [DOI] [PubMed] [Google Scholar]
- 24.Haggstrom AN, Lammer EJ, Schneider RA, Marcucio R, Frieden IJ. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics. 2006;117(3):698–703. doi: 10.1542/peds.2005-1092. [DOI] [PubMed] [Google Scholar]
- 25.Beslow LA, Jordan LC. Pediatric stroke: the importance of cerebral arteriopathy and vascular malformations. Childs Nerv Syst. 2010;26(10):1263–1273. doi: 10.1007/s00381-010-1208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson BA, Heiserman JE, Drayer BP, Keller PJ. Intracranial MR angiography: its role in the integrated approach to brain infarction. AJNR. 1994;15(5):901–908. [PMC free article] [PubMed] [Google Scholar]
- 27.Vasaiwala R, Saija K, Setabutr P. Novel management of the microphthalmic orbit in a patient with PHACE syndrome. Ophthal Plast Reconstr Surg. 2011;27(6):156–158. doi: 10.1097/IOP.0b013e318208319d. [DOI] [PubMed] [Google Scholar]
- 28.Solomon T, Ninnis J, Deming D, Merritt TA, Hopper A. Use of propranolol for treatment of hemangiomas in PHACE syndrome. J Perinatol. 2011;31(11):739–741. doi: 10.1038/jp.2011.28. [DOI] [PubMed] [Google Scholar]
- 29.Manunza F, Syed S, Laguda B, Linward J, Kennedy H, Gholam K, Glover M, Giardini A, Harper JI. Propranolol for complicated infantile hemangiomas: a case series of 30 infants. Br J Dermatol. 2010;162(2):466–468. doi: 10.1111/j.1365-2133.2009.09597.x. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Martin S, Lopez-Gutierrez JC, Lopez-Fernandez S, et al. Brain perfusion SPECT in patients with PHACES syndrome under propranolol treatment. Eur J Pediatr Surg. 2011 Nov 3; doi: 10.1055/s-0031-1291300. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Johnston GD, Finch MB, Shanks RG. Peripheral vascular effects of bufuralol in hypertensive and normal subjects: a comparison with propranolol and pindolol. Eur J Clin Pharmacol. 1986;30(6):649–652. doi: 10.1007/BF00608210. [DOI] [PubMed] [Google Scholar]
- 32.Joint Formulary Committee. British National Formulary. Vol. 54. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2007. [Google Scholar]
- 33.Stringer MD, Bentley PG. Peripheral gangrene associated with B-blockade. Br J Surg. 1986;73(12):1008. doi: 10.1002/bjs.1800731225. [DOI] [PubMed] [Google Scholar]
- 34.Vale JA, Jefferys DB. Peripheral gangrene complicating beta-blockade. Lancet. 1978;1(8075):1216. doi: 10.1016/s0140-6736(78)91014-0. [DOI] [PubMed] [Google Scholar]
- 35.Maguiness SM, Hoffman WY, McCalmont TH, Frieden IJ. Early white discoloration of infantile hemangioma: a sign of impending ulceration. Arch Dermatol. 2010;146(11):1235–1239. doi: 10.1001/archdermatol.2010.324. [DOI] [PubMed] [Google Scholar]
- 36.Hermans DJ, van Baynum IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen MH, van der Vleuten CJ. Propranolol, a very promising treatment of ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol. 2011;64(5):833–838. doi: 10.1016/j.jaad.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Webb AJ, Fischer U, Rothwell PM. Effects of B-blocker selectivity on blood pressure variability and stroke: a systematic review. Neurology. 2011;77(8):731–737. doi: 10.1212/WNL.0b013e31822b007a. [DOI] [PubMed] [Google Scholar]
- 38.Reiter MJ. Cardiovascular drug class specificity: B-blockers. Prog Cardiovasc Dis. 2004;47(1):11–33. doi: 10.1016/j.pcad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Webb AJS, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in BP and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375(9718):906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 40.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez Sabin J, Molins A, Turon A, Titus F, Codina A. [Migraine-infarct in patients treated with B-blockers] Rev Clin Esp. 1993;192(5):228–230. [PubMed] [Google Scholar]
- 42.Mendizabal JE, Greiner F, Hamilton DO, Rothrock JF. Migrainous stroke causing thalamic infarction and amnesia during treatment with propranolol. Headache. 1997;37(9):1594–1596. doi: 10.1046/j.1526-4610.1997.3709594.x. [DOI] [PubMed] [Google Scholar]
- 43.Bardwell A, Trott JA. Stroke in migraine as a consequence of propranolol. Headache. 1987;27(7):381–383. doi: 10.1111/j.1526-4610.1987.hed2707381.x. [DOI] [PubMed] [Google Scholar]


