Using recombinant antigen variant-expressing chronic LCMV strains, Zehn and colleagues showed that amount rather than antigen strength is a key determinant of inducing a chronic infection phenotype in T cells.
Abstract
Chronic infections induce T cells showing impaired cytokine secretion and up-regulated expression of inhibitory receptors such as PD-1. What determines the acquisition of this chronic phenotype and how it impacts T cell function remain vaguely understood. Using newly generated recombinant antigen variant-expressing chronic lymphocytic choriomeningitis virus (LCMV) strains, we uncovered that T cell differentiation and acquisition of a chronic or exhausted phenotype depend critically on the frequency of T cell receptor (TCR) engagement and less significantly on the strength of TCR stimulation. In fact, we noted that low-level antigen exposure promotes the formation of T cells with an acute phenotype in chronic infections. Unexpectedly, we found that T cell populations with an acute or chronic phenotype are maintained equally well in chronic infections and undergo comparable primary and secondary expansion. Thus, our observations contrast with the view that T cells with a typical chronic infection phenotype are severely functionally impaired and rapidly transition into a terminal stage of differentiation. Instead, our data unravel that T cells primarily undergo a form of phenotypic and functional differentiation in the early phase of a chronic LCMV infection without inheriting a net survival or expansion deficit, and we demonstrate that the acquired chronic phenotype transitions into the memory T cell compartment.
INTRODUCTION
Persisting viral infections remain a major global medical problem, as over 500 million people are infected long term with hepatitis B or C or HIV (Alter, 2006). Independent from the outcome, viral infections typically induce large numbers of pathogen-specific T cells (Murali-Krishna et al., 1998; Badovinac et al., 2007; Williams and Bevan, 2007; Virgin et al., 2009). Yet, it remains vaguely understood why effector CD8 T cells support the elimination of the majority of viral infections but frequently fail to eliminate hepatitis C virus or HIV. Data from human acute and chronic infections and studies performed in acute or chronic lymphocytic choriomeningitis virus (LCMV)–infected mice show tight correlations between the phenotype T cells acquire during a viral infection and success or failure in clearing it (Wherry et al., 2003; Klenerman and Hill, 2005; Day et al., 2006; Virgin et al., 2009; Schietinger and Greenberg, 2014; Speiser et al., 2014). In resolved infections, T cells typically acquire a so-called polyfunctional phenotype, which refers to the ability of T cells to cosecrete high levels of IFN-γ and TNF and, upon transition to memory, also IL-2 (Harari et al., 2008). In contrast, T cells in chronic infections are impaired in their ability to produce TNF, IFN-γ, or IL-2 (Wherry et al., 2007). Moreover, they typically retain expression of inhibitory receptors such as PD-1, LAG-3, 2B4, CD160, and Tim3, which are only temporarily expressed in acute infections (Wherry et al., 2007; Blackburn et al., 2009). These phenotypic particularities were first described in persisting LCMV infections. Subsequently, they were also found in HIV and hepatitis C virus infections (Day et al., 2006; Radziewicz et al., 2007; Wherry et al., 2007; Bengsch et al., 2010; Legat et al., 2013; Schietinger and Greenberg, 2014), but they are absent or occur much less prominently in EBV and latent CMV infections (Klenerman and Hill, 2005; Hertoghs et al., 2010). The presence of this chronic phenotype is typically viewed as a sign of a deteriorating T cell response, linked to a substantial loss of antiviral activity.
The concept that persisting infections exhaust a functional immune response is widely accepted (Moskophidis et al., 1993; Zajac et al., 1998; Wherry, 2011; Pauken and Wherry, 2015). Nonetheless, it contrasts with clinical and experimental observations, which indicate that critical levels of effector function are retained in persisting infections (Speiser et al., 2014; Zehn et al., 2016). This is underlined by observations that depleting CD8 T cells from established simian immunodeficiency virus infections in rhesus macaques leads to strong increases in virus titers (Jin et al., 1999; Schmitz et al., 1999) and by data showing that T cells express high granzyme B levels and can select epitope escape variants even in the chronic phase of LCMV clone-13 infections (Wherry et al., 2007; Johnson et al., 2015). The most prominent evidence of retained T cell function stems from the many studies demonstrating that T cell function and virus clearance can be significantly augmented by blocking inhibitory receptors (predominately PD-1; Barber et al., 2006; Blackburn et al., 2008; Nakamoto et al., 2009; Jin et al., 2010; Bengsch et al., 2014). These observations do not preclude that T cells in chronic infections are fundamentally different from T cells in acute infections, but they are difficult to align with the idea that T cells transition to a nonfunctional state. Instead, these examples question which functional properties are lost, maintained, or even gained by T cells in chronic infections. For instance, the expression of inhibitory receptors such as PD-1, which is typically seen as a sign of deterioration, might in fact reflect a gain of an additional feature, which is that the effector function of such T cells can be tuned (or attenuated) by PD-L1–expressing antigen-positive target cells (Frebel et al., 2012). Related to this, it was recently shown that PD-1 signaling serves a protective function and prevents a rapid deterioration of the T cell response (Odorizzi et al., 2015).
Along these lines, we previously reported that when T cells from chronic infections are adoptively transferred into naive host mice and reexpanded by an acute infection, they continue to display the cytokine and PD-1 expression profile of T cells in chronic infections (Utzschneider et al., 2013). This leads to the conclusion that T cells in chronic infections undergo a stable form of differentiation rather than transitioning to a nonfunctional stage. We therefore prefer to refer to them as T cells with a chronic phenotype rather than calling them exhausted T cells. What determines the extent to which T cells acquire this chronic phenotype, how significantly T cells with a chronic phenotype differ from acute effector T cells, and how stable these differences are beyond the previously described phenotypic markers remained unclear.
We addressed these fundamental questions by making extensive use of newly developed experimental systems, which are based on recombinant strains of the chronic LCMV clone-13 virus (Flatz et al., 2006). These allowed us to vary either the strength of stimulating the TCR or the amount of antigen to which T cells become exposed in chronic infections. Using these systems, we uncovered that antigen amount rather than antigen strength is a key determinant of generating T cells with a chronic phenotype, and we noted that key steps toward acquiring this phenotype occur early in persisting infections. We also provide clear evidence that T cell accumulation is uncoupled from the phenotype that T cells acquire in chronic infections, a notion that strongly supports the concept that T cells undergo phenotypic specialization when acquiring a chronic phenotype. This differentiation concept is further reinforced by showing that T cells can coexpress markers associated with dysfunction of T cells (PD-1) and of memory T cells (CD127). In conclusion, we provide critical new insights into CD8 T cell biology in chronic infection and provide highly relevant technological resources and approaches for studying T cell responses in chronic infection.
RESULTS
Low antigen quantity induces an acute T cell phenotype in chronic infections
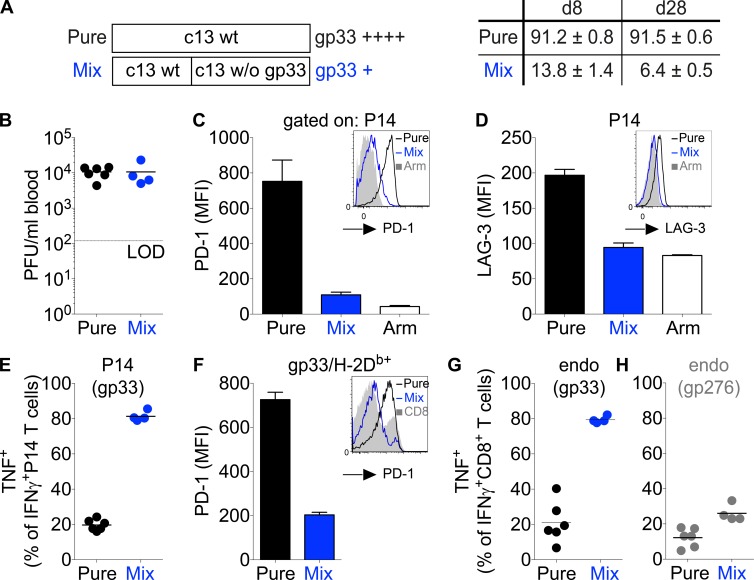
To investigate how antigen quantity impacts T cell responses in chronic infections, we devised an experimental setup in which P14 TCR-transgenic CD8 T cells (Pircher et al., 1990) are exposed to different amounts of the LCMV-derived gp33/Db epitope. Our aim was to create a minimally manipulated system in which we only alter the amount of presented gp33 peptide, whereas all other parameters, such as the magnitude of other LCMV-derived peptides, total virus load, and level of inflammation, remain constant. We achieved this by generating an LCMV clone-13 virus variant that expresses a known valine to alanine amino acid substitution, which results in a non–H-2Db–binding peptide KAAYNFATC (Puglielli et al., 2001). By mixing this gp33-deprived virus variant (c13 w/o gp33) with wild-type clone-13 virus, we could alter the amount of gp33 without impacting the infectious burden or presentation of any other epitopes (Fig. 1 A, left). Importantly, pure wild-type and mixed clone-13 infections contained similar total virus load at days 7 and 28 postinfection (p.i.; Fig. 1 B and not depicted). Using next generation sequencing techniques, we determined that our mixed infection experiments contained ∼14% wild-type clone-13 on day 8 and ∼6.5% on day 28, whereas in pure wild-type infections, the proportion of nonmutated clone-13 remained ∼91% (Fig. 1 A, right). These analyses were done with at least 15 mice per time point and condition. When CFSE-labeled naive P14 T cells were transferred into day 28 mixed infections, a fraction of the transferred P14 T cells began to proliferate, indicating that the gp33 peptide was still presented (not depicted). To exclude that this proliferation results only from inflammation-driven bystander activation (Chu et al., 2013), we transferred P14 T cells into mice 28 d p.i. with only the gp33-deficient clone-13 strain. In these hosts, we failed to detect the level of P14 proliferation seen in the mixed clone-13 infection (not depicted). Together, this demonstrates that some gp33-containing virus persists in the mixed group at 28 d p.i.
Figure 1.
Selective generation of effector T cells with an acute or chronic phenotype in chronic LCMV infections. (A, left) Schematic description of the infections applied to mice. C57BL/6 mice were infected either with 2 × 106 PFU wild-type (wt) LCMV clone-13 (pure group) or with a combined dose of 2 × 106 PFU consisting of 0.66 × 106 PFU wild-type LCMV clone-13 and 1.33 × 106 PFU LCMV clone-13 strain A3, which lacks (w/o) the gp33 epitope (mixed group). (Right) C57BL/6 mice grafted with CD45.1+ congenic P14 transgenic CD8+ T cells received the described pure or mixed LCMV infection. 8 or 28 d p.i., LCMV virus was received from blood samples and analyzed through deep sequencing. Shown are the mean percentages of wild-type gp33 (KAVYNFATC) obtained for all mice (n = 15 per time point and condition). (B–D and F) 4 wk p.i., virus titers in the blood (B) and PD-1 (C) and LAG-3 (D) expression among P14 T cells or in a series of experiments without P14 T cells among gp33-tetramer+ CD8+ T cells (F) were determined. Shown are representative histograms and mean fluorescence intensity (MFI) of P14 T cells compared with time-matched LCMV Armstrong–infected mice (white; C and D) or gp33-tetramer+ CD8+ T cells (F). Arm, Armstrong; LOD, limit of detection. (E, G, and H) At week 4 p.i., splenocytes were in vitro restimulated with gp33 or gp276 and analyzed for the intracellular expression of IFN-γ and TNF. Shown is the fraction of IFN-γ+ cells that coproduces TNF for P14 T cells (E) and endogenous (endo) T cells responding to gp33 (G) or gp276 (H). Each symbol in B, E, G, and H represents an individual mouse; small horizontal lines indicate the mean. Data are representative of at least three experiments with at least four mice per group. Error bars represent SEM.
As a next step, we analyzed the phenotype of T cells in the different infections. As expected, P14 T cells primed and expanded by high gp33 levels (pure clone-13 group) showed the expected exhausted or chronic phenotype; they expressed high levels of PD-1 and LAG-3, and only a small fraction of cells that produced IFN-γ in response to brief ex vivo gp33 restimulation cosecreted TNF (Fig. 1, C–E). In contrast, P14 T cells stimulated by low gp33 levels in mixed infection lacked expression of inhibitory receptors (PD-1 and LAG-3) and displayed a cytokine secretion profile (i.e., high ability to cosecrete IFN-γ and TNF) typically found among T cells in acute infections such as with LCMV Armstrong (Fig. 1, C–E). The same outcome was observed when C57BL/6 mice without a P14 transfer received a mixed infection. In this case, endogenous polyclonal T cells showed an acute phenotype and resembled the response of P14 cells (Fig. 1, F and G). In contrast, T cells specific for another LCMV glycoprotein-derived epitope (gp276–284) remained unaltered and showed a chronic phenotype in either mixed or pure infections (Fig. 1 H and not depicted). All these observations highlight that in the chosen experimental strategy, we indeed only influenced the response of gp33-specific T cells without altering the quality or quantity of the T cell response to other epitopes. Of note, the strict segregation into an acute or chronic phenotype critically depends on the ratio of c13 w/o gp33 and wild-type LCMV clone-13 virus. Increasing the proportion of wild-type virus augmented PD-1 expression by P14 T cells, whereas the fraction of IFN-γ– and TNF–coproducing cells declined (not depicted).
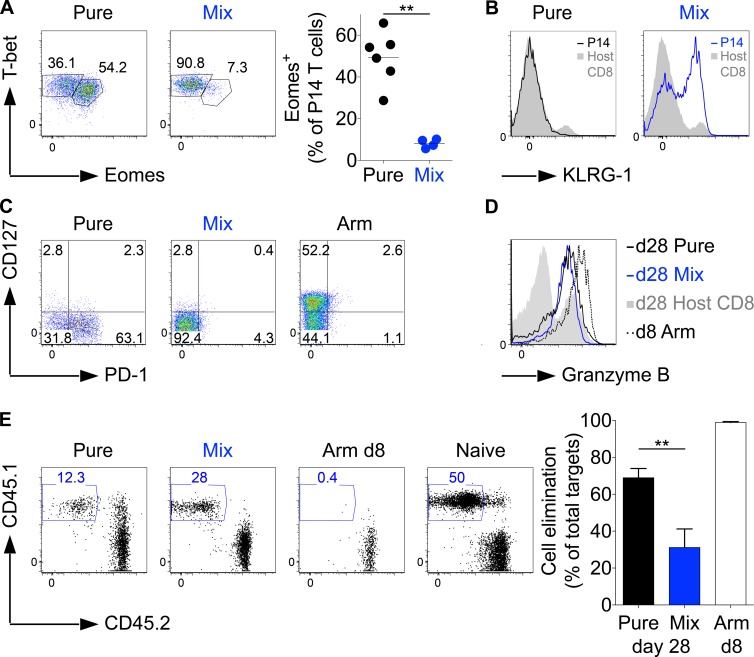
In line with the cytokine and PD-1 expression profiles, the majority of pure LCMV clone-13–primed P14 T cells were Eomes+ and T-betlow, whereas T cells primed by mixed infection were almost exclusively T-bethigh and Eomes− (Fig. 2 A). This resembles the T-box transcription factor profile that has previously been established for T cells in chronic or acute LCMV infections (Paley et al., 2012), except that we generated cells with an acute phenotype in a chronic infection. Interestingly, P14 T cells exposed to low gp33 levels in mixed infections expressed KLRG-1, whereas P14 T cells expanded in pure clone-13 wild-type infections behaved as expected from prior studies (Wherry, 2011; Speiser et al., 2014) and were KLRG-1 negative (Fig. 2 B; Wherry et al., 2007). Of additional interest is the observation that the vast majority of P14 T cells from both types of infection did not up-regulate expression of CD127 as in an LCMV Armstrong infection (Fig. 2 C), indicating that T cell populations with an effector phenotype can be maintained over periods much longer than the time span typically seen in acute infections. Most importantly, these long-term effector cells still expressed granzyme B, although to a lower extent than effector cells obtained from a day 8 Armstrong infection (Fig. 2 D). To test their effector capacity, we injected peptide-loaded target cells into pure and mixed week 4 clone-13–infected mice and, as a reference, into day 8 Armstrong-infected mice. Unexpectedly, after a 4–5-h observation period, we completely failed to detect residual target cells in any of the groups (not depicted). Even after 2 h, all cells were eliminated in the Armstrong group, whereas some target cells remained in both chronic groups, although more were eliminated from the pure infection group (Fig. 2 E). Given the extremely short (2 h) observation period, it remains unclear how relevant the measured difference is, but it is clear that T cells of either phenotype effectively exert effector function, and cells with the chronic phenotype might do so slightly better. We predominantly attribute the more effective elimination by the day 8 cells in Armstrong infection to the higher abundance of P14 T cells compared with the number of cells in the chronic infections and also to a higher activity level of cells at the acute compared with the chronic phase of an infection (see Fig. 5). This higher activity in day 8 Armstrong mice does not contradict our key finding that PD-1 expression levels and the cytokine profile do not reflect the cell-intrinsic cytolytic potential of T cells in chronic infections when acting against PD-L1–negative target cells in persisting infections.
Figure 2.
Properties of T cells with an acute or chronic phenotype in chronic LCMV infections. (A–D) C57BL/6 mice grafted with CD45.1+ congenic P14 T cells received a pure or mixed LCMV clone-13 infection (see Fig. 1 A for details). In some cases, an LCMV Armstrong (Arm) reference group was included. 4 wk p.i., P14 T cells were analyzed for T-bet and Eomes expression levels, whereby the plots show the percentage of Eomes-expressing cells (A); KLRG-1 expression (black line [left], pure P14; blue line [right], mixed P14; gray shadow, host CD8+ T cells; B); CD127 and PD-1 expression (C); and granzyme B expression (D; colors as in B plus P14 T cells from a day 8 Armstrong infection [dotted line]). (E) 4–6 wk p.i., C57BL/6 mice received gp33 peptide–pulsed CFSE+CD45.1+ and control CFSE+CD45.2+ splenocytes to test the cytolytic activity of P14 T cells in mixed and pure infection. Day 8 LCMV Armstrong–infected and naive mice served as positive and negative controls. The fraction of eliminated CFSE+CD45.1+ cells was enumerated 2 h after transferring the target cells. Data are representative of two (A, B, D, and E) or four (C) experiments with at least four mice per group and with similar results. Statistical analysis was by unpaired Student’s t test. **, P < 0.01. Error bars represent SEM.
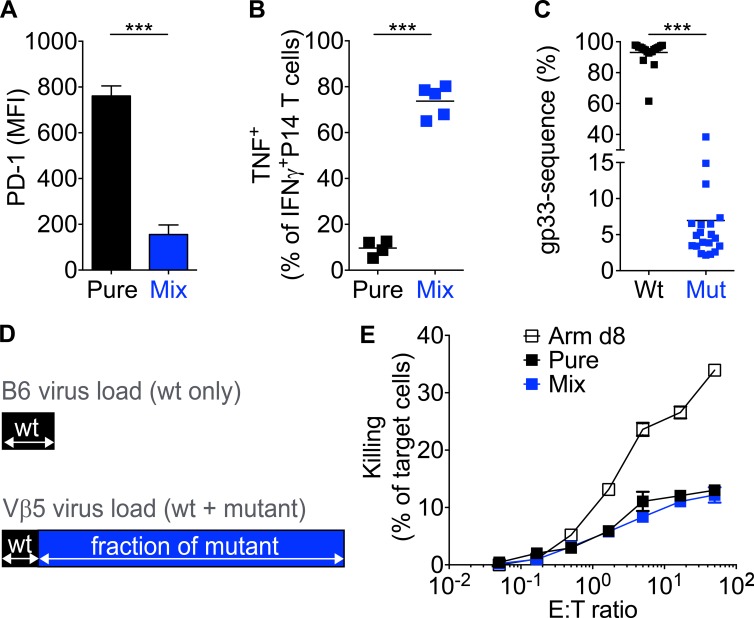
Figure 5.
Antigen dose during the early infection phase determines the T cell phenotype. (A and B) Vβ5 mice grafted with CD45.1+ congenic P14 T cells were infected with pure or mixed LCMV clone-13 infections (as described in Fig. 1 A). At week 4 p.i., the expression of PD-1 (A) and IFN-γ and TNF (B) were analyzed by FACS. MFI, mean fluorescence intensity. (C) Similar to that described in Fig. 1 A, 28 d after mixed infection, blood samples from Vβ5 mice were obtained and analyzed. Shown are the percentages of wild-type (Wt) gp33 (KAVYNFATC) or A3 mutant (Mut) gp33 (KAAYNFATC; n = 20 each). (D) Schematic illustration showing that wild-type (wt) gp33 (KAVYNFATC) viral load in day 28 pure-infected C57BL/6 mice (top, black bar) is similar to the wild-type gp33 portion of viral load in day 28 mixed-infected Vβ5 mice (bottom, black bar). (E) P14 T cells from week 4 infected Vβ5 mice were quantified and incubated with gp33-pulsed, 51Cr-labeled EL4 target cells for 5 h. P14 T cells obtained from day 8 LCMV Armstrong (Arm d8)–infected Vβ5 mice served as a positive control. Unpulsed cells served as a negative control (not depicted). The starting effector to target (E:T) ratio was adjusted for all infections to similar P14 numbers. 51Cr release was analyzed in triplicates and for at least four mice. Each symbol in B and C represents an individual mouse or the mean of all analyzed mice (E); horizontal lines indicate the mean. Data are representative of at least two experiments with at least three mice per group or a combination of four experiments (C). Statistical analysis was by unpaired Student’s t test. ***, P < 0.0001. Error bars represent SEM.
In summary, the data presented in this section demonstrate an experimental system that selectively generates in chronic LCMV clone-13 infections T cells with a phenotype similar to cells found in acute infections. Furthermore, the level of antigen exposure modulates whether T cells develop an acute or chronic phenotype within this system.
Proliferative capacity is uncoupled from the phenotype of T cells in chronic infections
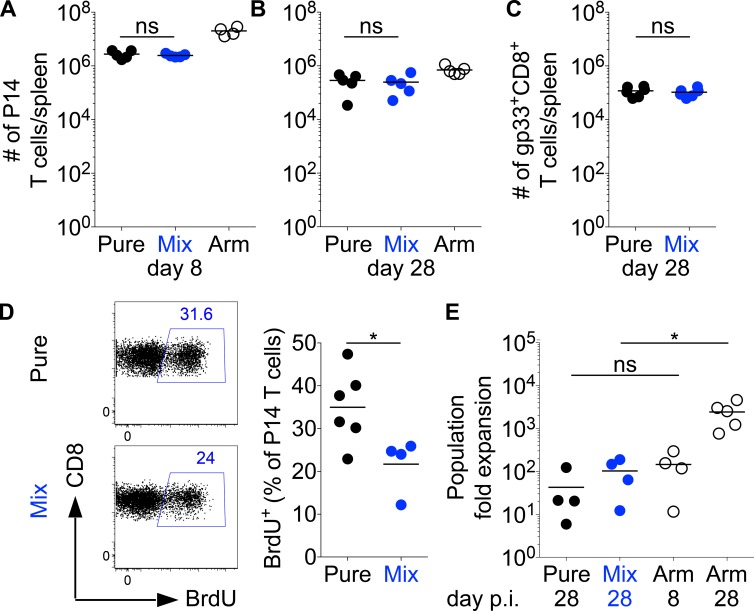
As a next step, we analyzed the proliferative capacity of T cells in pure and mixed LCMV infections. To our surprise, antigen amount impacted neither the number of P14 T cells at day 8 and 28 p.i. (Fig. 3, A and B) nor the number of endogenous gp33-specific T cells (Fig. 3 C). The day 28 outcome was very unexpected given that P14 T cells from pure and mixed infections strongly differed in PD-1 expression levels and because P14 T cells in the high antigen dose group showed a cytokine profile that is typically viewed to mark exhausted T cells (Fig. 1, C and E). By measuring BrdU incorporation, we observed that T cells continued to proliferate in both types of persisting infection, although at a slightly higher rate in pure infections (Fig. 3 D). Given the similar T cell numbers in both setups, we considered that this slightly higher proliferation rate is counterbalanced by a minor elevation in the cellular death rate. Overall, we therefore concluded that differences in the level of PD-1 expression had no major impact on the net balance between proliferation and T cell survival.
Figure 3.
Synchronous expansion kinetics of T cells with or without a chronic phenotype. (A–E) C57BL/6 mice were grafted with CD45.1+ congenic P14 T cells and infected with a pure or mixed LCMV clone-13 infection as indicated in Fig. 1 A. As a control, we also infected C57BL/6 mice grafted with CD45.1+ congenic P14 T cells with LCMV Armstrong (Arm). (A and B) Absolute numbers of splenic P14 T cells were measured on day 8 (A) or 28 (B) p.i. (C) Similarly, C57BL/6 mice (without P14) were infected with a pure and mixed LCMV clone-13 infection, and gp33-tetramer+ CD8+ T cell numbers were determined. (D) Representative flow cytometry (left) showing BrdU incorporation by P14 T cells between days 20 and 26 p.i. and percentage of BrdU+ among P14 T cells (right). (E) At week 4 p.i., P14 T cells were reisolated and transferred into naive secondary C57BL/6 hosts, which were then infected with LCMV Armstrong. P14 T cells harvested 8 or 28 d after an acute Armstrong infection were transferred as a reference. Shown is the calculated fold expansion of the P14 T cell population 8 d after the LCMV Armstrong challenge. The calculation estimates a 10% take of transferred P14 T cells. Symbols represent individual mice; horizontal lines show the mean. Data are representative of two (C) or at least four experiments with at least three mice per group. Statistical analysis was by unpaired Student’s t test. *, P < 0.05. ns, not significant.
When P14 T cell populations with an acute or chronic phenotype were isolated at day 28 p.i. from mixed or pure LCMV clone-13–infected mice and transferred into naive hosts, both populations showed in five replicate experiments a comparable reexpansion capacity 8 d after challenge that ranged from absolutely similar expansion to maximally fivefold higher expansion of T cells with an acute phenotype (Fig. 3 E). These minor differences might reflect a slightly lower reexpansion capacity of T cells with a chronic phenotype. Another nonmutually exclusive explanation is that T cell populations isolated from pure infections might contain a lower number of expandable precursor cells than those isolated from mixed infections. Compared with a similar-sized transfer of bona fide memory cells (isolated 28 d after an LCMV Armstrong infection), the reexpansion magnitude of T cells from both types of chronic infections appeared small. Nonetheless, their expansion capacity was comparable with that of a P14 T cell graft obtained from day 8 Armstrong-infected mice (Fig. 3 E).
Altogether, the observed response kinetics clearly indicate that the accumulation of T cells, either after primary or secondary challenge, is uncoupled from the phenotype T cells acquire in a chronic infection. Moreover, our data reveal that T cells with different phenotypes do not differ in their expansion capacity, which strongly suggests that they do not differ in their net proliferation or survival kinetics.
Early TCR stimulation critically impacts T cell differentiation in chronic infections
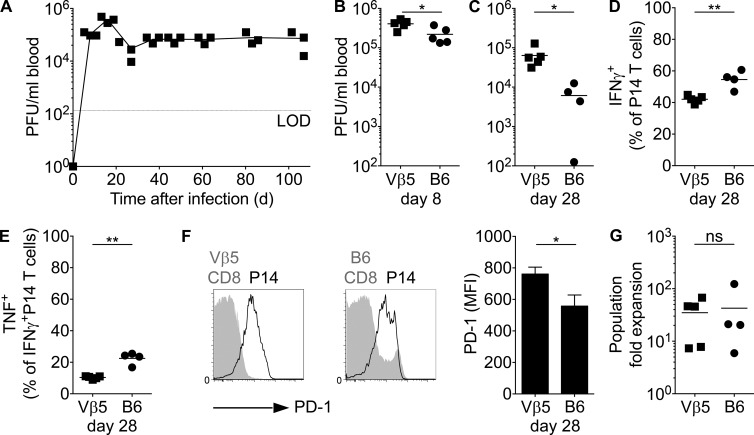
Virus load in LCMV clone-13–infected C57BL/6 mice declines over time and becomes typically undetectable 4–12 wk p.i. in the blood and slightly later in secondary lymphoid organs, but it persists long term in the kidneys (Matloubian et al., 1994). Consequently, T cells are normally exposed to low antigen levels in the late phase of a clone-13 infection. Having observed that antigen levels are critical in determining the acquisition of a chronic phenotype (Figs. 1, 2, and 3), we became interested in exploring what would happen if we sustained a high level of antigen exposure in the late stage of a clone-13 infection. To address this, we took advantage of Vβ5 TCR β chain–only transgenic mice. These mice express the same TCR β chain as H-2Kb/ovalbumin-specific OT-1 TCR-transgenic mice but endogenously rearranged TCR α chains (Zehn et al., 2007). This results in a polyclonal TCR repertoire that is biased toward recognizing ovalbumin and is severely impaired in responding to LCMV-derived antigens (not depicted; Utzschneider et al., 2013). When infected with LCMV clone-13, these mice show stable high virus titers (Fig. 4 A). Of particular importance is the fact that on day 8 p.i., they show a less than twofold difference in viral titers compared with C57BL/6 mice (Fig. 4 B). This difference becomes more prominent (10-fold) on day 28 p.i. (Fig. 4 C), as viremia persists in Vβ5 mice (Fig. 4 A). Of note, the presence of P14 T cells did not alter total virus load in Vβ5 mice (not depicted), and CFSE-labeled P14 T cells proliferated massively when transferred into Vβ5 mice at 28 d p.i. Moreover, when we recovered virus from chronically infected Vβ5 mice at day 28 and used it to infect naive P14-containing C57BL/6 mice, the P14 T cells expanded, indicating that they continued to present the gp33 peptide (not depicted). Comparing the phenotype of P14 T cells in low or high LCMV clone-13 titer C57BL/6 or Vβ5 hosts, we noted statistically significant differences in the level of IFN-γ secretion, the ability to coproduce TNF and IFN-γ, and the expression levels of PD-1 (Fig. 4, D–F). However, we argue that these are marginal differences compared with the profiles seen among cells with an acute phenotype in mixed LCMV infections. Thus, we concluded that despite the elevated antigen and virus exposure levels in chronically infected Vβ5 mice, we did not observe major phenotypic differences or signs of exhaustion in P14 T cells obtained from Vβ5 compared with P14 T cells in C57BL/6. Remarkably, even P14 T cell populations harvested 4 or 20 wk after infecting Vβ5 mice with LCMV clone-13 underwent robust secondary expansion after transfer into acutely infected C57BL/6 mice (Fig. 4 G and not depicted).
Figure 4.
High virus titer in the chronic phase of infection causes only minor changes in the T cell phenotype. (A) Vβ5 mice were infected with 2 × 106 PFU LCMV clone-13 infection. Virus titers in the blood were determined at various time points p.i. LOD, limit of detection. (B–G) Vβ5 or C57BL/6 mice grafted with CD45.1+ congenic P14 T cells were infected with LCMV clone-13. (B and C) Virus titers in the blood on day 8 (B) or 28 (C) p.i. (D and E) Week 4 splenocytes were in vitro restimulated with gp33 and analyzed for intracellular expression of IFN-γ and TNF. Shown are the frequencies of IFN-γ+ P14 T cells (D) and the fraction of IFN-γ+ cells that coproduce TNF (E). (F) PD-1 expression and mean fluorescence intensity (MFI) on P14 T cells (black) compared with the endogenous CD8+ T cell population in both hosts (gray shadows). Error bars represent SEM. (G) At 4 wk, P14 T cells were reisolated and transferred into naive secondary Vβ5 or C57BL/6 hosts, which were then infected with LCMV Armstrong. Shown is the fold expansion of the transferred P14 population assuming a 10% take of transferred cells. ns, not significant. Each symbol in A–E and G represents an individual mouse; small horizontal lines indicate the mean. Data are representative of at least two experiments with at least three mice per group. Statistical analysis was by unpaired Student’s t test. *, P < 0.05; **, P < 0.01.
We also applied the mixed infection strategy (Fig. 1 A) to Vβ5 mice. Similar to what was observed in C57BL/6 hosts, T cells retained an acute phenotype in mixed infections and a chronic one in pure infections (Fig. 5, A and B). The phenotypic outcome in the mixed infected mice surprised us because we calculated that the amount of wild-type virus that is present in day 28 mixed infected Vβ5 hosts is very similar to the amount of total LCMV virus in day 28 pure wild-type infected C57BL/6 hosts. This assessment takes into account that although wild-type virus is only 5–8% of total virus in mixed infected Vβ5 mice at day 28 (Fig. 5 C), they contain at this time point 10-fold more total virus than C57BL/6 mice (Fig. 4 C). Therefore, both infections contain in absolute terms a similar amount of wild-type clone-13 virus (Fig. 5 D).
Despite the similar level of virus and antigen exposure, P14 T cells in mixed infected Vβ5 mice maintain an acute phenotype, whereas P14 T cells in pure clone-13–infected C57BL/6 mice show a chronic phenotype. In contrast, P14 T cells in mixed infected Vβ5 or C57BL/6 mice are exposed to similar antigen and virus levels during the acute phase of the response (Fig. 4 B). We therefore conclude that the phenotype correlates with the levels of antigen exposure in the early but not in the late phase of chronic infection. Thus, the data shown in Figs. 4 and 5 provide strong evidence that the decision to acquire an acute or chronic phenotype is made at early time points in an infection.
Of note, we also assessed the effector capacity of P14 T cells obtained from 4–5-wk mixed and pure infected Vβ5 mice, but all target cells were readily eliminated (not depicted). Additionally, we assessed effector capacity using in vitro cytotoxicity assays, allowing us to precisely control the effector to target ratio. Interestingly, we found that day 28 T cells with an acute or chronic phenotype were equally effective but weaker than day 8 effector cells (Fig. 5 E). Although the reason for the lower effector capacity of the day 28 T cells remains unclear, the data further support our conclusion of a lack of correlation between the phenotype and intrinsic effector capacity of T cells in chronic infection.
Even persisting low-affinity TCR stimulation induces a chronic phenotype
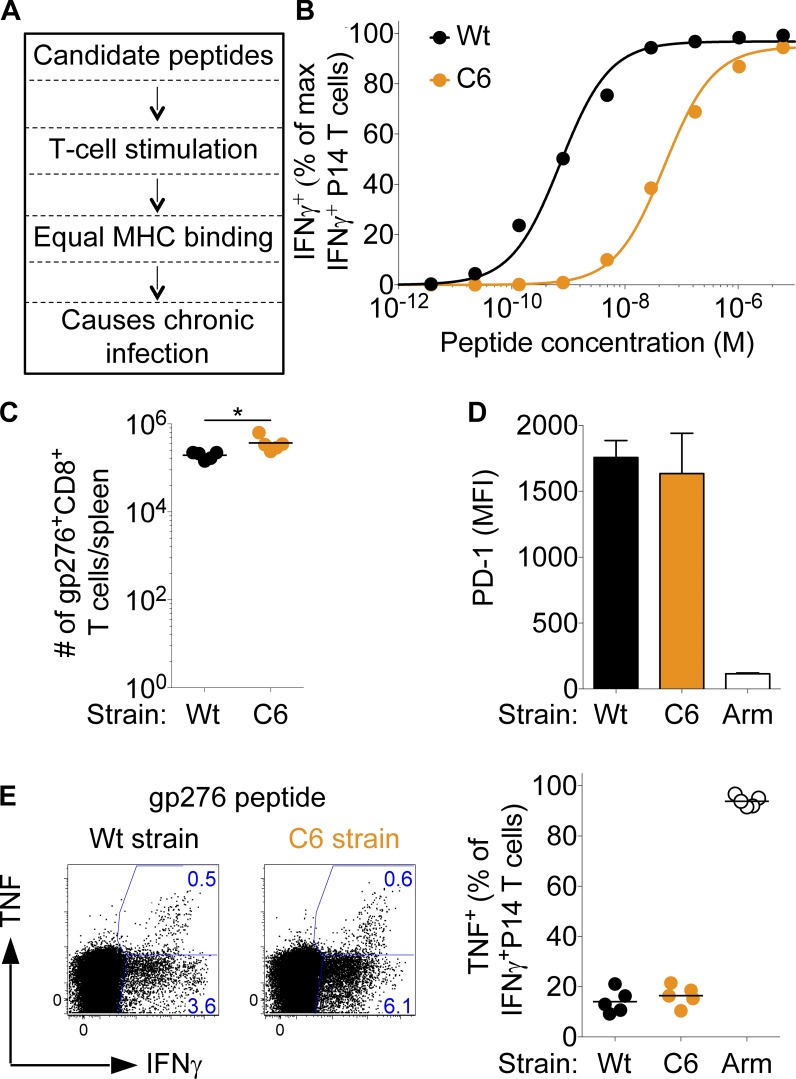
The population of T cells that responds to any foreign antigen is highly diverse and comprises a TCR repertoire in which individual receptors differ in their binding affinity to the stimulating peptide-MHC (pMHC). Such differences are known to critically impact T cell responses in acute infections (Zehn et al., 2009), but how pMHC recognition influences T cell differentiation and function in chronic infections has been a long-standing question. We followed the strategy illustrated in Fig. 6 A and developed a well-controlled experimental system, which allowed us to address this issue. We screened a pool of 95 single amino acid–substituted altered peptide ligands (APLs) of the LCMV-derived gp33 peptide. We selected 25 APLs binding similarly well to H-2Db but which differ in their potency of stimulating P14 T cells. The latter was determined by exposing P14 T cell lines to titrated peptide doses and measuring the concentration needed to induce half-maximum IFN-γ secretion (not depicted). Taking advantage of a previously described reverse genetics approach (Flatz et al., 2006, 2010), we developed 25 LCMV clone-13 strains encoding the selected APL in place of the wild-type sequence. Unsurprisingly, most mutant viruses failed to cause chronic infection in C57BL/6 mice because the gp33 peptide is derived from the glycoprotein that mediates LCMV uptake (Kunz et al., 2001). Nonetheless, we identified a variant virus, C6 (KAVYNFCTC), that carries a weak P14 T cell agonist (Fig. 6 B) and that shows a virus persistence pattern in the blood and kidneys that resembles the kinetics of wild-type LCMV clone-13 (not depicted). Of note, the C6 variant was the weakest ligand we identified that still induces P14 activation in vivo. An F4 variant was slightly weaker in stimulating P14 T cells in vitro but gave inconsistent results and in most cases failed to activate P14 T cells (not depicted). We therefore concluded that C6 is at the lowest affinity that still activates P14 T cells. Importantly, wild-type and C6 clone-13 induce similar numbers of gp276-specific T cells with identical PD-1 expression and cytokine secretion profiles (Fig. 6, C–E). This indicates that both strains cause a chronic infection of comparable severity.
Figure 6.
Generation of LCMV clone-13 strains expressing low-affinity APLs. (A) Schematic illustration of selecting LCMV clone-13–compatible, gp33-derived APLs for P14 TCR-transgenic T cells. 95 APLs were in vitro and in vivo screened for their potency to stimulate P14 T cell lines, their ability to bind to H-2Db (determined by MHC surface stabilization on TAP [transporter associated with antigen processing]-deficient RMA-S cells), and absence of interference with LCMV clone-13 infection kinetics. APLs showing a functional avidity distinct from the normal gp33 peptide but similar MHC binding were selected to generate recombinant LCMV clone-13 strains. With the exception of KAVYNCATC (C6), all other APLs were excluded because they did not meet at least one selection criteria. (B) IFN-γ dose–response curves of transgenic P14 T cells responding to normal gp33 or C6 peptide. (C–E) C57BL/6 mice were infected with 2–4 × 106 PFU LCMV clone-13 wild type (Wt) or C6. (C) 4 wk p.i., splenocytes were analyzed for absolute numbers of gp276 tetramer+ CD8+ T cells. (D) Mean fluorescence intensity (MFI) of PD-1 expression on gp276+ T cells 4 wk p.i. with wild-type and C6 clone-13 or LCMV Armstrong (Arm). Error bars represent SEM. (E) Splenocytes from the same experiment were in vitro restimulated with gp276 and analyzed for intracellular expression of IFN-γ and TNF. Shown are the frequencies of TNF+ cells among total endogenous IFN-γ+ T cells. Each symbol in B represents the mean of three individual titrations and in C and E, an individual mouse; horizontal lines indicate the mean. Data are representative of at least three independent experiments, each with at least four mice per group. Statistical analysis was by unpaired Student’s t test. *, P < 0.05.
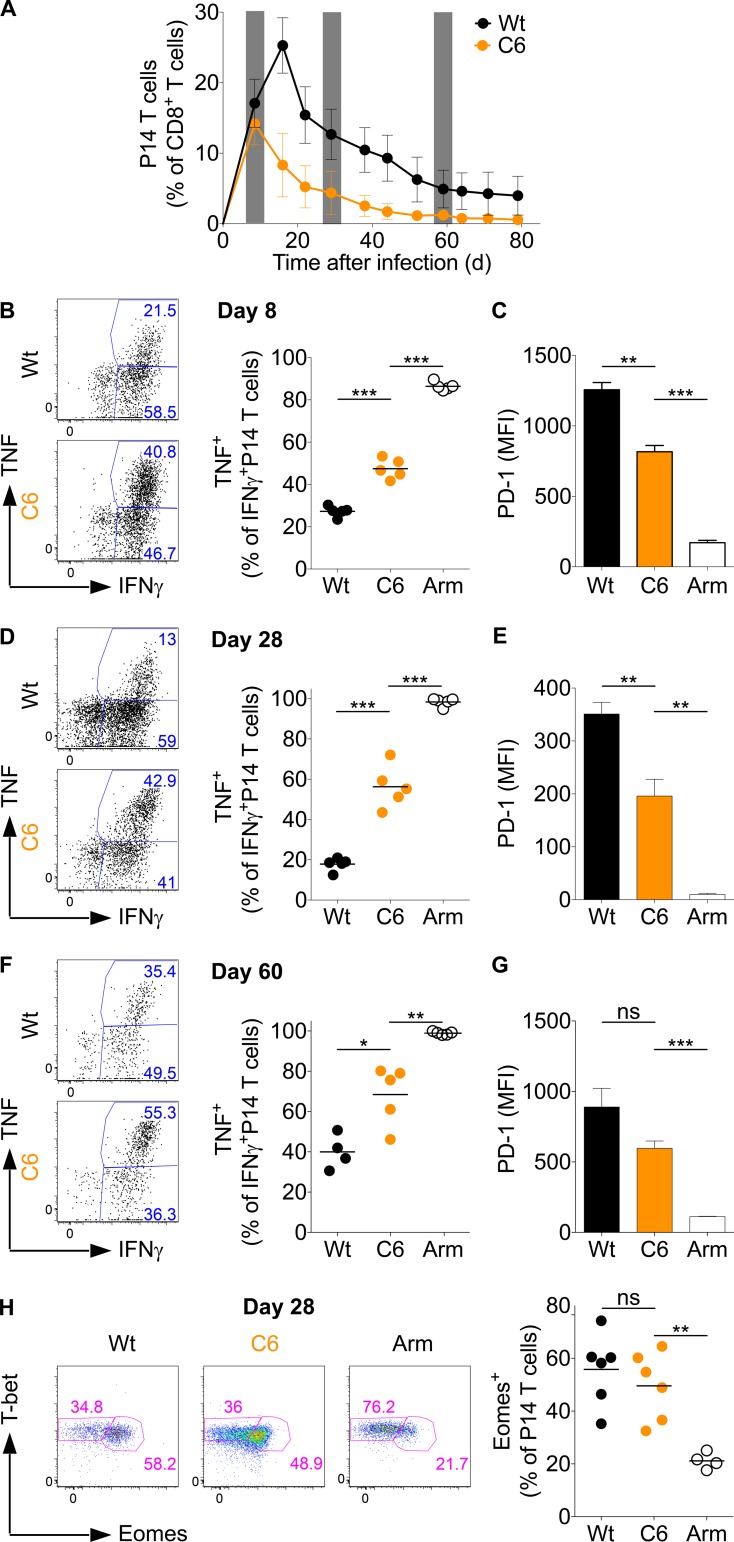
To study the T cell response to low-affinity epitopes in chronic infection, we transferred P14 T cells into C57BL/6 host mice and infected them with wild-type or C6 LCMV clone-13. High- and low-affinity T cells underwent similar expansion until day 8 p.i. Subsequently, the high-affinity cells expanded further, and expansion usually peaked between days 10 and 15 p.i., whereas low-affinity cells started to decline in numbers after day 8 p.i. (Fig. 7 A). This revealed that high- and low-affinity stimulated P14 T cells in chronic infections follow essentially the same kinetic pattern as high- and low-affinity T cells in acute infections, where it is known that initial T cell expansion is independent of the strength of TCR stimulation but that strength determines the time point when T cell numbers decline (Zehn et al., 2009, 2010). Phenotypic analysis performed at days 8, 28, and 60 p.i. revealed a higher degree of PD-1 expression and lower capacity to cosecrete TNF and IFN-γ at all time points among high- compared with low-affinity stimulated P14 T cells (Fig. 7, B–G). Together, the data indicate that strongly stimulated T cells with a chronic or exhausted phenotype expand more vigorously in a persisting LCMV infection than phenotypically less exhausted low-affinity stimulated T cells (Fig. 7 A). Furthermore, we noted that the relative difference in PD-1 expression and cytokine secretion capacity observed between high- and low-affinity stimulated P14 T cells did not change between days 8 and 60. We interpret this finding as additional strong evidence that critical steps toward acquiring a chronic phenotype are initiated in the early phase of a chronic infection. This notion of an early occurring differentiation is also in line with the data shown in Figs. 4 and 5, in which persisting high virus titers in the later phase of an LCMV clone-13 infection did not alter the phenotype or functional profile of T cells.
Figure 7.
Chronic low-affinity stimulation leads to an intermediate chronic phenotype. (A–H) C57BL/6 mice grafted with CD45.1+ congenic P14-transgenic CD8+ T cells were infected with wild-type (Wt) LCMV clone-13, the clone-13 strain containing the low-affinity gp33-C6 epitope (C6; described in Fig. 6), or LCMV Armstrong (Arm). (A) Frequency of P14 T cells among total CD8+ T cells in the blood. The gray columns indicate days 8, 28, and 60, which correspond to the analyses presented in B–G. (B, D, and F) Splenocytes were in vitro restimulated with gp33 and analyzed for intracellular expression of IFN-γ and TNF. (C, E, and G) PD-1 mean fluorescence intensity (MFI) on P14 T cells. (H) Eomes and T-bet expression in P14 T cells and frequency of Eomes+ P14 T cells 28 d p.i. Each symbol in A represents the mean of five mice and in B, D, F, and H, an individual mouse; small horizontal lines indicate the mean. Data are representative of two (A–C and F–H) or three (D and E) independent experiments with at least four mice per group. Statistical analysis was by unpaired Student’s t test. *, P < 0.05; **, P < 0.01; ***, P < 0.0001. Error bars represent SEM. ns, not significant.
Next, we investigated the levels of Eomes and T-bet expression by high- and low-affinity stimulated P14 T cells. Irrespective of the aforementioned variations in PD-1 expression and cytokine secretion, we saw no major differences, and about half of the P14 T cells in both conditions were Eomes+ T-betlow (Fig. 7 H). Taking into account that low-affinity T cells express high Eomes levels, express at least a low level of PD-1, and show an impaired cytokine secretion profile compared with LCMV Armstrong–stimulated cells, we conclude that even low-affinity stimulated T cells acquire cardinal features of the chronic infection phenotype. Moreover, it appears that the degree of showing the chronic phenotype is uncoupled from the Eomes expression level.
Collectively, our data show that low antigen amounts result in an acute T cell phenotype in chronic infections, whereas lowering the TCR stimulation strength still triggers the formation of T cells with a chronic phenotype. Thus, we conclude that epitope/antigen abundance is more critical for the induction of a chronic phenotype than the affinity by which the TCR becomes activated.
High- and low-affinity stimulation induce CD127+- and PD-1+–coexpressing T cells
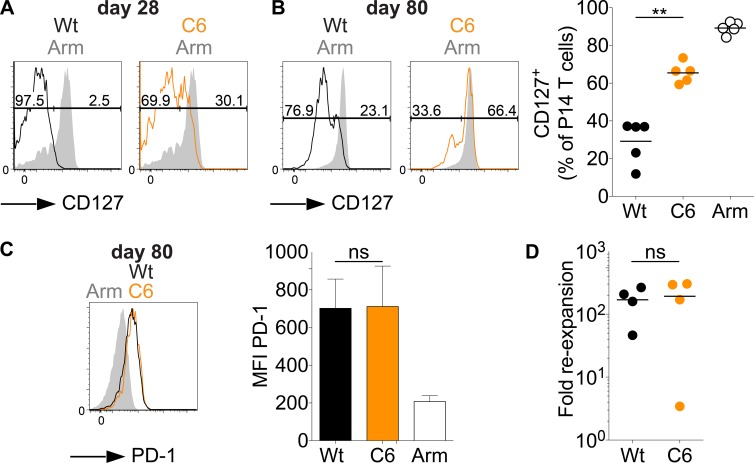
We noted that a significant fraction of day 28 low-affinity stimulated P14 T cells express CD127, a molecule that is typically expressed by naive or memory T cells in resolved infections (Fig. 8 A). CD127+ P14 T cells can also be detected in wild-type clone-13 infections, but high expression levels and a clearly separated population form at much later time points than after low-affinity stimulation (Fig. 8 B). Nonetheless, both observations show that viral persistence does not preclude that some cells reexpress the memory T cell marker CD127. Interestingly, even on day 80, the fraction of CD127+ cells was twice as large in low- compared with high-affinity stimulated T cells, indicating that low-affinity stimulation enhances the formation of T cells with a memory phenotype in chronic infections.
Figure 8.
CD127-expressing T cells in chronic infections coexpress PD-1. (A–D) C57BL/6 mice grafted with CD45.1+ congenic P14-transgenic CD8+ T cells were infected with LCMV Armstrong (Arm), LCMV clone-13 (wild type [Wt]), or LCMV clone-13 C6 (C6). (A and B) CD127 expression on splenic P14 T cells (black, wild type; orange, C6; gray, Armstrong) on day 28 (A) or day 80 (B). Shown are representative flow plots at days 28 and 80 and data for all mice in one experiment at day 80. (C) Representative flow cytometry histogram of PD-1 expression on the CD127+ P14 T cells shown in B and mean fluorescence intensity (MFI) of all mice. Error bars represent SEM. (D) 70–80 d p.i., CD127+ P14 T cells were sorted from the primary host and transferred into naive secondary C57BL/6 recipients, which were then infected with LCMV Armstrong. Depicted is the fold expansion of the CD127+ P14 population derived from wild-type or C6 LCMV clone-13–infected mice. Reexpansion was calculated estimating a 10% take of transferred P14 T cells. Symbols in B and D represent individual mice; horizontal lines indicate the mean. Data are representative of four (day 28) or two (day 70–80) independent experiments, with at least four mice per group. Statistical analysis was by unpaired Student’s t test. **, P < 0.01. ns, not significant.
To our surprise, we noted on day 80 p.i. that the CD127+ high- or low-affinity stimulated P14 T cells still expressed significant levels of PD-1 compared with LCMV Armstrong–derived bona fide memory T cells on day 80 p.i. (Fig. 8 C). This was also true for low-affinity stimulated CD127+ P14 T cells at day 28 (not depicted). Although this coexpression appears very unexpected at first glance, PD-1 expression by CD127+ T cells is consistent with the fact that when T cells from chronic infections are transferred into naive mice and reexpanded in an acute infection, they maintain high expression of PD-1 (Utzschneider et al., 2013). Despite the difference in numbers of CD127+ P14 T cells after high- and low-affinity stimulation, their PD-1 expression levels were similar at day 80 (Fig. 8 C). We then tested the ability of the high- and low-affinity stimulated CD127+ T cell populations to undergo secondary expansion. For this, we sorted low- and high-affinity stimulated CD127+ P14 T cells 70 d p.i., transferred them into naive mice, and infected them with LCMV Armstrong. At day 8, we noted a similar reexpansion magnitude for both CD127+ P14 T cell populations (Fig. 8 D). Thus, on a per cell basis, low- and high-affinity primed CD127+ T cells have similar reexpansion capacity.
Together, our observations indicate that the strength of the stimulus T cells receive early in a chronic infection impacts expansion magnitude and phenotype. In contrast, the differences level out in the emerging memory compartment as virus titers decline over time. Thus, low- and high-affinity stimulation in chronic infection generate T cells with comparable memory qualities.
DISCUSSION
T cells derived from persisting infections perform in many functional tests less well than T cells obtained from resolved infections. This leads to the conclusion that they are severely functionally impaired. However, such comparisons have several limitations and confounding factors, including finding a suitable time point for side-by-side evaluation. For instance, when week 4 Armstrong T cells are compared with T cells from a week 4 clone-13 infection, one is essentially comparing resting T cells with activated T cells from a persisting infection. Moreover, acute and chronic LCMV strains differ in the magnitude of inflammation they cause, the level of tissue destruction, antigen presentation kinetics, and tissue tropism of the virus (Sevilla et al., 2000; Smelt et al., 2001; Beura et al., 2015). All of these aspects make the comparison rather difficult to interpret. Thus, it has remained unclear to what degree functional differences between T cells from resolved or chronic infections relate to the particular effector phenotype they display (exhausted vs. acute phenotype) and how much of the response pattern can be attributed to the fact that the comparisons use cells at different stages of activation and from different environments.
While studying how differences in the magnitude of antigen exposure impact T cell differentiation in chronic infections, we noted that T cells can still acquire an acute effector phenotype if exposed to low quantities of antigen. Interestingly, these acute T cells persist without showing signs of transitioning into memory T cells and remain CD127− and KLRG-1+. This created a unique experimental situation and allowed us to compare acute and chronic phenotype T cells in exactly the same environment. Given that total virus titers, severity of infection, overall T cell response, and presentation of all LCMV epitopes except gp33 are identical in our setup, we argue that we are using a minimally manipulated experimental system. To our surprise, we uncovered that T cells with or without a chronic phenotype underwent similar primary expansion, were maintained in equal numbers, and even showed a similar secondary expansion potential. We therefore conclude that T cells in chronic infections undergo a form of differentiation that, at least initially, does not impact their net accumulation.
Our observations do not rule out that T cells with a chronic phenotype differ fundamentally from acute effector T cells when it comes to performing effector functions, for instance, against PD-L1 or other inhibitory ligands expressing target cells. In fact, we consider that the exhausted phenotype might have evolved because such T cells can be conditionally switched off by engaging the PD-1/PD-L1 axis or other inhibitory receptors. Moreover, the lower cytokine production capacity of cells with a chronic phenotype might render them less aggressive and less proinflammatory. Such effector T cells would be advantageous in preventing immunopathology because the strong and aggressive immune responses found in an acute infection could be detrimental in persistently infected hosts (Cornberg et al., 2013; Doedens et al., 2013). Thus, the exhausted phenotype might constitute an optimized effector phenotype that creates an equilibrium between virus control and tissue damage (Virgin et al., 2009) while having the option to block unwanted effector T cell function against vulnerable tissues or structures (Speiser et al., 2014). This principle has been well illustrated by studies showing that endothelial cell wall integrity during chronic infections critically depends on the presence of PD-L1+ endothelial cells (Frebel et al., 2012). Similarly, failure to acquire a chronic phenotype was shown to correlate with more severe pathology. For example, the transcription factor HIF1a is critical for generating T cells with an exhausted phenotype, and its absence correlates with strong immunopathology caused by retention of T cells with an acute phenotype (Doedens et al., 2013). This strongly supports the concept that attenuating T cell function through acquiring a chronic phenotype is essential for host integrity.
Of note, although day 28 cells of either phenotype showed similar effector capacity in in vitro tests, they were less effective than day 8 T cells obtained from LCMV Armstrong–infected mice. It is important to consider that day 8 and 28 T cells differ in their activation status. Moreover, they originate from host environments with distinct cytokine profiles, levels of inflammation, and metabolic profiles. Therefore, the implications of the day 8 versus day 28 comparisons remain unclear, and it needs to be determined whether the two day 28 populations are equally hypofunctional or equally functional against PD-L1–negative targets.
Another key observation of our study is the generation of CD127+-expressing pathogen-specific T cells in chronic LCMV infections. This observation, along with the finding that we can reexpand a fraction of T cells from chronic infection, indicates that long-term exposure to antigen and inflammation does not preclude T cells from segregating into antigen-dependent effector T cells and antigen-independent memory T cells. Nonetheless, there appears to be a strong link between total virus load and CD127 expression, as a discrete CD127+ population can only be seen in C57BL/6 mice at >4 wk of infection, when virus load has significantly dropped. In line with this, only very low levels of CD127+-expressing cells can be found in Vβ5 mice in which LCMV clone-13 persists at very high titers. Interestingly, the frequency of CD127+ cells correlates with the strength of TCR stimulation, as CD127 expression could be consistently detected at earlier time points and at higher frequencies among low-affinity stimulated T cells. In contrast, the amount of antigen (mixed vs. pure experiments) did not influence the kinetics of CD127 reexpression. Much to our surprise, we uncovered that CD127+ cells in chronic infections can coexpress PD-1, whereas memory T cells from acutely resolved infections remained PD-1−. As unexpected as this coexpression appears at first glance, it is well in line with our hypothesis that T cells undergo a stable form of differentiation and that the exhausted phenotype can be propagated through memory-equivalent T cells (Utzschneider et al., 2013; Speiser et al., 2014).
A long-standing question in the field has been what determines whether T cells acquire an exhausted phenotype. So far, high virus titers (Day et al., 2006), limited CD4 T cell help (Zajac et al., 1998; Blackburn et al., 2009), large numbers of antigen-positive target cells (Mueller and Ahmed, 2009), or the absence of IL-21 signaling (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009) has been shown to promote the generation of this phenotype. Following indications in the literature, we considered that the strength of pMHC–TCR interaction could be another key determining factor (Lichterfeld et al., 2007; Viganò et al., 2012). To address this in a well-defined experimental setup, we generated APL-expressing LCMV clone-13 strains. Indeed, we observed that low-affinity stimulation correlated with lower PD-1 expression and a better IFN-γ and TNF cosecretion profile, but we did not detect any differences in Eomes expression. This strongly contrasted with the phenotype seen after exposing T cells to low amounts of high-affinity antigen, which resulted in T cells that show an Eomes, cytokine, and PD-1 expression profile that resembles T cells found in acutely resolved infections. Lower antigen levels mean that a TCR engages less frequently with cognate pMHCs. We therefore conclude that frequency of TCR stimulation more critically impacts T cell differentiation in chronic infections than the actual strength of the TCR signal. How this exactly happens remains unclear, as lower antigen levels could mean reduced serial TCR triggering when a T cell interacts with one DC. Alternatively, the interval between engaging antigen-positive or -negative DCs or target cells could be reduced. In any case, linking T cell differentiation to antigen presentation levels means that T cells indirectly sense the pathogen load.
We also provide several lines of evidence that critical steps toward acquiring a chronic phenotype occur early and not selectively at late stages of chronic infections. Among them is the observation that the persisting high virus titers in Vβ5 host mice do not induce a more severe phenotype than in C57BL/6 mice. In this respect, it is important to consider that virus titers in C57BL/6 and Vβ5 mice are comparable during the first 7–10 d p.i., suggesting this interval is crucial for determining the phenotype T cells acquire. This model is also supported by the observation that low- and high-affinity stimulated T cells clearly already differ in PD-1 and cytokine expression levels on day 8 p.i., and the differences are subsequently stably maintained. Of note, P14 T cells responding to a low dose of antigen sustain over time their early acquired acute phenotype, precluding that chronic T cell stimulation induces an overall gradual loss of function. Our notion of an early differentiation contrasts at first glance with adoptive transfer experiments, which indicated that depriving T cells of antigen too early causes the population to revert back to an acute phenotype (Angelosanto et al., 2012). Nonetheless, those findings do not necessarily disagree with our conclusions. Rather, we propose that critical steps toward acquiring the chronic phenotype occur early and maybe between days 5 and 8 p.i. Nonetheless, there is a period of plasticity during which continuing antigen contacts are required to stably fix the phenotype and antigen deprivation might cause cells to revert back to an acute phenotype. Moreover, our notion that early antigen levels decide which phenotype T cells acquire has far-reaching consequences for vaccine design, as it suggests that too high antigen levels bear the risk of inducing the unwanted chronic phenotype in a vaccine setting.
Based on all these findings, we conclude that acquiring a chronic phenotype reflects an event of cellular differentiation and specialization and not primarily a transition into a terminal and nonfunctional stage. Thus, we establish that T cells can obtain either a polyfunctional PD-1− phenotype or a PD-1+, low cytokine–producing effector phenotype, and we demonstrate that the level of antigen exposure is the critical factor deciding which of the two phenotypes T cells obtain in chronic infections.
MATERIALS AND METHODS
Mice
C57BL/6 mice were obtained from Charles River. P14 TCR-α/β transgenic mice (Pircher et al., 1990) were provided by A. Oxenius (Eidgenössische Technische Hochschule, Zurich, Switzerland), and Vβ5 TCR-β–only transgenic mice (Zehn et al., 2007) were provided by P. Fink (University of Washington, Seattle, WA). Mice were bred and maintained in specific pathogen-free facilities and infected in conventional animal facilities of the University of Lausanne. Experiments were performed in at least 6-wk-old mice in compliance with the University of Lausanne institutional regulations and were approved by the veterinarian authorities of the Swiss Canton Vaud.
Infections and virus titer determination
Recombinant APL-expressing LCMV clone-13 strains were recovered from cDNA using a previously described reverse genetics approach (Flatz et al., 2006, 2010). Double nucleotide changes were introduced to mutate the H-2Db–restricted gp33 epitope KAVYNFATC (wild type) into KAVYNCATC (C6) or KAAYNFATC (A3), thus reducing the likelihood of viral mutational reversion in vivo. All strains were propagated in baby hamster kidney (BHK) cells and titrated on Vero cells following an established protocol (Battegay et al., 1991). Frozen stocks were diluted in PBS, and 2 × 105 PFU LCMV Armstrong was injected intraperitoneally, and 2 × 106 PFU wild-type LCMV clone-13 and 4 × 106 PFU LCMV clone-13-C6 were injected intravenously. Mixed LCMV infections were constituted of one third wild-type LCMV clone-13 (0.66 × 106 PFU) plus two thirds LCMV clone-13-A3 (1.33 × 106 PFU), which lacks a functional gp33 epitope (Puglielli et al., 2001). Blood samples from LCMV-infected mice were shock frozen. Diluted samples were used to infect Vero cells, and virus titers were determined using an LCMV focus–forming assay (Battegay et al., 1991).
Purification of mouse T cells and adoptive T cell transfer
Single-cell splenocyte suspensions were obtained by mashing total spleens through a 100-µm nylon cell strainer (BD), and red blood cells were lysed with a hypotonic ACK buffer. Transgenic CD8+ T cells were isolated using the mouse CD8 T cell enrichment kit (Miltenyi Biotech). 2–5 × 103 CD45.1+ naive congenic P14 T cells were transferred into naive CD45.2+ C57BL/6 mice. P14 T cells were reisolated from infected mice by staining total splenocytes with anti-CD8 (clone 53-6.7; eBioscience), CD45.1 (purified hybridoma supernatant; self-conjugated; clone A20), and CD127 (clone eBioSB/199; eBioscience). CD8+CD45.1+ or CD8+CD45.1+CD127+ cells were isolated by flow cytometry–assisted cell sorting. The purity of sorted cells was >99%. In all cases, we confirmed that T cells isolated from pure clone-13–infected mice exhibit the chronic phenotype.
Ex vivo T cell activation, expansion, and functional avidity tests
Similar to that previously described (Enouz et al., 2012), 106 P14 T cells were seeded into 24-well plates, stimulated with anti-CD3/CD28 Dynabeads (Invitrogen), and cultured in RPMI supplemented with 10% FCS, penicillin/streptomycin, 5 µM 2-ME, and 5 mM Hepes (all Invitrogen). Culture volumes were increased, and additional media were added as needed. 50 U/ml human IL-2 (Chiron) was added on day 0 and every other day. Cells were analyzed 6–7 d after the stimulation.
3 × 105 activated P14 T cells were seeded with 105 RMA cells (which functioned as APCs) into 96-well plates. The cells were stimulated with titrated doses of wild-type gp33 (KAVYNFATC) or gp33-C6 (KAVYNCATC) peptide (EMC Microcollections) in vitro for 30 min at 37°C before 7 µM brefeldin A (Sigma-Aldrich) was added for another 4.5-h incubation at 37°C. Afterward, cells were washed and surface and intracellularly stained.
Surface and intracellular antibody staining and flow cytometry cell sorting of mouse cells
Surface staining was performed for 30 min and with tetramer for 90 min at 4°C in PBS supplemented with 2% FCS and 0.01% azide (FACS buffer) using the following antibodies: anti-CD8α (53-6.7), CD127 (eBioSB/199), LAG-3 (C9B7W), and KLRG-1 (2F1; all eBioscience); anti–PD-1 (RMP1-30; BioLegend); gp276–286 tetramer (TCMetrix); and anti-CD4 (GK1.5), CD45.1 (A20), and CD45.2 (clone 104), obtained from or custom purified by Bio X Cell and coupled to Pacific blue, Alexa Fluor 647, or FITC using labeling reagents from Invitrogen. Cells were washed twice and fixed in PBS supplemented with 1% formaldehyde, 2% glucose, and 0.03% azide for 20 min. Then, cells were washed again and resuspended in FACS buffer. For intracellular cytokine staining, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD) and stained with anti–IFN-γ (XMG1.2), TNF (MP6-XT22; both from eBioscience), or granzyme B (GB12; Invitrogen). T-bet and Eomes staining was performed with a transcription factor staining kit (eBioscience) and stained with Eomes (Dan11mag) and T-bet (eBio4B10; both from eBioscience).
For flow cytometry sorting, living cells were stained in 10% FCS RPMI media and sorted on a FACSAria instrument (BD).
BrdU administration and staining
LCMV-infected mice were fed with 0.8 mg/ml BrdU (Sigma-Aldrich) in drinking water between days 20 and 26 and received a 2-mg BrdU bolus i.p. injection on days 20 and 24 p.i. On day 26 p.i., splenocytes were intracellularly stained for incorporated BrdU with the BD BrdU staining kit.
In vivo cytotoxicity assay
LCMV-infected (weeks 4–6) or naive mice received CFSE+CD45.1+ splenocytes pulsed with gp33 peptide and a nonpulsed CFSE+CD45.2+ reference population. 5 µM CFSE was used to label the cell populations. Cells were pulsed for 60 min with 5 µM gp33 peptide. 2 h after the transfer of the target cells, total splenocytes were harvested and stained with anti-CD45.1 (A20; eBioscience) and self-conjugated anti-CD45.2 (custom purified from hybridoma 104; Bio X Cell) and analyzed by flow cytometry. Using the no-peptide reference population, the fraction of eliminated peptide-containing target cells was calculated.
Ex vivo cytotoxicity assay
P14 T cells obtained from LCMV-infected Vβ5 mice were quantified and adjusted to equal numbers. As target cells, EL4 cells were labeled with 200 µCi Cr-51 (PerkinElmer) for 60–90 min at 37°C. Cells were washed twice and incubated with the adjusted number of P14 T cells in the presence or absence of gp33 peptide (EMC Microcollections) for 5 h at 37°C. Maximum release (MR) was measured with target cells plus 500 mM HCl, and spontaneous release (SR) was measured with target cells only. After incubation, supernatant was taken from each well, and chromium content was measured using a LumaPlate (PerkinElmer). Specific lysis was calculated according to the following formula: percent specific lysis = (cpmSample − cpmSR)/(cpmMR − cpmSR) × 100.
Next generation sequencing
Blood samples were taken at day 8 or 28 from LCMV-infected mice and used to propagate LCMV virus by infecting BHK cells. RNA extracted from the infected BHK cells was isolated using the RNeasy Micro kit (QIAGEN) and transcribed into cDNA with a Sensiscript Reverse Transcription kit (QIAGEN) using an LCMV-specific primer (5′-CACAGGACCTGCCAGCC-3′). The relevant section of the LCMV glycoprotein, which contains the gp33 epitope, was PCR amplified with the following primers: 5′-GGTGATCAACATTGTCATTATTGTGC-3′ and 5′-xxxxxxxxCACAGGACCTGCCAGCC-3′. All primers contained a unique nucleotide barcode (eight nucleotides long, indicated by xxxxxxxx in the primer) allowing us to pool different samples for next generation sequencing analysis using an Illumina sequencing platform (paired-end analysis; 25 million reads).
Data analyses
Flow cytometry measurements of cells were performed on an LSR-II flow cytometer (BD). All data were analyzed using FlowJo (Tree Star). Graphs were prepared and EC50 concentrations were determined with Prism (GraphPad Software). The error bars show SEM.
ACKNOWLEDGMENTS
We thank M.J. Bevan, M. Prlic, S. Voght, and P. Romero for helpful discussions and critical review of the manuscript.
This work was supported by funds provided by the Swiss Vaccine Research Institute to D. Zehn, the Swiss National Science Foundation (grants CRSII3-141789 and PP00P3_144883 to D. Zehn and grants PP00P3_135442/1 and 310030_149340/1 to D.D. Pinschewer), and the European Union (European Research Council starting grant 337043-ProtecTC to D. Zehn).
D.D. Pinschewer is a cofounder and consultant to Hookipa Biotech AG, which commercializes arenavirus-based vector technology. The authors declare no other competing financial interests.
Footnotes
Abbreviations used:
- APL
- altered peptide ligand
- BHK
- baby hamster kidney
- LCMV
- lymphocytic choriomeningitis virus
- p.i.
- postinfection
- pMHC
- peptide-MHC
References
- Alter M.J. 2006. Epidemiology of viral hepatitis and HIV co-infection. J. Hepatol. 44:S6–S9. 10.1016/j.jhep.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Angelosanto J.M., Blackburn S.D., Crawford A., and Wherry E.J.. 2012. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86:8161–8170. 10.1128/JVI.00889-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac V.P., Haring J.S., and Harty J.T.. 2007. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8+ T cell response to infection. Immunity. 26:827–841. 10.1016/j.immuni.2007.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., and Ahmed R.. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Battegay M., Cooper S., Althage A., Bänziger J., Hengartner H., and Zinkernagel R.M.. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods. 33:191–198. 10.1016/0166-0934(91)90018-U [DOI] [PubMed] [Google Scholar]
- Bengsch B., Seigel B., Ruhl M., Timm J., Kuntz M., Blum H.E., Pircher H., and Thimme R.. 2010. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6:e1000947 10.1371/journal.ppat.1000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengsch B., Martin B., and Thimme R.. 2014. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J. Hepatol. 61:1212–1219. 10.1016/j.jhep.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Beura L.K., Anderson K.G., Schenkel J.M., Locquiao J.J., Fraser K.A., Vezys V., Pepper M., and Masopust D.. 2015. Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution. J. Leukoc. Biol. 97:217–225. 10.1189/jlb.1HI0314-154R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Freeman G.J., and Wherry E.J.. 2008. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl. Acad. Sci. USA. 105:15016–15021. 10.1073/pnas.0801497105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn S.D., Shin H., Haining W.N., Zou T., Workman C.J., Polley A., Betts M.R., Freeman G.J., Vignali D.A., and Wherry E.J.. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37. 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T., Tyznik A.J., Roepke S., Berkley A.M., Woodward-Davis A., Pattacini L., Bevan M.J., Zehn D., and Prlic M.. 2013. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Reports. 3:701–708. 10.1016/j.celrep.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M., Kenney L.L., Chen A.T., Waggoner S.N., Kim S.K., Dienes H.P., Welsh R.M., and Selin L.K.. 2013. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front. Immunol. 4 10.3389/fimmu.2013.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- Doedens A.L., Phan A.T., Stradner M.H., Fujimoto J.K., Nguyen J.V., Yang E., Johnson R.S., and Goldrath A.W.. 2013. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol. 14:1173–1182. 10.1038/ni.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H., Sauer K., and Brooks D.G.. 2009. IL-21 is required to control chronic viral infection. Science. 324:1569–1572. 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enouz S., Carrié L., Merkler D., Bevan M.J., and Zehn D.. 2012. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J. Exp. Med. 209:1769–1779. 10.1084/jem.20120905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Bergthaler A., de la Torre J.C., and Pinschewer D.D.. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. USA. 103:4663–4668. 10.1073/pnas.0600652103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Hegazy A.N., Bergthaler A., Verschoor A., Claus C., Fernandez M., Gattinoni L., Johnson S., Kreppel F., Kochanek S., et al. 2010. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat. Med. 16:339–345. 10.1038/nm.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel H., Nindl V., Schuepbach R.A., Braunschweiler T., Richter K., Vogel J., Wagner C.A., Loffing-Cueni D., Kurrer M., Ludewig B., and Oxenius A.. 2012. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209:2485–2499. 10.1084/jem.20121015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich A., Kisielow J., Schmitz I., Freigang S., Shamshiev A.T., Weber J., Marsland B.J., Oxenius A., and Kopf M.. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 324:1576–1580. 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- Harari A., Bart P.A., Stöhr W., Tapia G., Garcia M., Medjitna-Rais E., Burnet S., Cellerai C., Erlwein O., Barber T., et al. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63–77. 10.1084/jem.20071331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertoghs K.M., Moerland P.D., van Stijn A., Remmerswaal E.B., Yong S.L., van de Berg P.J., van Ham S.M., Baas F., ten Berge I.J., and van Lier R.A.. 2010. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Invest. 120:4077–4090. 10.1172/JCI42758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.T., Anderson A.C., Tan W.G., West E.E., Ha S.J., Araki K., Freeman G.J., Kuchroo V.K., and Ahmed R.. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 107:14733–14738. 10.1073/pnas.1009731107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Bauer D.E., Tuttleton S.E., Lewin S., Gettie A., Blanchard J., Irwin C.E., Safrit J.T., Mittler J., Weinberger L., et al. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus–infected macaques. J. Exp. Med. 189:991–998. 10.1084/jem.189.6.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Bergthaler A., Graw F., Flatz L., Bonilla W.V., Siegrist C.A., Lambert P.H., Regoes R.R., and Pinschewer D.D.. 2015. Protective efficacy of individual CD8+ T cell specificities in chronic viral infection. J. Immunol. 194:1755–1762. 10.4049/jimmunol.1401771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P., and Hill A.. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879. 10.1038/ni1241 [DOI] [PubMed] [Google Scholar]
- Kunz S., Sevilla N., McGavern D.B., Campbell K.P., and Oldstone M.B.. 2001. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 155:301–310. 10.1083/jcb.200104103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legat A., Speiser D.E., Pircher H., Zehn D., and Fuertes Marraco S.A.. 2013. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front. Immunol. 4 10.3389/fimmu.2013.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichterfeld M., Yu X.G., Mui S.K., Williams K.L., Trocha A., Brockman M.A., Allgaier R.L., Waring M.T., Koibuchi T., Johnston M.N., et al. 2007. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J. Virol. 81:4199–4214. 10.1128/JVI.01388-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Concepcion R.J., and Ahmed R.. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Lechner F., Pircher H., and Zinkernagel R.M.. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 362:758–761. 10.1038/362758a0 [DOI] [PubMed] [Google Scholar]
- Mueller S.N., and Ahmed R.. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA. 106:8623–8628. 10.1073/pnas.0809818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K., Altman J.D., Suresh M., Sourdive D.J., Zajac A.J., Miller J.D., Slansky J., and Ahmed R.. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. 10.1016/S1074-7613(00)80470-7 [DOI] [PubMed] [Google Scholar]
- Nakamoto N., Cho H., Shaked A., Olthoff K., Valiga M.E., Kaminski M., Gostick E., Price D.A., Freeman G.J., Wherry E.J., and Chang K.M.. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 5 10.1371/journal.ppat.1000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi P.M., Pauken K.E., Paley M.A., Sharpe A., and Wherry E.J.. 2015. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212:1125–1137. 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley M.A., Kroy D.C., Odorizzi P.M., Johnnidis J.B., Dolfi D.V., Barnett B.E., Bikoff E.K., Robertson E.J., Lauer G.M., Reiner S.L., and Wherry E.J.. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 338:1220–1225. 10.1126/science.1229620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken K.E., and Wherry E.J.. 2015. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36:265–276. 10.1016/j.it.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., and Zinkernagel R.M.. 1990. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 346:629–633. 10.1038/346629a0 [DOI] [PubMed] [Google Scholar]
- Puglielli M.T., Zajac A.J., van der Most R.G., Dzuris J.L., Sette A., Altman J.D., and Ahmed R.. 2001. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb. J. Virol. 75:5099–5107. 10.1128/JVI.75.11.5099-5107.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziewicz H., Ibegbu C.C., Fernandez M.L., Workowski K.A., Obideen K., Wehbi M., Hanson H.L., Steinberg J.P., Masopust D., Wherry E.J., et al. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545–2553. 10.1128/JVI.02021-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A., and Greenberg P.D.. 2014. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 35:51–60. 10.1016/j.it.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J.E., Kuroda M.J., Santra S., Sasseville V.G., Simon M.A., Lifton M.A., Racz P., Tenner-Racz K., Dalesandro M., Scallon B.J., et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 283:857–860. 10.1126/science.283.5403.857 [DOI] [PubMed] [Google Scholar]
- Sevilla N., Kunz S., Holz A., Lewicki H., Homann D., Yamada H., Campbell K.P., de La Torre J.C., and Oldstone M.B.. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249–1260. 10.1084/jem.192.9.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt S.C., Borrow P., Kunz S., Cao W., Tishon A., Lewicki H., Campbell K.P., and Oldstone M.B.. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448–457. 10.1128/JVI.75.1.448-457.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D.E., Utzschneider D.T., Oberle S.G., Münz C., Romero P., and Zehn D.. 2014. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat. Rev. Immunol. 14:768–774. 10.1038/nri3740 [DOI] [PubMed] [Google Scholar]
- Utzschneider D.T., Legat A., Fuertes Marraco S.A., Carrié L., Luescher I., Speiser D.E., and Zehn D.. 2013. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14:603–610. 10.1038/ni.2606 [DOI] [PubMed] [Google Scholar]
- Viganò S., Utzschneider D.T., Perreau M., Pantaleo G., Zehn D., and Harari A. Functional avidity: a measure to predict the efficacy of effector T cells? Clin. Dev. Immunol. 2012;2012 doi: 10.1155/2012/153863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W., Wherry E.J., and Ahmed R.. 2009. Redefining chronic viral infection. Cell. 138:30–50. 10.1016/j.cell.2009.06.036 [DOI] [PubMed] [Google Scholar]
- Wherry E.J. 2011. T cell exhaustion. Nat. Immunol. 12:492–499. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., and Ahmed R.. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Ha S.J., Kaech S.M., Haining W.N., Sarkar S., Kalia V., Subramaniam S., Blattman J.N., Barber D.L., and Ahmed R.. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 27:670–684. 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Williams M.A., and Bevan M.J.. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192. 10.1146/annurev.immunol.25.022106.141548 [DOI] [PubMed] [Google Scholar]
- Yi J.S., Du M., and Zajac A.J.. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 324:1572–1576. 10.1126/science.1175194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., and Ahmed R.. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213. 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Bevan M.J., and Fink P.J.. 2007. Cutting edge: TCR revision affects predominantly Foxp3 cells and skews them toward the Th17 lineage. J. Immunol. 179:5653–5657. 10.4049/jimmunol.179.9.5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Lee S.Y., and Bevan M.J.. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 458:211–214. 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Turner M.J., Lefrançois L., and Bevan M.J.. 2010. Lack of original antigenic sin in recall CD8+ T cell responses. J. Immunol. 184:6320–6326. 10.4049/jimmunol.1000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Utzschneider D.T., and Thimme R.. 2016. Immune-surveillance through exhausted effector T-cells. Curr. Opin. Virol. 16:49–54. 10.1016/j.coviro.2016.01.002 [DOI] [PubMed] [Google Scholar]