Abstract
BACKGROUND
Mechanical ventilation (MV) is a life-saving intervention used to provide adequate pulmonary ventilation in patients suffering from respiratory failure. However, prolonged MV is associated with significant diaphragmatic weakness resulting from both myofiber atrophy and contractile dysfunction. Although several signaling pathways contribute to diaphragm weakness during MV, it is established that oxidative stress is required for diaphragmatic weakness to occur. Therefore, identifying the site(s) of MV-induced reactive oxygen species (ROS) production in the diaphragm is important.
OBJECTIVE
These experiments tested the hypothesis that elevated mitochondrial ROS emission is required for MV-induced oxidative stress, atrophy, and contractile dysfunction in the diaphragm.
DESIGN
Cause and effect was determined by preventing MV-induced mitochondrial ROS emission in the diaphragm of rats using a novel mitochondrial-targeted antioxidant (SS-31).
MEASUREMENTS AND MAIN RESULTS
Compared to mechanically ventilated animals treated with saline, animals treated with SS-31 were protected against MV-induced mitochondrial dysfunction, oxidative stress, and protease activation in the diaphragm. Importantly, treatment of animals with the mitochondrial antioxidant also protected the diaphragm against MV-induced myofiber atrophy and contractile dysfunction.
CONCLUSIONS
These results reveal that prevention of MV-induced increases in diaphragmatic mitochondrial ROS emission protects the diaphragm MV-induced diaphragmatic weakness. This important new finding indicates that mitochondria are a primary source of ROS production in the diaphragm during prolonged MV. These results could lead to the development of a therapeutic intervention to impede MV-induced diaphragmatic weakness.
Keywords: atrophy, respiratory muscles, weaning, oxidative stress, antioxidant, redox signaling
Introduction
Mechanical ventilation (MV) is used clinically to achieve adequate pulmonary gas exchange in patients suffering from hypoxemia and in patients who are incapable of maintaining sufficient alveolar ventilation. Common indications for MV include respiratory failure, heart failure, surgery, drug overdose, and spinal cord injuries (1). Even though MV is a life-saving measure for patients in respiratory failure, problems with weaning patients from the ventilator are common. Indeed, weaning difficulties are an important clinical problem as 20–30% of mechanically ventilated patients experience weaning difficulties (2). Although the failure to wean patients may be due to several factors, respiratory muscle weakness is predicted to play an important role (3). In reference to the causes of respiratory muscle weakness, numerous studies demonstrate that prolonged MV (>12 hours) promotes a rapid development of diaphragmatic weakness due to both contractile dysfunction and atrophy (4–8).
Oxidative damage to diaphragmatic myofibrillar lipids and proteins is a hallmark of MV-induced diaphragmatic weakness (9, 10). Importantly, our work reveals that prevention of MV-induced oxidative stress via antioxidants can avert the diaphragmatic atrophy and contractile dysfunction that occurs during prolonged MV (11, 12). These findings indicate that the MV-induced production of reactive oxygen species (ROS) is an essential up-stream trigger to initiate signaling events leading to diaphragmatic weakness. To develop an appropriate therapeutic target to prevent MV-induced diaphragmatic weakness, it is important to identify the site(s) of ROS production in the diaphragm during prolonged MV. Previous efforts to identify the location of MV-induced ROS generation in the diaphragm indicate that xanthine oxidase, NADPH oxidase, and mitochondria produce ROS during prolonged MV (13–15). However, inhibition of xanthine oxidase activity does not completely protect the diaphragm from MV-induced oxidative stress and atrophy (15). Further, although prolonged MV activates NADPH oxidase in the diaphragm, MV-induced increases in NADPH activity is small (i.e., 5%) (14). In contrast, our recent work indicates that prolonged MV results in a significant increase in diaphragmatic mitochondrial ROS emission (13). Together, these findings suggest that mitochondria may be an important source of ROS production in the diaphragm during prolonged MV. Therefore, to determine the physiological significance of mitochondrial ROS emission in the development of MV-induced diaphragmatic weakness, these experiments employed a mitochondria-targeted antioxidant (SS-31) to test the hypothesis that mitochondrial ROS emission plays a dominant role in MV-induced diaphragmatic oxidative stress, proteolysis, atrophy, and contractile dysfunction. SS-31 (D-Arg-2′6′dimethylTyr-Lys-Phe-NH2) is a synthetic aromatic cationic tetrapeptide that has been shown to selectively target and concentrate ~1000 fold in the inner mitochondrial membrane (16). Tyrosine and tyrosine-containing peptides can scavenge oxyradicals (17), and the mitochondrial targeting of SS-31 provides selective scavenging of mitochondrial oxyradicals. SS-31 has no effect on NAHPH oxidase activity (unpublished observations). Numerous studies in isolated mitochondria, cultured cells, and animal models have shown that SS-31 can selectively scavenge mitochondrial ROS and protect mitochondrial function (18, 19). Furthermore, the protection provided by SS-31 is similar to that achieved by overexpression of catalase targeted to mitochondria (18).
Methods
Animals and Institutional Approval
Young adult (~6 month old) female Sprague-Dawley (SD) rats were used in these experiments. The Institutional Animal Care and Use Committee of the University of Florida approved these experiments.
Experimental Design
To investigate the role that mitochondrial ROS emission plays in MV-induced diaphragmatic weakness, we performed two separate experiments.
Experiment #1
This experiment was performed to determine the effect of a mitochondrial-targeted antioxidant (SS-31) on diaphragmatic contractile function, fiber cross sectional area (CSA), and mitochondrial function in awake and spontaneously breathing rats. Animals (n=6/group) were randomly assigned into one of two experimental groups: 1) Control group-injected with saline (i.p.) at three hour intervals for 12 hours; and 2) Mitochondrial antioxidant group-injected (i.p.) with SS-31 every three hours for 12 hours. At the completion of the 12-hour treatment periods, we measured diaphragmatic contractile function, fiber CSA, mitochondrial ROS emission, and mitochondrial respiratory function. Our results reveal that compared to control, treatment of spontaneous breathing animals with SS-31 did not alter any of these dependent measures (see results). Therefore, we then performed a second experiment using SS-31 to determine the role that mitochondrial ROS emission plays in MV-induced diaphragmatic weakness during 12-hours of MV.
Experiment #2
To test the hypothesis that mitochondrial ROS emission plays a critical role in MV-induced diaphragmatic oxidative stress and weakness, rats were randomly assigned to one of three experimental groups (n =12/group): 1) an acutely anesthetized control group; 2) a 12-hour MV group (MV); and 3) a 12-hour MV group treated with the mitochondrial-targeted antioxidant SS-31 (MVSS). Because of the large tissue requirement for our numerous dependent measures, six animals from each experimental group were used for the mitochondrial measures and the remaining six animals in each group were employed in all other biochemical assays.
Experimental Protocol for Experiment 2
Acutely anesthetized controls
Animals in the control group were acutely anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (60 mg/kg body weight). After reaching a surgical plane of anesthesia, the diaphragms were quickly removed. In one group of animals (n=6), a strip of the medial costal diaphragm was immediately used for in vitro contractile measurements, a separate section was stored for histological measurements, and the remaining portions of the costal diaphragm were rapidly frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses. In a second group of animals (n=6), the entire costal diaphragm was rapidly removed and used to isolate mitochondria for measurements of mitochondrial respiration and ROS emission.
Mechanical ventilation
All surgical procedures were performed using aseptic techniques. Animals in the MV groups were anesthetized with an IP injection of sodium pentobarbital (60 mg/kg body weight), tracheostomized, and mechanically ventilated with a pressure-controlled ventilator (Servo Ventilator 300, Siemens) for 12 hours with the following settings: upper airway pressure limit: 20 cm H2O, typical pressure generation above PEEP was 6–9 cm H2O, respiratory rate: 80 bpm; and PEEP: 1 cm H2O.
The carotid artery was cannulated to permit the continuous measurement of blood pressure and the collection of blood during the protocol. Arterial blood samples (100 μl per sample) were removed periodically and analyzed for arterial pO2, pCO2 and pH using an electronic blood-gas analyzer (GEM Premier 3000; Instrumentation Laboratory, Lexington, MA). Ventilator adjustments were made if arterial PCO2 exceeded 40 mm Hg. Arterial PO2 was maintained > 60 mmHg throughout the experiment by increasing the FIO2 (22–26% oxygen).
A venous catheter was inserted into the jugular vein for continuous infusion of sodium pentobarbital (~10 mg/kg/hr) and fluid replacement. Body temperature was maintained at 37°C by use of a heating blanket and heart rate was monitored via a lead II electrocardiograph. Continuous care during the MV protocol included lubricating the eyes, expressing the bladder, removing airway mucus, rotating the animal, and passively moving the limbs. Animals also received a intramuscular injection of glycopyrrolate (0.08 mg/kg; loading dose) and (0.04 mg/kg) every two hours during MV to reduce airway secretions. Upon completion of MV, diaphragm removal and storage was completed using the same protocol as previously described for the controls.
Mitochondrial-targeted antioxidant: Chemical details and experimental delivery
We selected a mitochondria-targeted antioxidant designated as “SS-31” for use in the current experiments. This molecule belongs to a family of small, water soluble peptides that contain an alternating aromatic-cationic motif and selectively target to the mitochondria (20).
In experiment 1, the mitochondrial-targeted antioxidant SS-31 was dissolved in saline and delivered via four subcutaneous injections during the 12-hour experimental period. The first bolus (loading) dose (3 mg/kg; subcutaneous injection) was administered at the onset of the experiment. We then delivered SS-31 (0.05 mg/kg/hr) via subcutaneous injections staged every three hours during the 12-hour experiment. Importantly, animals in experiment 1 received the same total amount of SS-31 during 12 hours as the animals in experiment 2.
In experiment 2, the mitochondrial-targeted antioxidant SS-31 was dissolved in saline and delivered in a bolus (loading) dose (3 mg/kg; subcutaneous injection) 15 min prior to initiation of MV. A constant intravenous infusion (0.05 mg/kg/hr) of SS-31 was maintained throughout MV.
Biochemical Measures
Isolation of mitochondria
Approximately 500 mg of costal diaphragm muscle was used to isolate diaphragmatic mitochondria using the methods of Makinen and Lee (21) with minor modifications (13).
Mitochondrial respiration
Mitochondrial oxygen consumption was measured using previously described techniques (13). The maximal respiration (state 3) and state 4 respiration (basal respiration) were measured as described previously (22). The respiratory control ratio (RCR) was calculated by dividing state 3 by state 4 respiration.
Mitochondrial ROS emission
Diaphragmatic mitochondrial ROS emission was determined using Amplex™ Red (Molecular Probes, Eugene, OR). Details of this assay have been described previously (13). Mitochondrial ROS production was measured using the creatine kinase energy clamp technique to maintain respiration at steady state using previously described methods (23).
Western blot analysis
Protein abundance was determined in diaphragm samples via Western Blot analysis using previously described methods (24). After electrophoresis, the proteins were transferred to nitrocellulose membranes and incubated with primary antibodies directed against the protein of interest. 4-hydroxynonenal (4-HNE) (Abcam) was probed as a measurement indicative of oxidative stress while proteolytic activity was assessed by analyzing murf1 (ECM Biosciences), atrogin1 (ECM Biosciences), cleaved (active) calpain-1 (Cell Signaling) and cleaved (active) caspase-3 (Cell Signaling). Further, α-II spectrin (Santa Cruz) calpain specific cleavage (145kDa cleavage product) and caspase-3 specific cleavage (120kDa cleavage product) were measured to obtain an additional measurement of both calpain-1 and caspase-3 activity during MV. The protein abundance of actin (Santa Cruz) was measured as an index of overall proteolysis in the diaphragm. Finally, α-tubulin was used as a loading control for all western blots to verify equal protein loading and transfer.
Assessment of protein oxidation via reactive carbonyl derivatives
The levels of reactive carbonyl derivatives in the myofibrillar protein samples were assessed as an index of the magnitude of protein modification. This was accomplished using the Oxyblot Oxidized Protein Detection Kit from Chemicon International (Temecula, Ca) as described previously (13).
RNA isolation and cDNA synthesis
Total RNA was isolated from muscle tissue with TRIzol Reagent (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. RNA content (μg/mg muscle) was evaluated by spectrophotometry. RNA (5 μg) was then reverse transcribed with the Superscript III First-Strand Synthesis System for RT-PCR (Life Technologies), using oligo(dT)20 primers and the protocol outlined by the manufacturer.
Real-time polymerase chain reaction
One μl of cDNA was added to a 25 μl PCR reaction for real-time PCR using Taqman chemistry and the ABI Prism 7000 Sequence Detection system (ABI, Foster City, CA). Relative quantification of gene expression was performed using the comparative computed tomography method (ABI, User Bulletin #2). β-Glucuronidase, a lysosomal glycoside hydrolase, was chosen as the reference gene based on previous work showing unchanged expression with our experimental manipulations (25). MAFbx (GenBank NM AY059628) and MuRF-1 (GenBank NM AY059627, NM BC061824) mRNA transcripts were assayed using predesigned rat primer and probe sequences commercially available from Applied Biosystems (Assays-on-Demand).
20S proteasome activity
A section of the ventral costal diaphragm was homogenized and the in vitro chymotrypsin-like activity (substrate: Z-Leu-Leu-Leu-AMC) of the 20S proteasome was measured fluorometrically using techniques described by Stein and co-workers (26).
Functional Measures
Measurement of in vitro diaphragmatic contractile properties
At the completion of the experimental periods, the entire diaphragm was removed and placed in a dissecting chamber containing a Krebs-Hensleit solution equilibrated with 95% O2-5% CO2 gas. A muscle strip (~3mm wide) was suspended vertically between two lightweight Plexiglas clamps with one end connected to an isometric force transducer (model FT-03, Grass Instruments, Quincy, MA) within a jacketed tissue bath. The muscle was electrically stimulated to contract and the force output was recorded via a computerized data-acquisition system as previously described (5). For comparative purposes, diaphragmatic force production was normalized as fiber cross sectional area (i.e., specific force production).
Histological Measures
Myofiber cross-sectional area
Sections from frozen diaphragm samples were cut at 10 microns using a cryotome (Shandon Inc., Pittsburgh, PA) and stained for dystrophin, myosin heavy chain (MHC) I and MHC type IIa proteins for fiber cross-sectional area analysis (CSA) as described previously (12). CSA was determined using Scion software (NIH).
Statistical Analysis
Comparisons between groups for each biochemical dependent variable were made by a one-way analysis of variance (ANOVA) and, when appropriate, a Tukey HSD (honestly significant difference) test was performed post-hoc. Further, using a one-way ANOVA, we compared the group differences in diaphragmatic specific force production at each stimulation frequency. If statistically significant differences existed, we then performed a Tukey HSD post-hoc test to determine which groups differed. Significance was established at p < 0.05. Data are presented as means ± SEM.
Results
SS-31 does not impact diaphragmatic fiber CSA or function in spontaneously breathing animals
To determine the impact of the mitochondrial antioxidant SS-31 on diaphragmatic contractile function, fiber cross sectional area (CSA), and mitochondrial function in awake and spontaneously breathing rats, we treated animals for 12-hours with the same levels of SS-31 that were provided to the mechanically ventilated animals during the 12-hour MV period. Our results indicate that compared to control animals, the treatment of animals with SS-31 does not influence diaphragmatic fiber size, contractile function, mitochondrial ROS emission, and mitochondrial respiratory function (Table 1).
Table 1.
| 1A) Fiber cross-sectional area (CSA) in diaphragm muscle fibers from both control (treated with saline injections) and awake and spontaneously breathing animals treated with the mitochondrial-targeted antioxidant SS-31. No significant differences in diaphragmatic fiber CSA existed between the Control and SS-31 groups in any fiber type. Values are means ± SEM. | ||
|---|---|---|
| Diaphragm muscle fiber type |
Control Group Fiber CSA (μm2) |
SS-31 Group Fiber CSA (μm2) |
| Type I | 1186±71 | 1280±44 |
| Type IIa | 1211±143 | 1267±49 |
| Type IIx/B | 3092±230 | 3007±304 |
| 1B) Effects of a mitochondrial targeted antioxidant (SS-31) on the diaphragmatic force-frequency response (in vitro) in control (saline injected) and SS-31 treated animals. No significant differences in diaphragmatic force production existed between the control and SS-31 groups at any stimulation frequency. Values are means ± SEM. | ||
|---|---|---|
| Diaphragm stimulation frequency (Hz) |
Control Group Diaphragm force production (Newtons/cm2)) |
SS-31 Group Diaphragm force production (Newtons/cm2) |
| 15 | 14.1±0.7 | 15.0±0.7 |
| 30 | 20.4±0.5 | 21.0±0.3 |
| 60 | 24.1±0.4 | 24.2±0.3 |
| 100 | 24.7±0.5 | 25.0±0.4 |
| 160 | 24.6±0.5 | 24.8±0.3 |
| 1C) Effects of a mitochondrial targeted antioxidant (SS-31) on diaphragm mitochondrial hydrogen peroxide emission and the mitochondrial respiratory function in control (saline injected) and SS-31 treated animals. These data were obtained using pyruvate/malate as substrate. VO2 = mitochondrial oxygen consumption; RCR = respiratory control ratio. Units for state-3 and state-4 VO2 are nmoles oxygen/mg protein/minute. Values are means ± SEM * = different from control at p<0.05. | ||||||
|---|---|---|---|---|---|---|
| Group (N=4/group) |
H2O2 Emission State 3 (pmoles/min/mg) |
H2O2 Emission State 4 (pmoles/min/mg) |
State-3 VO2 | State-4 VO2 | ADP/O ratio | RCR |
| Control Group | 51±3.6 | 661±21 | 282±28 | 67±5 | 2.2±0.2 | 4.3±0.3 |
| SS-31 Group | 54±4.5 | 652±18 | 237±15 | 49±2* | 2.7±0.2 | 4.8±0.2 |
Physiological responses to prolonged MV
To determine whether our MV protocol was successful in maintaining homeostasis, we measured arterial blood pressures, arterial PCO2, arterial PO2 and arterial pH in all animals at the beginning of the experiments and at various time intervals during MV. Our results confirm that arterial blood pressure and blood-gas/pH homeostasis were well-maintained during MV (Table 2).
Table 2.
Animal heart rates, systolic blood pressure, and arterial blood gas tension/pH and at the completion of 12 hours of mechanical ventilation.
| Physiological variable | MV | MVSS |
|---|---|---|
| Heart rate (beats/min) | 339±10 | 347±7 |
| Systolic blood pressure (mm/Hg) | 105±6 | 108±5 |
| Arterial PO2 (mm/Hg) | 73±2 | 75±5 |
| Arterial PCO2 | 45±0.8 | 46±1 |
| Arterial pH | 7.41 ±0.01 | 7.41 ±0.01 |
Values are means± SEM. No significant differences existed between the two experimental groups in any of these physiological variables.
Our data also indicate that our animals did not develop infection during MV as evidenced by the observations that our MV animals were afebrile, no detectable bacteria were in the blood, and postmortem examination of the lungs and peritoneal cavity yielded no detectable abnormalities.
A mitochondrial-targeted antioxidant impedes MV-induced ROS emission from diaphragmatic mitochondria
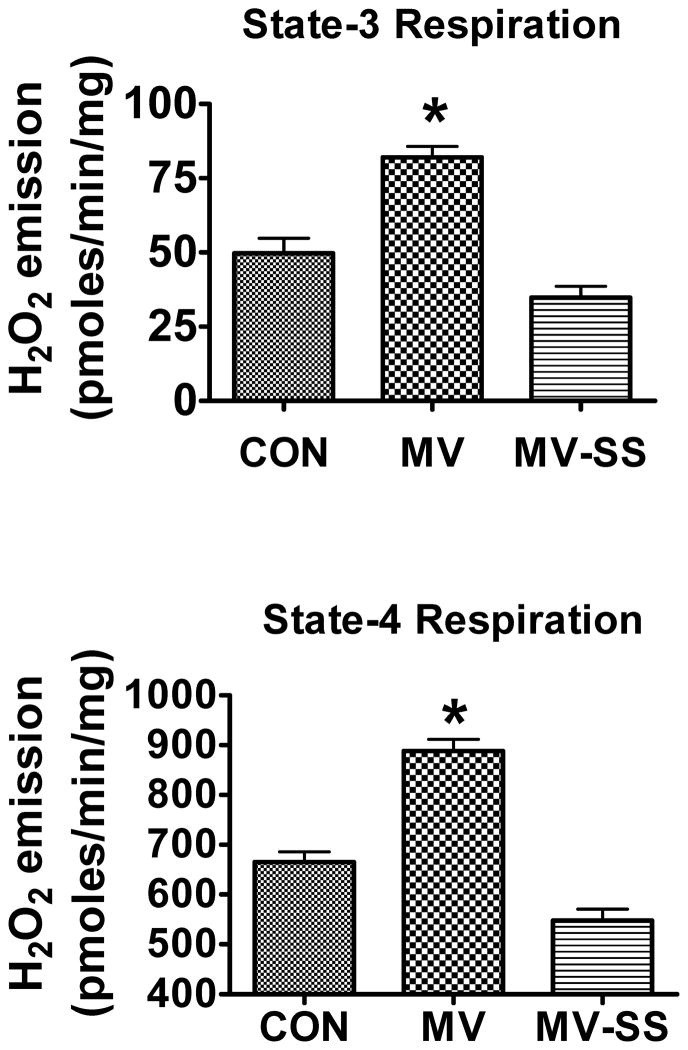
To determine if mitochondrial-derived ROS emission is required for MV-induced oxidative damage, contractile dysfunction, and atrophy in the diaphragm, we treated rats with a mitochondrial-targeted antioxidant (SS-31) to prevent MV-induced ROS emission from diaphragm mitochondria. Our results indicate that treatment with SS-31 prevented the MV-induced increase in diaphragmatic mitochondrial H2O2 release during both state 3 and 4 respiration (Figure 1).
Figure 1.
Rates of hydrogen peroxide (H2O2) release from mitochondria isolated from diaphragms of control (CON), mechanically ventilated (MV), and mechanically ventilated rats treated with the mitochondrial-targeted antioxidant SS-31 (MVSS). Note that compared to CON, 12 hours of MV resulted in a significant increase in mitochondrial H2O2 emission during both state-3 and state-4 respiration. Importantly, treatment of animals with SS-31 significantly reduced the rates of H2O2 release from the mitochondria following prolonged MV. Values are mean ± SEM. * = CON different (p<0.05) from MV; (n =6/group).
Our prior work indicates that prolonged MV results in damage to mitochondria as indicated by impaired coupling (i.e., lower respiratory control ratios) in mitochondria isolated from the diaphragm of MV animals (13). Therefore, we asked whether treatment of animals with SS-31 would protect diaphragmatic mitochondria from MV-induced mitochondrial uncoupling. As shown in Table 3, treatment with SS-31 was successful in averting diaphragmatic mitochondrial uncoupling that occurs following prolonged MV.
Table 3.
State-3 respiration, State-4 respiration, and respiratory control ratio (RCR) in mitochondria isolated from diaphragms of control (CON), mechanically ventilated (MV), and mechanically ventilated animals treated with the mitochondrial antioxidant, SS-31 (MVSS). These data were obtained using pyruvate/malate as substrate. Units for state-3 and state-4 oxygen consumption (VO2) are nmoles oxygen/mg protein/minute.
| Parameter | Control | MV | MVSS |
|---|---|---|---|
| State-3 VO2 | 235.9±10 | 212.4±11 | 193.1±9 |
| State-4 VO2 | 61.8±3 | 77.6±5* | 42.4±3 |
| RCR | 4.7±0.2 | 2.7±0.3* | 4.6±0.2 |
| ADP/O ratio | 2.1±0.2 | 2.3±0.2 | 2.3±0.2 |
Values are means ± SEM.
different (p<0.05) from both CON and MVSS.
MV-induced oxidative stress is mediated by mitochondrial ROS emission
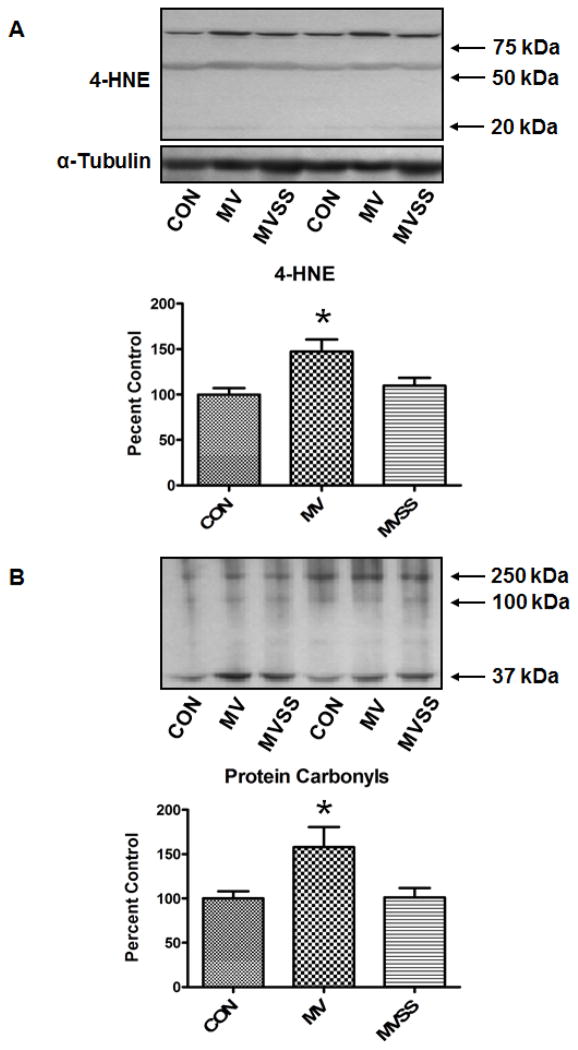
To determine if mitochondrial ROS emission is required for MV-induced oxidative stress in the diaphragm, we measured two biomarkers of oxidative damage. Our results reveal that treatment of animals with SS-31 protected the diaphragm against the ROS-induced increase in both protein carbonyls and 4-HNE-conjugated proteins normally associated with prolonged MV (Figure 2).
Figure 2.
Levels of oxidatively modified proteins in the diaphragm of control (CON), mechanically ventilated (MV), and mechanically ventilated rats treated with the mitochondrial-targeted antioxidant SS-31 (MVSS). A) Levels of 4-hydroxyl-nonenal-conjugated proteins in the diaphragm of the three experimental groups. The image above the histograph is a representative western blot of data from the three experimental groups. B) Levels of protein carbonyls in the diaphragm of the three experimental groups. The image above the histograph is a representative western blot of data from the three experimental groups. * = different (p<0.05) from both CON and MVSS (n = 6/group).
Increased mitochondrial ROS emission promotes MV-induced diaphragmatic contractile dysfunction and fiber atrophy
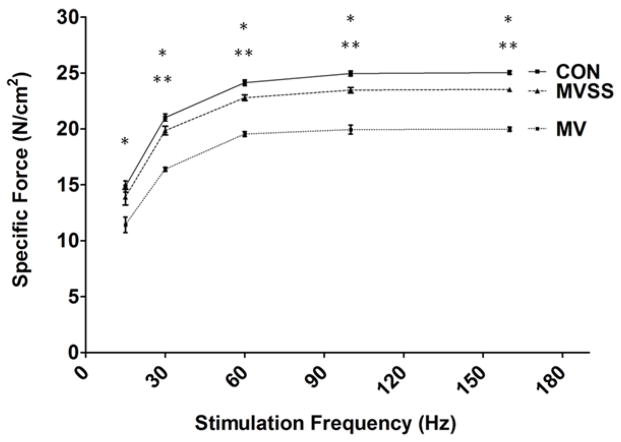
To determine the role that mitochondrial ROS emission plays in MV-induced diaphragmatic contractile dysfunction, we measured diaphragmatic contractile performance in vitro using strips of diaphragm muscle obtained from control, MV, and MV animals treated with SS-31. Prevention of MV-induced mitochondrial ROS emission using SS-31 was successful in diminishing the normally observed MV-induced diaphragmatic contractile dysfunction. Note, however, that treatment of animals with SS-31 did not completely retard the detrimental effects of prolonged MV on diaphragmatic force production at stimulation frequencies >15 HZ (Figure 3). Nonetheless, while treatment with SS-31 did not completely eliminate the MV-induced diaphragmatic contractile dysfunction, the difference in diaphragmatic force generation between controls and MV animals treated with SS-31 were small (~5–6%) at all stimulation frequencies. At present, it is unclear if these small differences are physiologically significant.
Figure 3.
Effects of prolonged MV on the diaphragmatic force-frequency response (in vitro) in control and mechanically ventilated rats with/without mitochondrial targeted antioxidants. No significant differences in diaphragmatic force production existed between the CON and MVSS groups at any stimulation frequency. Values are means ± SEM. Note that some of the SEM bars are not visible because of the small size. * = MV different (p<0.05) from both CON and MVSS; ** = MVSS different (p<0.05) from CON (n = 6/group).
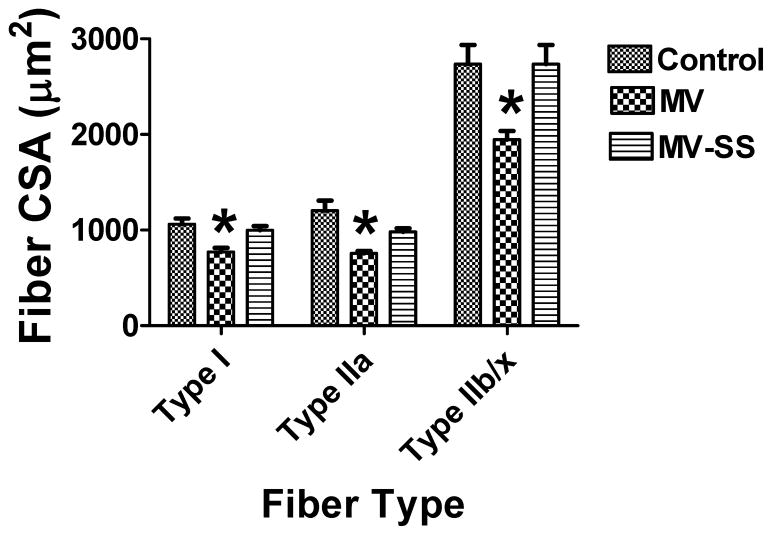
Previous work reveals that MV-induced oxidative stress is a requirement for the diaphragmatic fiber atrophy that is associated with prolonged MV (11, 12). Therefore, we asked if MV-induced mitochondrial ROS emission is a requirement for MV-induced diaphragmatic atrophy. Myofiber cross-sectional area was determined for individual fiber types for all treatment groups. Our data reveal that prevention of the MV-induced increase in mitochondrial ROS emission protects the diaphragm from MV-induced fiber atrophy (Figure 4).
Figure 4.
Fiber cross-sectional area (CSA) in diaphragm muscle myofibers from control (CON) and mechanically ventilated rats with (MVSS) and without mitochondrial targeted antioxidants (MV). Note that no significant differences in diaphragmatic fiber CSA existed between the CON and MVSS groups in any fiber type. Values are means ± SEM. * = different (p<0.05) from both CON and MVSS (n = 6/group).
MV-induced mitochondrial ROS emission promotes diaphragmatic protease activation and proteolysis
Ubiquitin-proteasome system
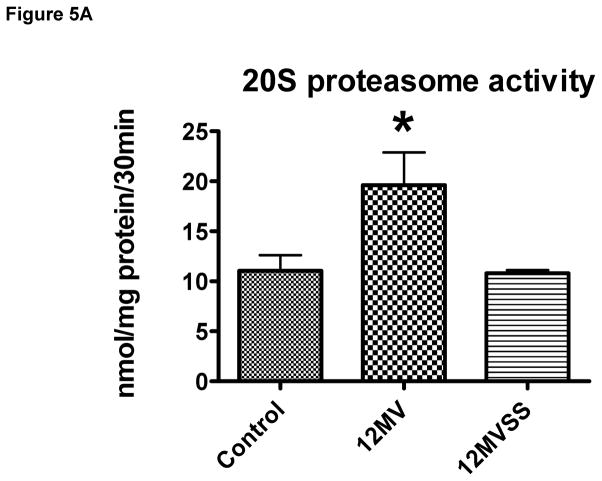
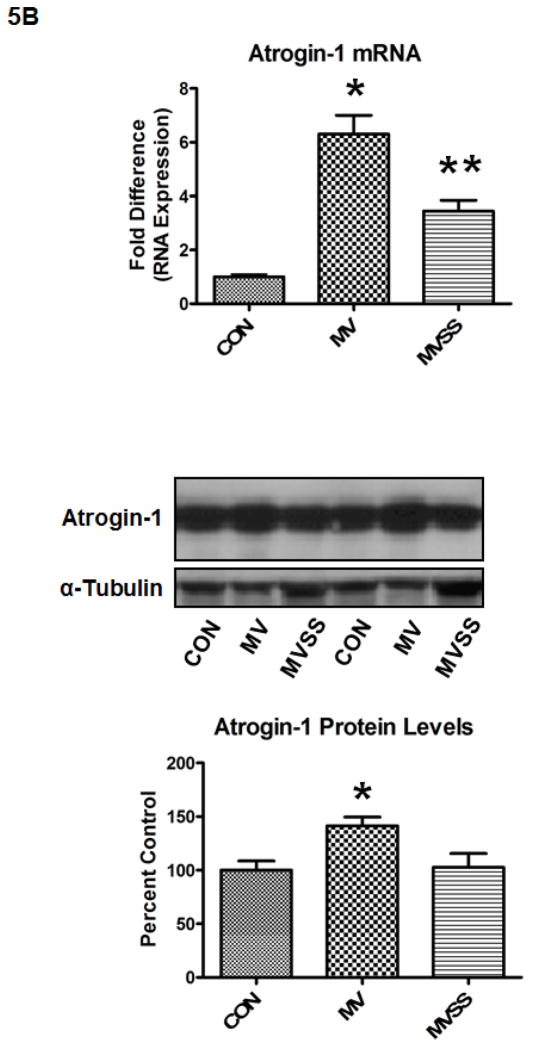
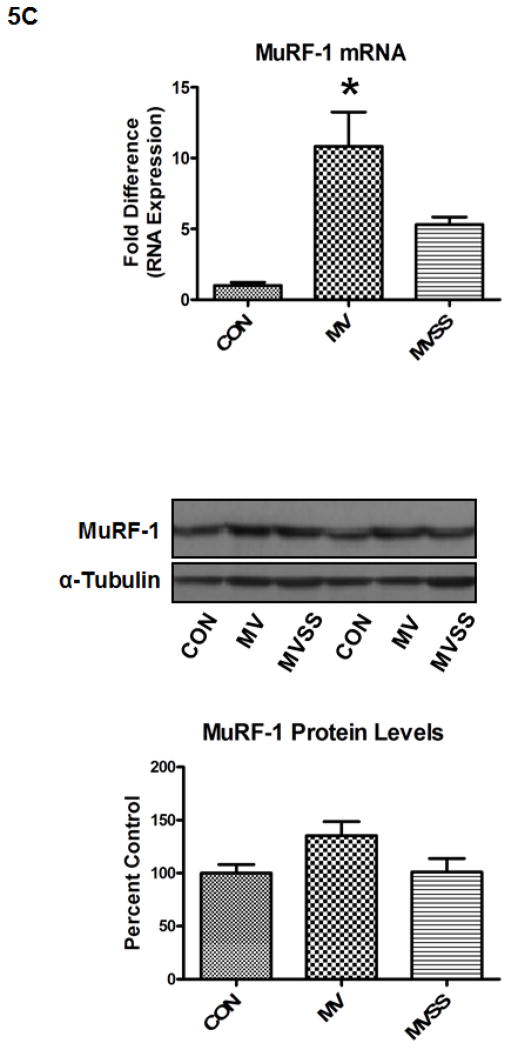
The ubiquitin-proteasome system of proteolysis is activated in the diaphragm during prolonged MV and therefore, is predicted to contribute to MV-induced diaphragmatic protein breakdown. Therefore, we measured 20S proteasome activity along with both mRNA and protein levels of two important muscle specific E3 ligases (i.e., atrogin-1/MAFbx and MuRF-1) in the diaphragm. Our results reveal that prevention of MV-induced mitochondrial ROS release via SS-31 prevented the MV-induced increase in 20S proteasome activity in the diaphragm (Figure 5A). Further, our data indicate that prolonged MV resulted in a significant increase in atrogin-1/MAFbx mRNA levels in the diaphragm of both MV groups; however, treatment of animals with SS-31 significantly blunted the MV-induced increase in atrogin-1/MAFbx protein levels in the diaphragm (Figure 5B). Further, prolonged MV resulted in a significant increase in MuRF-1 mRNA levels in the diaphragm and although MuRF-1 proteins levels tended to increase in the diaphragm of mechanically ventilated animals, these differences did not reach significance (Figure 5C).
Figure 5.
Activity of the 20S proteasome (5A), mRNA and protein levels of both atrogin-1(5B) and MuRF-1 (5C) in the diaphragm from control (CON) and mechanically ventilated animals with (MVSS) and without mitochondrial-targeted antioxidants (MV). The images above the histograms in Figures 5B and 5C are representative western blots of data from the three experimental groups. Values are means ± SEM. * = different (p<0.05) from both CON and MVSS. ** = different (p<0.05) from both CON and MV (n = 6/group).
Calpain and caspase-3 activation
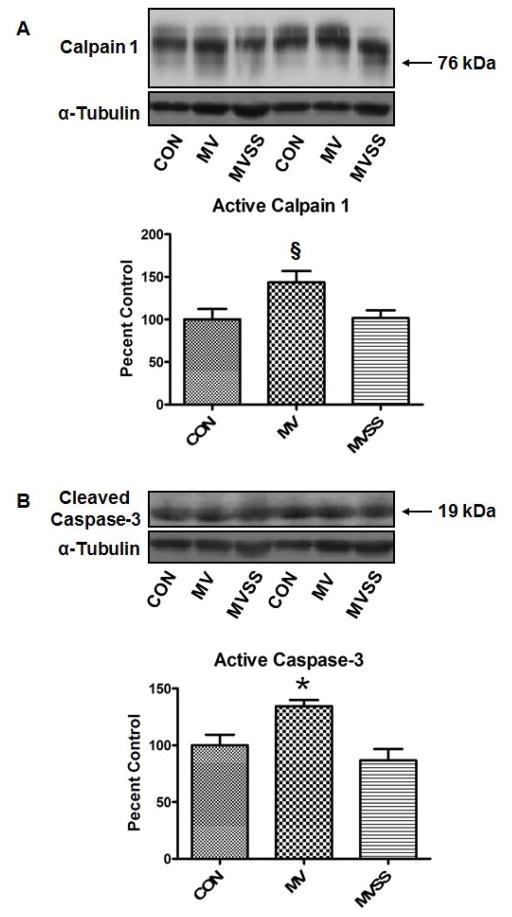
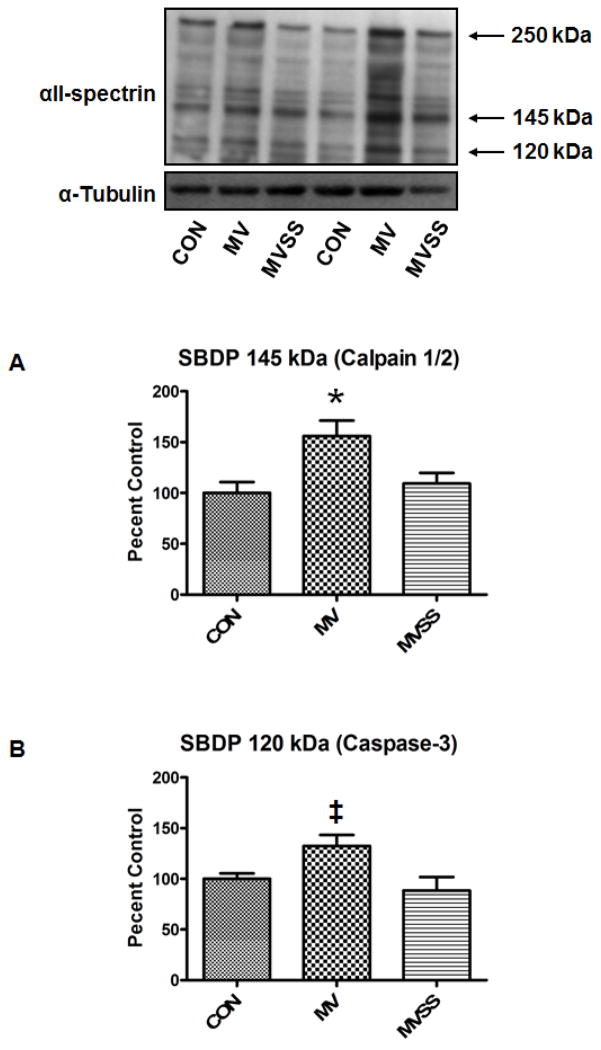
Our prior work indicates that calpain and caspase-3 activation in the diaphragm plays an important role in MV-induced diaphragmatic atrophy and contractile dysfunction (24, 27). We assayed diaphragmatic calpain and caspase-3 activity using two different but complimentary methods. First, we determined active calpain-1 and caspase-3 levels in the muscle via Western blotting to detect the cleaved and active forms of calpain 1 and caspase-3 (Figure 6). We also measured the calpain and caspase-3 specific degradation products of αII-spectrin as these breakdown products provide a signature product in vivo that can be detected via western analysis (Figure 7). This technique provides an index of in vivo calpain and caspase-3 activity in the diaphragm over a prolonged period of time during MV. Collectively, our results reveal that treatment of animals with SS-31 protected the diaphragm against the MV-induced activation of both calpain (Figure 6A and 7A) and caspase-3 (Figure 6B and 7B).
Figure 6.
Calpain 1 and Caspase 3 activity in the diaphragm from control (CON) and mechanically ventilated animals with (MVSS) and without mitochondrial-targeted antioxidants (MVSS). A) The active form of calpain 1 in diaphragm muscle at the completion of 12 hours of MV. B) The cleaved and active band of caspase-3 in diaphragm muscle at the completion of 12 hours of MV. The images above the histograms in Figure 6A and 6B are representative western blots of data from the three experimental groups. Values are means ± SEM. §= different (p<0.05) from CON; * = different (p<0.05) from both CON and MVSS (n=6/group).
Figure 7.
Calpain and caspase-3 activity in the diaphragm from control (CON) and mechanically ventilated animals with (MVSS) without mitochondrial-targeted antioxidants (MV). A) Levels of the 145 kDa α-II-spectrin break-down product (SBPD) in diaphragm muscle following 12 hours of MV. Note that the SBDP 145 kDa is an α-II-spectrin break-down product that is specific to calpain cleavage of intact α-II-spectrin and therefore, the cellular level of SBDP 145 kDa can be used as a biomarker of in vivo calpain activity. B) Levels of the 120 kDa α-II-spectrin break-down product (SBPD 120 kDa) in diaphragm muscle following 12 hours of MV. Note that the SBDP 120 kDa is a α-II-spectrin break-down product that is specific to caspase-3 cleavage of intact α-II-spectrin and therefore, the cellular levels of SBDP 120 kDa can be used as a biomarker of caspase-3 activity. The images above the histograms in Figure 7A and 7B are representative western blots of data from the three experimental groups. Values are means ± SEM. * = different (p<0.05) from both CON and MVSS. ‡= different (p<0.05) from MVSS (n=6/group).
Mitochondrial-targeted antioxidants protect against MV-induced diaphragmatic proteolysis
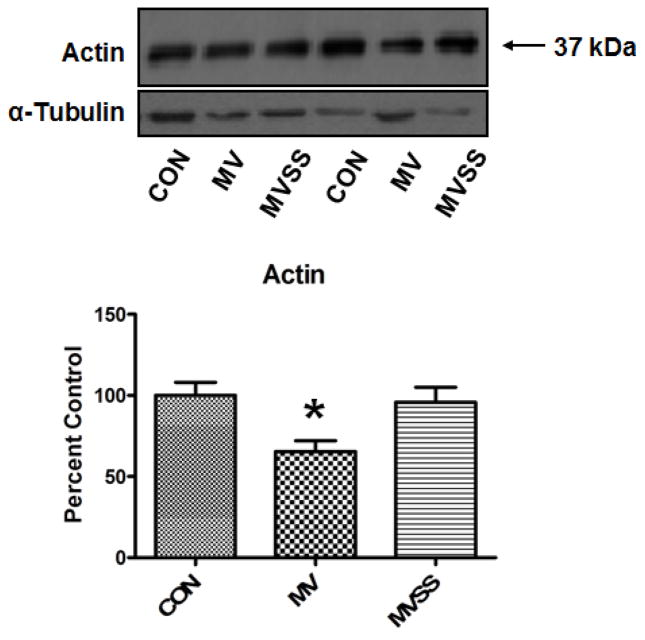
After demonstrating that prevention of MV-induced increases in mitochondrial ROS emission protects the diaphragm against protease activation, we then measured the relative abundance of the sarcomeric protein actin in the diaphragm as a marker of disuse-induced muscle proteolysis (28). Our results reveal that, compared to diaphragm muscle from both control and MV-SS animals, the actin abundance was significantly reduced in diaphragm muscle from animals exposed to prolonged MV without mitochondrial antioxidants (Figure 8). Therefore, prevention of MV-induced mitochondrial ROS emission protected against both MV-induced protease activation and diaphragmatic proteolysis.
Figure 8.
Ratio of actin to total sarcomeric protein levels in the diaphragm from control (CON) and mechanically ventilated animals with (MVSS) without mitochondrial-targeted antioxidants (MV). Given that actin is preferentially degraded during disuse muscle atrophy, assessment of the ratio of actin to total sarcomeric protein levels provides a relative index of diaphragmatic proteolysis during prolonged MV. The image above the histogram is a representative western blot of data from the three experimental groups. Values are means ± SEM. * = different (p<0.05) from both CON and MVSS (n=6/group).
Discussion
Overview of major findings
These experiments provide several new and important findings relative to the mechanism(s) responsible for the rapid onset of MV-induced diaphragmatic atrophy and contractile dysfunction. First, our data reveal that mitochondria are a dominant source of oxidant production in the diaphragm during prolonged MV. Second, our findings clearly demonstrate that mitochondrial ROS emission is a required up-stream signal for the MV-induced activation of key proteases (i.e., calpain and caspase-3) in the diaphragm. Finally, our results also show that prevention of MV-induced increases in mitochondrial ROS emission in the diaphragm can protect the diaphragm from MV-induced diaphragmatic atrophy and contractile dysfunction. A critique of our experimental model and a detailed discussion of the significance of these results follow.
Critique of experimental model
Because of the invasive nature of obtaining diaphragm muscle samples from humans, an animal model is required to perform mechanistic experiments to explore the signaling pathways that promote MV-induced diaphragmatic weakness. We selected the rat as the experimental animal in these experiments for several reasons. First, the anatomical features and function of the rat diaphragm are both similar to the human diaphragm (29, 30). Second, the fiber type composition of the rat and human costal diaphragm are comparable (4, 31, 32). Finally, the time course of MV-induced atrophy in the rat and human diaphragm are also similar (4, 10).
Investigating the role of mitochondrial ROS emission as a signaling molecule in the diaphragm requires a mitochondria-targeted antioxidant to “buffer” this increased ROS emission. To achieve this goal, we selected a new and innovative mitochondria-targeted antioxidant (i.e., SS-31) for use in the current experiments. This compound selectively concentrates in the mitochondria and can provide radical scavenging of H2O2, OH·, and ONOO− on a dose-dependent basis (20, 33). Importantly, SS-31 does not alter mitochondrial membrane potential (20) and our results reveal that SS-31 does not influence diaphragmatic mitochondrial function, myofiber size, and contractile function. Because of these collective properties, this mitochondrial-targeted antioxidant is ideal for use in our current experiments.
Our experiments investigated the impact of prolonged MV on diaphragm mitochondria function using isolated mitochondria. The isolation of mitochondria from diaphragm muscle disrupts the normal in vivo arrangement of these organelles as skeletal muscle mitochondria in vivo are arranged in an interconnected reticulum (34, 35). This in vivo architecture of muscle mitochondria suggests that this structural pattern may have functional implications for these organelles (36). It follows that isolation of muscle mitochondria would disrupt this normal structure and potentially impair mitochondrial function. Nonetheless, in the current study, the respiratory control ratios of isolated mitochondria from control animals are similar to those reported in preparations of permeabilized rat muscle fibers which permit mitochondria to remain in their reticular structure (37). This observation is consistent with the notion that careful isolation of skeletal muscle mitochondria does not alter normal mitochondrial function and coupling.
Mitochondria are a key source of MV-induced ROS production in the diaphragm
It is well established that prolonged MV results in increased ROS production in the diaphragm, disturbed redox signaling, and oxidative damage to diaphragmatic biomolecules (9, 10, 38). However, the primary site(s) of MV-induced ROS production in the diaphragm remain unknown. To investigate the role that mitochondria play in MV-induced ROS production in the diaphragm, we treated animals with a mitochondrial-targeted antioxidant (SS-31) and isolated mitochondria from the diaphragm of control and mechanically ventilated animals with and without the mitochondrial antioxidant. Our results clearly indicate that prolonged MV promotes a significant increase in mitochondrial ROS emission and that treatment of animals with SS-31 prevents this increase (Figure 1). Further, providing animals with SS-31 prevented the MV-mediated oxidative damage to diaphragmatic proteins (Figure 3). Collectively, these data indicate that mitochondrial ROS are the dominant contributor to oxidative damage to the diaphragm during prolonged MV.
Mitochondrial ROS emission is required for MV-induced diaphragmatic atrophy and contractile dysfunction
Our prior work reveals that MV-induced oxidative stress is a requirement for MV-induced diaphragmatic atrophy and contractile dysfunction (11, 12). Further, we have shown that MV-induced oxidative stress is essential for the activation of key proteases in the diaphragm including the 20S proteasome, calpain, and caspase-3 (11, 39). Activation of calpain and caspase-3 is important because both proteases are vital contributors to MV-induced diaphragmatic proteolysis and weakness (24, 27). Therefore, identifying the ROS producing pathways responsible for activation of these diaphragmatic proteases during prolonged MV is important. The current experiments demonstrate that mitochondrial ROS emission is a required upstream signal for MV-induced activation of both calpain and caspase-3. Although it is tempting to speculate that mitochondrial ROS emission activates calpain via increased levels of cytosolic free calcium, our experiments do not provide direct evidence for or against this supposition. Further, although oxidative stress has been shown promote caspase-3 activation in myotubes, our study does not distinguish the redox-sensitive signaling pathway that is responsible for this activation. Therefore, the ROS triggered signaling pathways responsible for activation of calpain and caspase-3 in the diaphragm during MV remain unknown and are an important direction for future work.
Further, note that prevention of MV-induced mitochondrial ROS emission also protected against activation of the 20-S proteasome and the increased expression of atrogin-1 in the diaphragm. The cell signaling link to connect mitochondrial ROS emission and the activation of these key proteasome proteins remains unknown.
Importantly, our results also show that prevention of the MV-induced increase in mitochondrial ROS emission not only prevented protease activation, this intervention attenuated MV-induced diaphragmatic atrophy and contractile dysfunction as well. Indeed, treatment of animals with the mitochondrial-targeted antioxidant SS-31 was successful in preventing the MV-induced atrophy in type I, IIa, and IIx/b fibers in the diaphragm (Figure 4). Further, prevention of MV-induced increases in mitochondrial ROS emission also provided partial protection against MV-induced decreases in diaphragmatic specific force production at both sub-maximal and maximal stimulation frequencies (Figure 3). Together, these novel results indicate that protection against MV-induced mitochondrial ROS emission in the diaphragm protects this key inspiratory muscle from MV-induced weakness.
Conclusions and clinical implications
From a clinical perspective, the most important result from these experiments is the finding that treatment of animals with the mitochondrial-targeted antioxidant SS-31 prevents the rapid onset of MV-induced diaphragmatic atrophy and contractile dysfunction. Given that MV-induced diaphragmatic weakness may contribute to the failure-to-wean syndrome, this finding has significant clinical implications and suggests that mitochondrial-targeted antioxidants may have therapeutic potential in protecting the diaphragm from MV-induced weakness and reduce weaning problems in some patients. SS-31 is currently undergoing clinical development as a novel mitochondria-targeted therapy by Stealth Peptides Inc. The results of a recent Phase 1 clinical trial with intravenous infusion of SS-31 demonstrated safety and tolerability with predictable linear pharmacokinetics that included doses exceeding the expected patient dose by several fold. Clearly the use of SS-31 in prevention of MV-induced diaphragmatic weakness is an exciting possibility that warrants clinical investigation.
Acknowledgments
Financial support: This work was support by a grant from the National Institutes of Health (R01HL087839) awarded to Scott K. Powers.
Dr. Szeto consulted for Stealth Peptides Inc., and holds equity interest and stock ownership with Stealth Peptides Inc. Dr. Szeto also received a sponsored research grant from Stealth Peptides Inc and has pending patents from the Cornell Research Foundation. Dr. Szeto is the inventor of SS-31. The technology was licensed by the Cornell Research Foundation to Stealth Peptides Inc. for clinical development.
Footnotes
The remaining authors have not disclosed any potential conflicts of interest.
Disclosures
The SS peptides technology has been licensed for commercial development by the Cornell Research Foundation (CRF), and both CRF and Hazel H. Szeto have financial interests.
References
- 1.Hess D, Kacmarek R. Essentials of Mechanical Ventilation. New York, NY: McGraw-Hill; 1996. [Google Scholar]
- 2.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 3.Laghi F, Cattapan SE, Jubran A, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med. 2003;167:120–127. doi: 10.1164/rccm.200210-1246OC. [DOI] [PubMed] [Google Scholar]
- 4.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 5.Powers SK, Shanely RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 6.Shanely RA, Coombes JS, Zergeroglu AM, et al. Short-duration mechanical ventilation enhances diaphragmatic fatigue resistance but impairs force production. Chest. 2003;123:195–201. doi: 10.1378/chest.123.1.195. [DOI] [PubMed] [Google Scholar]
- 7.Radell PJ, Remahl S, Nichols DG, et al. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med. 2002;28:358–364. doi: 10.1007/s00134-002-1207-8. [DOI] [PubMed] [Google Scholar]
- 8.Gayan-Ramirez G, Testelmans D, Maes K, et al. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33:2804–2809. doi: 10.1097/01.ccm.0000191250.32988.a3. [DOI] [PubMed] [Google Scholar]
- 9.Zergeroglu MA, McKenzie MJ, Shanely RA, et al. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol. 2003;95:1116–1124. doi: 10.1152/japplphysiol.00824.2002. [DOI] [PubMed] [Google Scholar]
- 10.Shanely RA, Zergeroglu MA, Lennon SL, et al. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- 11.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170:1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 12.McClung JM, Kavazis AN, Whidden MA, et al. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavazis AN, Talbert EE, Smuder AJ, et al. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClung JM, Van Gammeren D, Whidden MA, et al. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37:1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whidden MA, McClung JM, Falk DJ, et al. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol. 2009;106:385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao K, Luo G, Giannelli S, et al. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Winterbourn CC, Parsons-Mair HN, Gebicki S, et al. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem J. 2004;381:241–248. doi: 10.1042/BJ20040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Z, Varadharaj S, Giedt RJ, et al. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther. 2009;329:94–101. doi: 10.1124/jpet.108.145557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao K, Zhao GM, Wu D, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 21.Makinen MW, Lee CP. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phosphorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys. 1968;126:75–82. doi: 10.1016/0003-9861(68)90561-4. [DOI] [PubMed] [Google Scholar]
- 22.Estrabrook R. Mitochondrial respiratory control and the polarographic measurement of ADP/O ratios. Methods Enzymology. 1967;10:41–47. [Google Scholar]
- 23.Messer JI, Jackman MR, Willis WT. Pyruvate and citric acid cycle carbon requirements in isolated skeletal muscle mitochondria. Am J Physiol Cell Physiol. 2004;286:C565–572. doi: 10.1152/ajpcell.00146.2003. [DOI] [PubMed] [Google Scholar]
- 24.McClung JM, Kavazis AN, Deruisseau KC, et al. Caspase-3 Regulation of Diaphragm Myonuclear Domain during Mechanical Ventilation-induced Atrophy. Am J Respir Crit Care Med. 2007;175:150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deruisseau KC, Shanely RA, Akunuri N, et al. Diaphragm Unloading via Controlled Mechanical Ventilation Alters the Gene Expression Profile. Am J Respir Crit Care Med. 2005;172:1267–1275. doi: 10.1164/rccm.200503-403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein RL, Melandri F, Dick L. Kinetic characterization of the chymotryptic activity of the 20S proteasome. Biochemistry. 1996;35:3899–3908. doi: 10.1021/bi952262x. [DOI] [PubMed] [Google Scholar]
- 27.Maes K, Testelmans D, Powers S, et al. Leupeptin Inhibits Ventilator-Induced Diaphragm Dysfunction in Rats. Am J Respir Crit Care Med. 2007;175:1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Moylan JS, Chambers MA, et al. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2009;297:C706–714. doi: 10.1152/ajpcell.00626.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Troyer A. Respiratory muscles. In: Crystal RaJW., editor. The lung: scientific foundations. New York: Raven press; 1991. pp. 869–883. [Google Scholar]
- 30.Greene E. Anatomy of the rat. New York: Hafner Publishing company; 1963. [Google Scholar]
- 31.Powers SK, Demirel HA, Coombes JS, et al. Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med Sci Sports Exerc. 1997;29:1573–1579. doi: 10.1097/00005768-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Powers SK, Criswell D, Lawler J, et al. Regional training-induced alterations in diaphragmatic oxidative and antioxidant enzymes. Respir Physiol. 1994;95:227–237. doi: 10.1016/0034-5687(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 33.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 34.Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Vendelin M, Beraud N, Guerrero K, et al. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol. 2005;288(3):C757–767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe MP. The machinery of mitochondrial inheritance and behavior. Science. 1999;283:1493–1497. doi: 10.1126/science.283.5407.1493. [DOI] [PubMed] [Google Scholar]
- 37.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290:C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 38.Falk DJ, Deruisseau KC, Van Gammeren DL, et al. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol. 2006;101:1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- 39.Whidden MA, Smuder AJ, Wu M, et al. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108:1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]