Abstract
The endocannabinoid 2-AG is highly susceptible to its hydrolysis into AA, which activates neutrophils through de novo LTB4 biosynthesis, independently of CB activation. In this study, we show that 2-AG and AA stimulate neutrophils to release antimicrobial effectors. Supernatants of neutrophils activated with nanomolar concentrations of 2-AG and AA indeed inhibited the infectivity of HSV-1 and RSV. Additionally, the supernatants of 2-AG- and AA-stimulated neutrophils strongly impaired the growth of Escherichia coli and Staphylococcus aureus. This correlated with the release of a large amount (micrograms) of α-defensins, as well as a limited amount (nanograms) of LL-37. All the effects of AA and 2-AG mentioned above were prevented by inhibiting LTB4 biosynthesis or by blocking BLT1. Importantly, neither CB2 receptor agonists nor antagonists could mimic nor prevent the effects of 2-AG, respectively. In fact, qPCR data show that contaminating eosinophils express ~100-fold more CB2 receptor mRNA than purified neutrophils, suggesting that CB2 receptor expression by human neutrophils is limited and that contaminating eosinophils are likely responsible for the previously documented CB2 expression by freshly isolated human neutrophils. The rapid conversion of 2-AG to AA and their subsequent metabolism into LTB4 promote 2-AG and AA as multifunctional activators of neutrophils, mainly exerting their effects by activating the BLT1. Considering that nanomolar concentrations of AA or 2-AG were sufficient to impair viral infectivity, this suggests potential physiological roles for 2-AG and AA as regulators of host defense in vivo.
Keywords: leukotriene, cannabinoid receptor, endocannabinoid, eicosanoid, GPR55, 5-lipoxygenase
Introduction
Neutrophils play an important role in host defense by participating in pathogen clearance. They fulfil this important function notably by releasing antimicrobial peptides, by producing ROS, and by ingesting and killing microbes. Neutrophils represent a rich source of antimicrobial peptides, such as α-defensins and the cathelicidin LL-37. α-Defensins are small, cationic peptides accounting for ~5% of neutrophil proteins, and LL-37 is an α-helix peptide containing 37 aa that originates from the C-terminal domain of the cathelicidin human cationic antimicrobial protein-18. In addition to their antimicrobial properties against viruses and bacteria [1–4], α-defensins and LL-37 recruit and activate leukocytes [5–13].
One of the main bioactive ingredients of cannabis, THC was previously shown to increase the susceptibility of mice to bacterial, protozoan, and viral infections [14–17]. This effect of THC is linked to CB2 receptor activation, decreased phagocytosis by macrophages, down-regulation of proinflammatory cytokine production, and the polarization of T cells toward a Th2 phenotype [18–23]. Although CB2 receptor activation is usually considered anti-inflammatory, endocannabinoids, such as AEA and 2-AG, can enhance cell functions, such as chemotaxis, phagocytosis, and the release of soluble mediators [21, 24–33].
The pro- and anti-inflammatory effects of endocannabinoids are likely explained by a differential expression of CB receptors among leukocytes and the activation of additional cell surface receptors by endocannabinoid metabolites. 2-AG and AEA are indeed the respective precursors of other bioactive lipids, such as PGs-glycerol and prostamides [34, 35], as well as being a significant source of AA, leading to the biosynthesis of eicosanoids [36, 37]. We indeed documented that 2-AG was rapidly hydrolyzed to AA and further converted into LTB4 by human neutrophils [36]. Given that LTB4 promotes the ingestion and killing of microbes and the release of antimicrobial peptides [38–42], we postulated that 2-AG and AA would have similar effects. In this study, we investigated the antimicrobial activities of human neutrophils activated by 2-AG or AA.
MATERIALS AND METHODS
Material
2-AG ether, 2-AG, AA, AEA, SR144528, LTB4, and MAFP were purchased from Cayman Chemical (Ann Arbor, MI, USA). O-2050, AM-251, and DPI were obtained from Tocris Bioscience (Ellisville, MO, USA). ADA was purchased from Roche Applied Science (Indianapolis, IN, USA). fMLP, cytochalasin B, DMSO, dextran, and methyl cellulose were obtained from Sigma Chemical Co. (St. Louis, MO, USA). HBSS, lymphocyte separation medium, M199, FBS, penicillin, streptomycin, and amphotericin B were obtained from Wisent Laboratories (St-Bruno, Quebec, Canada). The magnetic bead-conjugated anti-CD16 mAb and MACS were purchased from Miltenyi Biotec (Auburn, CA, USA). Purified human LL-37 and α-defensins, which consist of a 5:4:1 mixture of α-defensin-1, -2, and -3, as well as LL-37 and α-defensin ELISA kits were obtained from Hycult Biotechnologies (Uden, The Netherlands). The FLAP antagonist MK-0591 was provided by Dr. Denis Riendeau from Merck Frosst (Kirkland, Quebec, Canada). E. coli (clinical isolate; ATCC #25922), S. aureus (clinical isolate; ATCC #25923), Vero cells (ATCC #CCL-81), and HSV-1 (MacIntyre strain; ATCC #VR-539) were obtained from the American Type Culture Collection (Manassas, VA, USA). rRSV encoding GFP was kindly provided by Dr. Peter Collins (U.S. National Institutes of Health, Bethesda, MD, USA). Shaphylokinase and aureolysin were, respectively, obtained from Cedarlane (Burlington, Ontario, Canada) and Axxora (Farmingdale, NY, USA).
Ethics Committee approval
This work required the use of human cells from healthy volunteers and was approved by the Institutional Ethics Committee from the Institut Universitaire de Cardiologie et de Pneumologie de Québec. All of the experiments were conducted with the understanding and the signed consent of each participant.
Isolation of human neutrophils
Venous blood was obtained from healthy volunteers in K3 EDTA tubes, and neutrophils were purified, as described before with slight modifications [43]. In brief, blood was centrifuged (17 min; 250 g) to remove the platelet-rich plasma. Erythrocytes then were sedimented with 3% dextran. PBMCs were discarded from the granulocytes by centrifugation on a discontinuous gradient by using lymphocyte separation medium cushions. A hypotonic lysis with sterile water was next performed on the granulocyte pellet to eliminate the residual erythrocytes. Neutrophils were finally purified from the granulocyte suspension by positive selection with anti-CD16-conjugated magnetic beads, according to the manufacturer’s instructions. The purity and viability of the resulting neutrophil and eosinophil suspensions were always ≥98.5 %, as assessed by Diff-Quick staining and trypan blue exclusion, respectively.
Analysis of α-defensins and LL-37
Supernatant of human neutrophils was analyzed for α-defensins and LL-37 content by ELISA (Hycult Biotechnologies), according to the manufacturer’s instructions.
Analysis of CB2 expression by RT-PCR and qPCR
Total RNA of granulocytes, neutrophils, and eosinophils was extracted with TRIzol, according to the manufacturer’s instructions. For the analysis of CB2 expression by RT-PCR, experiments were performed in a single tube using Qiagen OneStep RT-PCR kit. In brief, 50 ng total RNA was reverse-transcribed for 30 min and then amplified on a Peltier Thermal Cycler (PTC-200; MJ Research, Watertown, MA, USA) during 28 cycles with the following parameters: denaturation at 94°C for 1 min (2 min for the first cycle), annealing at 60°C for 30 s, and extension at 72°C for 1 min (5 min for the last cycle). After cycling, the amplicons were resolved by electrophoresis on ethidium bromide-stained 2% agarose gels, and the fluorescent bands were visualized using a UV transilluminator. The chemigenius software (Syngene, Frederick, MD, USA) was then used to capture the images. Primer sequences were CB2 (CNR2; NM_001841.2) forward, 5′-CCACAACACAACCCAAAGC-3′; CB2 reverse, 5′-GCAGAGGTATCGGTCAATGG-3′. For qPCR analyses, total RNA was extracted using TRIzol, followed by a treatment with the RNAse-free DNAse set (Qiagen, Valencia, CA, USA) to eliminate residual genomic DNA. Samples were cleaned and concentrated with the RNeasy MinElute cleanup kit (Qiagen) and then quantified spectrophotometrically at 260 nm using a Synergy H1 microplate reader and the Take3 trio plate (BioTek, Winooski, VT, USA). cDNAs were obtained using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). qPCR reactions were performed with the iQ SYBR Green Supermix (Bio-Rad) on a CFX96 Bio-Rad thermal cycler instrument. cDNA samples, obtained from 8 ng total RNA, were used in a reaction volume of 25 μl with specific primers for human CB2 (NM_001841) and human 18S rRNA (X03205) from Qiagen.
Dialysis of ADA and removal of endogenous adenosine
ADA was dialyzed using a Slide-A-Lyzer dialysis device (3.5 kDa MW cutoff; Thermo Fisher Scientific, Waltham, MA, USA). Sterile dialysis was performed in a beaker containing 0.9% NaCl and 10 mM Hepes (pH 7.4) with constant stirring. The buffered saline solution was changed after 1.5 and 3 h, and then the content was dialyzed overnight at 4°C. ADA was harvested from the dialysis device and then assessed for its ability to hydrolyze adenosine. To prevent the extracellular buildup of adenosine observed in isolated neutrophil suspensions, which results in the inhibition of neutrophil functions, ADA (0.3 U/ml) was always added to samples 10 min prior to stimulation [44].
Stimulation of human neutrophils
Prewarmed human neutrophil suspensions (37°C; 2×107 cells/ml) in HBSS, containing 1.6 mM CaCl2, were treated with 1 μM cytochalasin B for 15 min and then activated with LTB4, fMLP, 2-AG, AA, AEA, or 2-AG ether for the indicated times and concentrations (see figure legends). Incubations were stopped by placing the samples in an ice-water bath for 5 min. Samples then were centrifuged (4°C; 5 min; 700 g), and the cell-free supernatants were collected and frozen until further analyses. Addition of enzyme inhibitors or receptor antagonists was performed 5 min before the addition of the stimuli.
O2− release
The release of O2− by human neutrophils was assessed by cytochrome c reduction, as described previously with minor modifications [45]. In brief, prewarmed human neutrophil suspensions (37°C; 2×107 cells/ml) in HBSS, containing 1.6 mM CaCl2, were treated with 10 μM cytochalasin B for 15 min and then activated with 2-AG (3 μM), AA (3 μM), LTB4 (100 nM), or fMLP (100 nM) for 5 min. Addition of 130 μM cytochrome c was done 5 min before the addition of the stimuli. Incubations were stopped by placing the samples in an ice-water bath for 15 min. Samples were next centrifuged (4°C; 5 min; 700 g), and cell-free supernatants were analyzed for O2− production using the following formula: (OD550−OD540) × 47.4 = nmol O2−/106 cells/time units.
Bacterial growth and bactericidal assay
Inocula of E. coli or S. aureus were grown overnight (18 h, 37°C) in TSB. The obtained cultures were diluted 1/100 in fresh TSB and incubated at 37°C until the OD600 reached 0.5. Aliquots of the culture broths (250 μl for E. coli and 500 μl for S. aureus) were washed twice and then suspended in 1 ml sodium phosphate buffer (10 mM; pH 7.4) containing 1% TSB. Solutions then were diluted with the same buffer (1/3500 for E. coli and 1/1750 for S. aureus) to obtain suspensions of 10,000 CFU/ml. The resulting bacterial suspensions were then diluted with an equal volume of supernatant from activated neutrophils at 37°C for 4 h. Samples were next diluted 1/300 in sodium phosphate buffer containing 1% TSB, plated on LB agar plates, and incubated overnight to allow the growth of colonies and their enumeration.
Epithelial cell culture and virucidal assays
For the production of HSV-1, Vero cells were cultured in M199 medium, supplemented with 10% FBS, penicillin, streptomycin, and amphotericin B. Cells were passaged by trypsinization and grown until they reached 80% confluence. Vero cells were then infected with HSV-1. When cellular lysis was almost complete (5 days), the supernatant was collected, filtered (0.45 μm), and pelleted (30,000 g; 180 min). The viral pellet was suspended in 5 ml HBSS, aliquoted, and frozen at −80°C. HSV-1 titration was determined by a standard plaque assay on Vero cells. Viral preparations only underwent one freeze-thaw cycle. For the analysis of HSV-1 infectivity, assays were performed, as described before with slight modifications [39]. In brief, the HSV-1 stock solution was diluted in M199 medium to a final working concentration of 1200 PFU/ml and kept on ice until further use. In parallel, 15 μl neutrophil supernatant was added to 85 μl M199 medium and then mixed with 50 μl of the HSV-1 working solution and incubated at 37°C for 10 min. Samples were then mixed with 350 μl M199, and the mixture was added to each well of a 12-well plate containing 90% confluent Vero cells. The plates then were incubated for 60 min at 37°C, after which, the media were replaced with M199 containing 1.5% methylcellulose. After a 3-day incubation at 37°C, wells were washed twice with PBS, fixed with 5% formaldehyde for 10 min, then stained with 0.8% crystal violet in 50% ethanol for 2 min, and finally, washed with sterile water to allow the determination of HSV-1 infectivity by plaque count.
For the production of rRSV encoding GFP, amplification and purification were performed, as described previously [46]. In brief, amplification was done in human epidermoid cancer cell at a multiplicity of infection of 0.1 until a 50% cytopathic effect was observed. Virus was purified by ultra-centrifugation on 30% sucrose cushion centrifugation at 4°C (18 h; 8000 g). RSV titration was determined by a standard plaque assay on Vero cells. Viral preparations only underwent one freeze-thaw cycle. For the analysis of RSV infectivity, treatment with neutrophil supernatants was performed, as described for HSV-1, except that DMEM medium was used. Infection of Vero cells was performed for 2 h in DMEM containing 2% FBS. The medium was then replaced with DMEM containing 1% methylcellulose and 2% FBS. Infection was pursued for 7 days, and fluorescent lysis plaques were visualized using a Typhoon apparatus (Molecular Dynamics, Sunnyvale, CA, USA).
Statistical analyses
Variables were log-transformed to stabilize variance and analyzed with a multiple comparison using the Tukey method. Results were considered significant if P values were <0.05. The data were analyzed using the statistical package program SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
RESULTS
2-AG and AA induce the release of antimicrobial effectors by cytochalasin B-treated neutrophils
As our initial work on human neutrophils indicated that 2-AG was stimulatory rather than inhibitory [36], we first assessed whether endocannabinoids would also induce the release of antimicrobial effectors. Neutrophil suspensions were incubated with increasing concentrations of 2-AG or AA for 5 min, and then the cell-free supernatants were assayed for their potency to impair HSV-1 or RSV infectivity using Vero cells. The supernatants of 2-AG- and AA-stimulated neutrophils inhibited HSV-1 infectivity with similar efficacies and potencies, with 50% protection at ~30 nM (Fig. 1A). Additionally, 1 μM 2-AG or AA significantly decreased RSV infectivity of Vero cells by ~45%, which was similar to the effect of 100 nM LTB4 (Fig. 1B).
Figure 1. 2-AG and AA induce the release of antiviral activities.
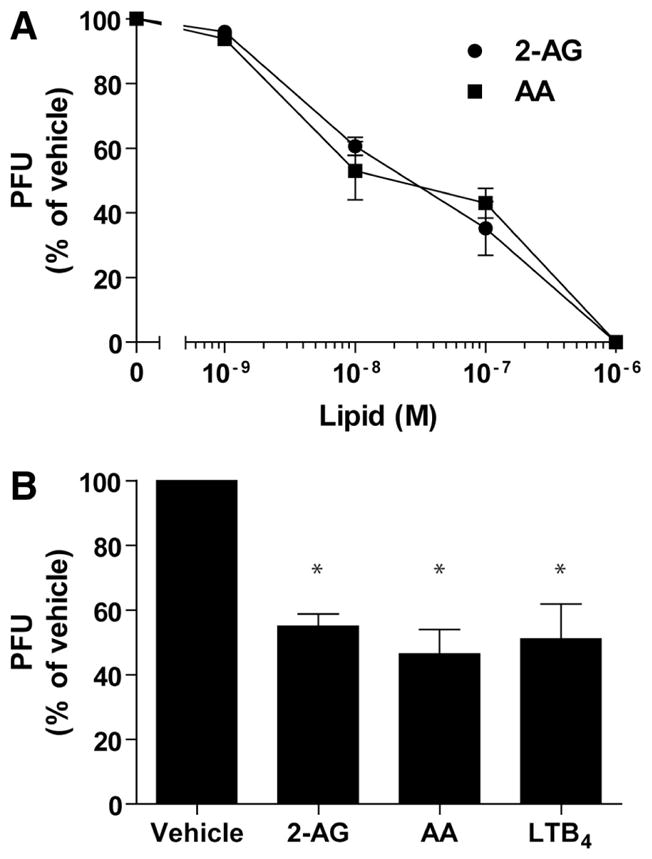
Human neutrophil suspensions (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 2-AG or AA for 5 min. Incubations were stopped by placing the samples in an ice-water bath. Cell-free supernatants were diluted in M199 medium (10:90) and incubated with (A) HSV-1 or (B) RSV for 10 min before infection, as described in Materials and Methods. Data are the mean (±SEM) of four experiments, each performed in duplicate. Statistical analyses were done using mixed-effects ANOVA, followed by the Tukey a posteriori test and were considered significant when P values were <0.05. *Statistically significant difference compared with vehicle (DMSO).
Given that 2-AG and AA levels are increased significantly during sepsis [47], we investigated whether they would also impair bacterial growth. We consequently performed another series of experiments in which the supernatants of 2-AG- and AA-treated neutrophils were incubated with exponentially growing E. coli or S. aureus suspensions. 2-AG and AA induced the release of a bactericidal activity from neutrophils, although the EC50 were one order of magnitude higher than what we observed with HSV-1 (Fig. 2). The maximal effects of 2-AG and AA were obtained with 3 μM 2-AG or AA, which inhibited the growth of S. aureus and E. coli by ~95% and ~85%, respectively. Importantly, the low concentration of LTB4, AA, or 2-AG that we used did not impact virus infectivity or bacterial growth [48, 49].
Figure 2. 2-AG and AA induce the release of antibacterial activities.
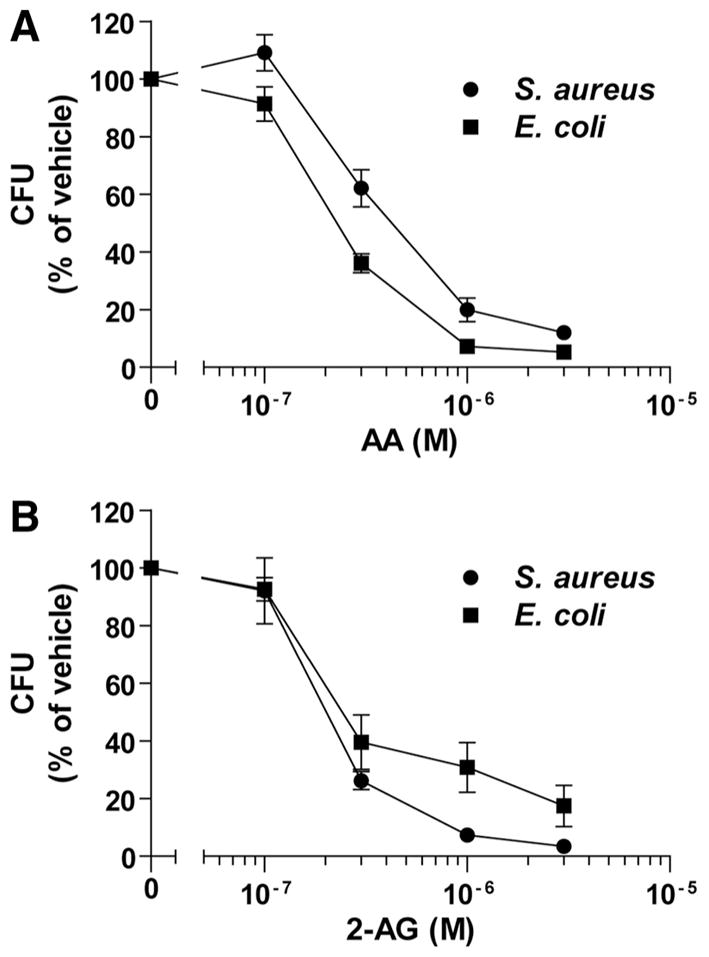
Human neutrophils (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with (A) AA or (B) 2-AG for 5 min. Incubations were stopped by placing the samples in an ice-water bath and then centrifuged. Cell-free supernatants were incubated (1:1) with exponentially growing E. coli or S. aureus suspensions (10,000 CFU/ml) in sodium phosphate buffer containing 1% TSB for 4 h at 37°C. Samples then were spread on LB agar plates and incubated overnight at 37°C, followed by CFU enumeration. The results represent the mean (±SEM) of four independent experiments, each performed in triplicate. Vehicle represents an amount of DMSO that equals the volume used in each experiment involving 2-AG or AA.
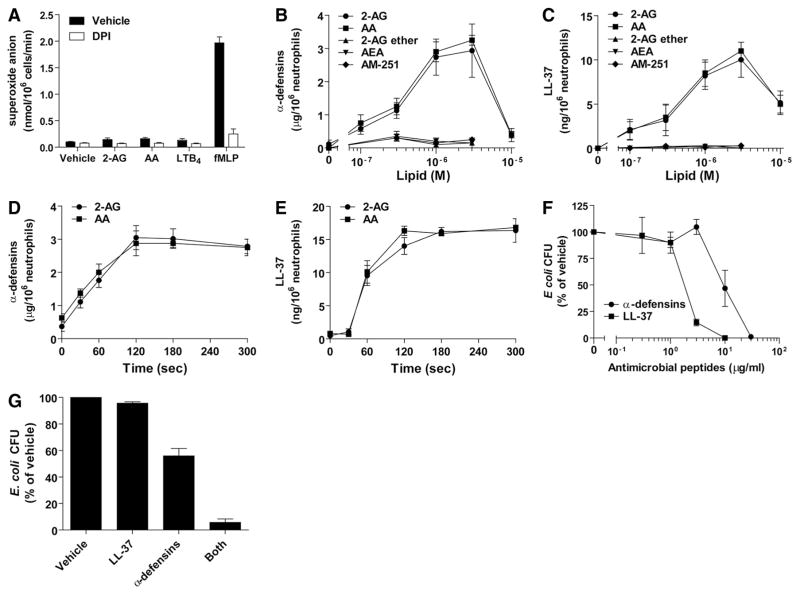
2-AG and AA induce the release of α-defensins and LL-37
As the antimicrobial effects of AA- and 2-AG-activated neutrophil supernatants were observed during short-term incubations (5 min), we postulated that the mediators involved were rapidly produced or stored in neutrophil granules. 2-AG, AA, and LTB4 were poor inducers of O2− release compared with fMLP (Fig. 3A). Indeed, 100 nM fMLP induced the release of O2−, 5× greater than that observed with LTB4, 2-AG, or AA. Moreover, the NADPH oxidase inhibitor DPI, which blocked O2− production (Fig. 3A), did not prevent the effects of 2-AG and AA on bacterial growth (data not shown). In contrast, 2-AG and AA induced an important release of α-defensins (Fig. 3B) and LL-37 (Fig. 3C) in a concentration-dependent manner. This began at a 2-AG or AA concentration of 300 nM, which is also the concentration at which supernatants begin to exert their bactericidal effects (Fig. 2) and was maximal at 3 μM. Interestingly, this mimicked the dose-response curves obtained for the biosynthesis of LTB4 induced by 2-AG or AA [36]. The release of antimicrobial peptides induced by 2-AG and AA was rapid (Figs. 3D and E), with most of the peptides released within 2 min. AEA, the GPR55 agonist (and CB1 antagonist) AM-251, as well as 2-AG ether (a nonhydrolyzable form of 2-AG) did not induce the release of α-defensin or LL-37 (Figs. 3B and C). The amounts of antimicrobial peptides released upon neutrophil activation were different for both classes of peptides. Indeed, whereas α-defensins were detected in the microgram range, LL-37 was found in the nanogram range (~250:1 ratio). In an attempt to dissect the contribution of α-defensins and LL-37 in the antimicrobial effects that we observed, additional experiments were performed by treating neutrophil supernatants with commercially available staphylokinase and aureolysin to impair the efficacy of α-defensins and LL-37. This strategy was, however, unsuccessful, as both protein preparations directly inhibited the growth of our bacterial suspensions (data not shown). We, nonetheless, investigated the potency of purified α-defensins and LL-37 on bacterial growth in our experimental model. Purified peptides effectively inhibited the growth of E. coli (Fig. 3F), with LL-37 being more potent by one order of magnitude. Essentially similar results were obtained for S. aureus (data not shown). Interestingly, when 10 μg/ml α-defensins were combined with 40 ng/ml LL-37 (250:1 ratio), the antimicrobial peptides prevented the growth of E. coli suspension in a synergistic manner (Fig. 3G).
Figure 3. 2-AG and AA induce the release of antimicrobial factors.
(A) Human neutrophils (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 3 μM 2-AG, 3 μM AA, 100 nM LTB4, or 100 nM fMLP for 5 min in the presence or absence of 10 μM DPI. Incubation was stopped by placing the samples in an ice-water bath and analyzed for O2− production, as described in Materials and Methods. (B and C) Human neutrophils (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were stimulated with 2-AG, AA, 2-AG ether, AEA, or AM-251 at the indicated concentration for 5 min. Incubations were stopped by placing the samples in an ice-water bath, and then cell-free supernatants were analyzed for their content in α-defensins or LL-37, as described in Materials and Methods. (D and E) Human neutrophils (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were stimulated with 3 μM 2-AG or AA for the indicated times. Incubations were stopped by placing the samples in an ice-water bath, and then the cell-free supernatants were analyzed for their content in α-defensins or LL-37, as described in Materials and Methods. (F) Purified LL-37 or α-defensins were incubated with exponentially growing E. coli suspensions (10,000 CFU/ml) in sodium phosphate buffer containing 1% TSB for 4 h at 37°C. (G) Purified LL-37 (40 ng/ml), α-defensins (10 μg/ml), or both were incubated with exponentially growing E. coli suspensions (10,000 CFU/ml) in sodium phosphate buffer containing 1% TSB for 4 h at 37°C. (F and G) Samples were diluted 1/300 in incubation medium, plated on LB agar plates, and incubated overnight at 37°C to allow CFU enumeration. (A–G) The data represent the mean (±SEM) of at least three independent experiments, each performed in duplicate.
CBs are not involved in the 2-AG-induced release of antimicrobial peptides
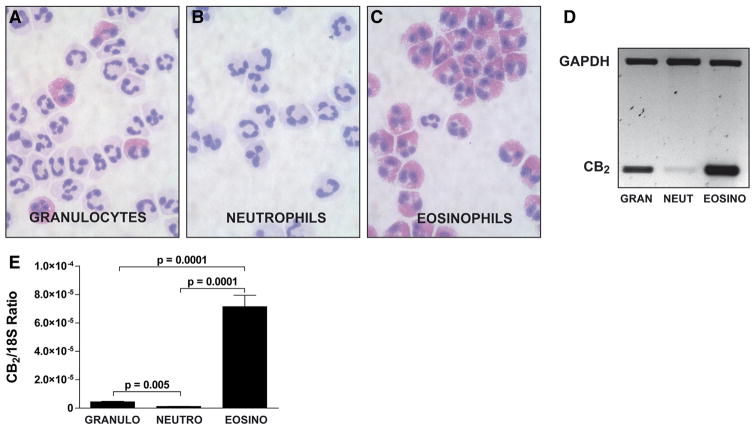
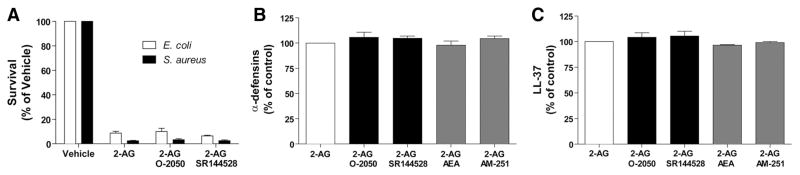
The results obtained using AEA and 2-AG ether (Fig. 3B and C), which activate CBs and GPR55 [50–55], suggested that the effects of 2-AG were likely independent of GPR55 or CB activation. The expression of CBs by neutrophils is controversial. Neutrophils were reported to express the CB2 receptor [56–59], whereas others [27] and we [36] reported otherwise. Interestingly, the absence of CB2 receptor mRNA by human neutrophils coincided with the removal of contaminating eosinophils. This prompted us to determine the expression of the CB2 receptor in isolated granulocytes (which many research groups use as purified neutrophils), eosinophil-depleted neutrophils, and eosinophils. As shown in Fig. 4, CB2 receptor mRNA expression was detected by RT-PCR in isolated granulocytes and eosinophils, in sharp contrast to purified (eosinophil-depleted) neutrophils. Additional experiments using qPCR (Fig. 4E) also yielded similar results: the removal of contaminating eosinophils significantly decreased the CB2 mRNA signal found in granulocytes. Importantly, the expression of CB2 receptor mRNA by human eosinophils was ~100-fold higher than that observed with highly purified neutrophils. We also undertook a series of experiments, in which human neutrophils were pretreated with the CB1 receptor antagonist O-2050 or the CB2 antagonist SR144528. Both antagonists did not prevent the bactericidal effects of 2-AG (Fig. 5A) nor did they prevent the release of antimicrobial peptides (Fig. 5B and C). Additionally, AEA and the GPR55 agonist (and CB1 antagonist) AM-251 did not inhibit nor enhance the 2-AG-induced release of α-defensins or LL-37. Altogether, the lack of effect of AEA and 2-AG ether combined with the lack of efficacy of CB antagonists to block the 2-AG-induced antimicrobial peptide release and bactericidal effect indicate that these phenomena are likely independent of CB1, CB2, or GPR55 activation.
Figure 4. Expression of CB2 by granulocytes, eosinophil-depleted neutrophils, and eosinophils.
Granulocytes (A) were purified further into neutrophils (CD16+; B) and eosinophils (CD16−; C) and then stained with Diff-Quik. Total RNA was extracted from the cell pellets, and amplification of the CB2 receptor mRNA by (D) RT-PCR or by (E) RT-qPCR was performed, as described in Materials and Methods. (D) The data represent a typical RT-PCR result obtained using the mRNA from cells of the same donor. (E) Data are the mean (±SEM) of six independent experiments, each performed in triplicate. Statistical analyses were done using mixed-effects ANOVA, followed by the Tukey a posteriori test. 18S refers to 18S rRNA and is used as an expression control.
Figure 5. The 2-AG-mediated bacterial killing and antimicrobial peptide release are independent of CB activation.
(A) Neutrophil suspensions (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 1 μM 2-AG for 5 min. Incubations were stopped by adding 1 vol ice-cold buffer, immediately placing the samples in an ice-water bath for 5 min, and then centrifuged. Cell-free supernatants then were incubated with exponentially growing E. coli or S. aureus for 4 h and then plated on LB agar plates overnight to allow CFU quantification, as described in Materials and Methods. (B and C) Neutrophil suspensions (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 1 μM 2-AG for 5 min. Incubations were stopped by adding 1 vol cold incubation buffer and then rapidly centrifuged. Supernatants then were assessed for their content in (B) α-defensins 1–3 and (C) LL-37 by ELISA, according to the manufacturer’s instructions. Addition of 1 μM AEA, the GPR55 agonist (and CB1 antagonist) AM-251, the CB1 antagonist O-2050, or the CB2 antagonist SR144528 was done 5 min before stimulating neutrophils with 2-AG in all experimental settings. The data represent the mean (±SEM) of three independent experiments performed in duplicate.
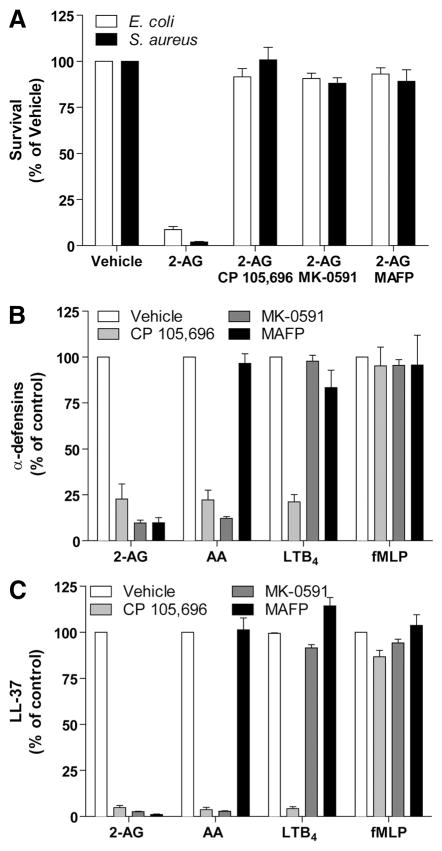
2-AG hydrolysis, AA, and LTB4 biosynthesis are involved in the antimicrobial effects of 2-AG
The similarity between the effects induced by 2-AG and AA and the absence of response of AEA and 2-AG ether suggested that the effects of 2-AG and AA were mediated through their metabolism. Importantly, 2-AG and AA induce a robust biosynthesis of LTB4 by human neutrophils [36, 60], and LTB4 is an important regulator of host defense [38–42, 61] Blockade of 2-AG hydrolysis to AA (MAFP), LTB4 biosynthesis (MK-0591), or BLT1 activation (CP 105,696) inhibited the effects of 2-AG on the release of antimicrobial activity (Fig. 6A) and antimicrobial peptides (Figs. 6A–C). LT modifiers, but not MAFP, also prevented the effect of AA on antimicrobial peptide release and the antimicrobial effects observed. Importantly, the release of α-defensins and LL-37, induced by fMLP, was not affected by any of the tested compounds. These results demonstrate that the release antimicrobial effects of 2-AG and AA relies on de novo LTB4 biosynthesis, as opposed to the effects of fMLP. They clearly underscore the essential role of LTB4 in the release of the antimicrobial activity by 2-AG and AA. Moreover, they underline the potential of 2-AG as a source of AA for neutrophils in vivo.
Figure 6. Involvement of LTB4 in the effects of 2-AG and AA on bacterial growth and antimicrobial peptide release by neutrophils.
(A) Neutrophil suspensions (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 3 μM 2-AG for 5 min. Incubations were stopped by placing the samples in an ice-water bath and then centrifuged. Cell-free supernatants were incubated with exponentially growing E. coli or S. aureus for 4 h and then plated on LB agar plates overnight to allow CFU quantification, as described in Materials and Methods. (B and C) Neutrophil suspensions (2×107 cells/ml) in HBSS containing 1.6 mM CaCl2 were incubated with 1 μM cytochalasin B for 15 min and then stimulated with 3 μM 2-AG, 3 μM AA, 100 nM LTB4, or 100 nM fMLP for 5 min. Incubations were stopped by adding 1 vol cold incubation buffer and then rapidly centrifuged. Supernatants were next assessed for their content in (B) α-defensins 1–3 and (C) LL-37 by ELISA, according to the manufacturer’s instructions. The addition of the BLT1 antagonist CP 105,696 (100 nM), the FLAP antagonist MK-0591 (100 nM), and the 2-AG hydrolysis inhibitor MAFP (30 nM) was done 5 min prior to the stimulation with 2-AG, AA, LTB4, or fMLP in all experimental settings. The results represent the mean (±SEM) of at least three independent experiments, each performed in duplicate.
DISCUSSION
In the present study, we evaluated the effects of AEA, 2-AG, and AA on the activation of neutrophil functions related to host defense. We found that in contrast to AEA, very low (mid-nanometer) concentrations of 2-AG and AA induce the release of antimicrobial effectors against S. aureus, E. coli, HSV-1, and RSV. The activation of neutrophils by 2-AG and AA was associated with: 1) the release of α-defensins and LL-37; 2) a lack of CB involvement; and 3) an essential role of endogenous LTB4 and BLT1 activation. This suggests that low 2-AG concentration possibly plays an important role in host defense by promoting human neutrophil functions.
Plasma and tissue endocannabinoid levels are usually in the low nanomolar range [62, 63] but increase following cell activation. Many inflammatory mediators, present at infectious sites, such as LPS, platelet-activating factor, and M-CSF, induce the biosynthesis of endocannabinoids by leukocytes [64–67]. In this respect, endocannabinoid levels in sepsis and inflamed tissues are in the micromolar range [47, 68], well above the EC50 that we observed in this study (Figs. 1 and 2). Importantly, experiments performed with HSV-1 and RSV were done in the presence of only 10% (v/v) of neutrophil supernatants, possibly underestimating the efficacy of 2-AG and AA by up to one order of magnitude.
Studies investigating the involvement of CBs at regulating neutrophil functions are limited. Moreover, inconsistencies are found regarding the expression of the CB receptors [27, 36, 56, 57, 69–77]. Importantly, studies that reported a lack of CB2 were performed using cell preparations in which contaminating eosinophils were removed [27, 36]. The comparative experiments among granulocytes (neutrophils and eosinophils), neutrophils, and eosinophils, which we provided here, demonstrate that >95% of CB2 mRNA for CB2 originates from eosinophils rather than neutrophils (Fig. 4). The 100-fold higher CB2 receptor expression (mRNA) that we observed in human eosinophils indicates that even a 1% contamination of neutrophil suspensions with eosinophils is enough to yield a false-positive signal when performing RT-PCR or qPCR analyses. Furthermore, the inefficacy of other endocannabinoids (AEA, 2-AG ether) or CB antagonists to, respectively, mimic or block the effects of 2-AG supports an activation mechanism independent of CB receptors.
Following its uptake, 2-AG can be hydrolyzed to AA by serine hydrolases, such as monoacylglycerol lipase, or metabolized into other bioactive lipids by eicosanoid biosynthetic enzymes [34, 35, 78–81]. The data presented here (Fig. 5) indicate that 2-AG and AA induce the release of antimicrobial activities through their metabolism into LTB4 and the activation of BLT1, in agreement with our previous study [36]. This again supports the physiological role of LTB4 in host defense. Indeed, LTB4 enhances host defense in vivo, promoting antimicrobial peptide release and enhancing phagocyte functions through BLT1 receptor activation [39, 40].
AA is usually esterified at the sn-2 position of glycerophospholipid. One of the dogmata regarding AA release is that upon cell activation, the group IVA PLA2 translocates to membranes, where it releases AA. Interestingly, other PLA2, notably the secreted PLA2 groups V, X, and XII, are also participating in AA release [82]. The important impact of exogenously added AA on the release of antimicrobial peptides and antimicrobial effects that we observed indicates that secreted PLA2 might also play an important role in the regulation of host defense. This, however, fosters additional studies to delineate the putative role of the secreted PLA2 in these processes.
We also attempted to delineate the exact contribution of some antimicrobial products. Our data indicate that 2-AG and AA induced a modest production of ROS compared with fMLP and that the inhibition of NADPH oxidase with DPI did not blunt the effect of 2-AG and AA (Fig. 3, and data not shown). Interestingly, LL-37 and α-defensins were documented to mediate the LTB4-induced antimicrobial effects of neutrophils [8, 39]. Our data show that there is an ~250:1 ratio between α-defensins and LL-37 release following the activation of human neutrophils by 2-AG or AA. Furthermore, our data indicate that LL-37 is more potent than α-defensins against E. coli and S. aureus by one order of magnitude (ED50 of 1 vs. 10 μg/ml). Importantly, we provided evidence that α-defensins and LL-37 acted synergistically rather than redundantly (Fig. 3G) in agreement with a previous study [83]. This suggests that the bactericidal effect of 2-AG and AA is likely the consequence of LL-37 and α-defensins. Interestingly, the inhibitory effect of 2-AG- and AA-stimulated neutrophil supernatants on HSV-1 infectivity does not correlate with the release of LL-37 or α-defensins (Figs. 1 and 3). This suggests that other soluble mediators might also participate in the virucidal effect of 2-AG- and AA-stimulated neutrophil supernatants and fosters additional studies.
Cytochalasin B acts by disrupting the actin cytoskeleton, which enhances many neutrophil functions, including degranulation. Cytoskeleton-disrupting agents are not necessary in vivo, as adherence to entothelium, which is linked to cytoskeleton rearrangement, is sufficient to prime neutrophils and promote the release of granule components (reviewed in ref. [39]). To better mimic the fate of in vivo neutrophils, we treated our neutrophil suspensions with 1 μM cytochalasin B to enhance their cellular responses. In the absence of cytochalasin B, only a minimal release of LL-37 and α-defensins was observed (data not shown).
Finally, our experiments were performed under serum-free conditions, given that serum can trap fatty acids. For example, the use of serum or BSA has long been used to analyze AA release by stimulated cells ex vivo. Moreover, the incubation of isolated neutrophils in plasma results in the trapping of de novo-synthesized LTA4 [84]. Nonetheless, LTB4 can be found in activated whole blood under many circumstances. In our own hands, we still observed a stimulatory effect of 2-AG and AA, with up to 2% BSA (data not shown), which represents the protein content of an aqueous solution containing 5% serum [85]. We favored using BSA over plasma or serum to avoid the stimulatory effect that TGF-β has on the LT pathway [86]. That being said, AA-specific secreted PLA2 enzymes have many biological functions involving eicosanoids, albeit they release AA in the extracellular space.
In conclusion, we provide evidence that 2-AG and AA are potential regulators of human neutrophil functions associated to host defense. Importantly, this occurred at physiological (nanomolar) concentrations of 2-AG and AA and was associated with the release of LL-37 and α-defensins from cytochalasin B-treated neutrophils. Numerous events associated with the innate and adaptive immune responses are linked to antimicrobial peptides: they recruit and activate leukocytes [5–13], the complement cascade [87], and the phagocytosis by macrophages. Consequently, our data support that 2-AG and AA may be important physiological regulators of the innate and adaptive immune responses in humans. Additional studies will be needed to establish the roles of 2-AG and AA as regulators of neutrophil-mediated innate immunity in vivo.
Acknowledgments
This work was supported by grants to N.F. from the CIHR and the Natural Sciences and Engineering Research Council of Canada. F.C. was the recipient of a doctoral training award from the CIHR. N.F. and N.G. are the recipients of scholarships from the Fonds de Recherche du Québec-Santé.
Abbreviations
- 2-AG
2-arachidonoyl-glycerol
- AA
arachidonic acid
- ADA
adenosine deaminase
- AEA
N-arachidonylethanolamine
- AM-251
N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole 3-carboxamide
- BLT1
leukotriene B4 receptor 1
- CB
cannabinoid receptor
- CIHR
Canadian Institutes of Health Research
- DPI
diphenyleneiodonium
- FLAP
5-lipoxygenase-activating protein
- GPR55
GPCR 55
- HSV-1
herpes simplex virus 1
- LT
leukotriene
- MAFP
methyl-arachidonyl-fluorophosphonate
- O-2050
(6aR,10aR)-3-(1-methane-sulfonyl-amino-4-hexyn-6-yl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzopyran
- O2−
superoxide anion
- qPCR
quantitative PCR
- RSV
respiratory syncytial virus
- SR144528
5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-1H-pyrazole-3-carboxamide
- THC
(−)-Δ9-tetrahydrocannabinol
- TSB
tryptic soy broth
Footnotes
AUTHORSHIP
F.C., C.T., M-C.L., X.G., S.P., L.B., and V.P. performed experiments. F.C., C.T., L.F., N.G., and N.F. designed the experiments. F.C. and N.F. wrote the paper. All authors reviewed the paper.
References
- 1.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeianov V, Scott K, Reid G. Activity of cecropin P1 and FA-LL-37 against urogenital microflora. Microbes Infect. 2000;2:773–777. doi: 10.1016/s1286-4579(00)90359-9. [DOI] [PubMed] [Google Scholar]
- 4.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 5.Grigat J, Soruri A, Forssmann U, Riggert J, Zwirner J. Chemoattraction of macrophages, T lymphocytes, and mast cells is evolutionarily conserved within the human α-defensin family. J Immunol. 2007;179:3958–3965. doi: 10.4049/jimmunol.179.6.3958. [DOI] [PubMed] [Google Scholar]
- 6.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan M, Sabirsh A, Wetterholm A, Agerberth B, Haeggstrom JZ. Leukotriene B4 triggers release of the cathelicidin LL-37 from human neutrophils: novel lipid-peptide interactions in innate immune responses. FASEB J. 2007;21:2897–2905. doi: 10.1096/fj.06-7974com. [DOI] [PubMed] [Google Scholar]
- 9.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil α-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 10.Khine AA, Del SL, Vaschetto R, Voglis S, Tullis E, Slutsky AS, Downey GP, Zhang H. Human neutrophil peptides induce interleukin-8 production through the P2Y6 signaling pathway. Blood. 2006;107:2936–2942. doi: 10.1182/blood-2005-06-2314. [DOI] [PubMed] [Google Scholar]
- 11.Koczulla R, von DG, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 14.Klein TW, Newton C, Friedman H. Resistance to Legionella pneumophila suppressed by the marijuana component, tetrahydrocannabinol. J Infect Dis. 1994;169:1177–1179. doi: 10.1093/infdis/169.5.1177-a. [DOI] [PubMed] [Google Scholar]
- 15.Cabral GA, Marciano-Cabral F. Cannabinoid-mediated exacerbation of brain infection by opportunistic amebae. J Neuroimmunol. 2004;147:127–130. doi: 10.1016/j.jneuroim.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Morahan PS, Klykken PC, Smith SH, Harris LS, Munson AE. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infect Immun. 1979;23:670–674. doi: 10.1128/iai.23.3.670-674.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishkin EM, Cabral GA. Δ-9-Tetrahydrocannabinol decreases host resistance to herpes simplex virus type 2 vaginal infection in the B6C3F1 mouse. J Gen Virol. 1985;66:2539–2549. doi: 10.1099/0022-1317-66-12-2539. [DOI] [PubMed] [Google Scholar]
- 18.Friedman M, Cepero ML, Klein T, Friedman H. Suppressive effect of Δ9-tetrahydrocannabinol in vitro on phagocytosis by murine macrophages. Proc Soc Exp Biol Med. 1986;182:225–228. doi: 10.3181/00379727-182-42332. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Cepero M, Friedman M, Klein T, Friedman H. Tetrahydrocannabinol-induced suppression of macrophage spreading and phagocytic activity in vitro. J Leukoc Biol. 1986;39:679–686. doi: 10.1002/jlb.39.6.679. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard DK, Newton C, Klein TW, Stewart WE, Friedman H. In vitro and in vivo suppressive effects of Δ9-tetrahydrocannabinol on interferon production by murine spleen cells. Int J Immunopharmacol. 1986;8:819–824. doi: 10.1016/0192-0561(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, Terminelli C, Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- 22.Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by Δ9-tetrahydrocannabinol injection. Infect Immun. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Δ9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-Arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J Biol Chem. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–223. doi: 10.1093/jb/mvi021. [DOI] [PubMed] [Google Scholar]
- 26.Maestroni GJ. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18:1914–1916. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- 27.Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EOL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002–1009. doi: 10.1189/jlb.0404252. [DOI] [PubMed] [Google Scholar]
- 28.Shiratsuchi A, Watanabe I, Yoshida H, Nakanishi Y. Involvement of cannabinoid receptor CB2 in dectin-1-mediated macrophage phagocytosis. Immunol Cell Biol. 2008;86:179–184. doi: 10.1038/sj.icb.7100121. [DOI] [PubMed] [Google Scholar]
- 29.Stefano GB, Bilfinger TV, Rialas CM, Deutsch DG. 2-Arachidonyl-glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol Res. 2000;42:317–322. doi: 10.1006/phrs.2000.0702. [DOI] [PubMed] [Google Scholar]
- 30.Gokoh M, Kishimoto S, Oka S, Metani Y, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, enhances the adhesion of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes. FEBS Lett. 2005;579:6473–6478. doi: 10.1016/j.febslet.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Kishimoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J Biochem. 2004;135:517–524. doi: 10.1093/jb/mvh063. [DOI] [PubMed] [Google Scholar]
- 32.Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. doi: 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- 33.Lau AH, Chow SS. Effects of cannabinoid receptor agonists on immunologically induced histamine release from rat peritoneal mast cells. Eur J Pharmacol. 2003;464:229–235. doi: 10.1016/s0014-2999(03)01430-4. [DOI] [PubMed] [Google Scholar]
- 34.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 35.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 36.Chouinard F, Lefebvre JS, Navarro P, Bouchard L, Ferland C, Lalancette-Hebert M, Marsolais D, Laviolette M, Flamand N. The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J Immunol. 2011;186:3188–3196. doi: 10.4049/jimmunol.1002853. [DOI] [PubMed] [Google Scholar]
- 37.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey MJ, Phare SM, Peters-Golden M. Role of leukotrienes in killing of Mycobacterium bovis by neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2004;71:185–190. doi: 10.1016/j.plefa.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Flamand L, Tremblay MJ, Borgeat P. Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J Immunol. 2007;178:8036–8045. doi: 10.4049/jimmunol.178.12.8036. [DOI] [PubMed] [Google Scholar]
- 40.Mancuso P, Nana-Sinkam P, Peters-Golden M. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect Immun. 2001;69:2011–2016. doi: 10.1128/IAI.69.4.2011-2016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaudreault E, Gosselin J. Leukotriene B4-mediated release of antimicrobial peptides against cytomegalovirus is BLT1 dependent. Viral Immunol. 2007;20:407–420. doi: 10.1089/vim.2006.0099. [DOI] [PubMed] [Google Scholar]
- 42.Gaudreault E, Gosselin J. Leukotriene B4 induces release of antimicrobial peptides in lungs of virally infected mice. J Immunol. 2008;180:6211–6221. doi: 10.4049/jimmunol.180.9.6211. [DOI] [PubMed] [Google Scholar]
- 43.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 44.Flamand N, Boudreault S, Picard S, Austin M, Surette ME, Plante H, Krump E, Vallee MJ, Gilbert C, Naccache P, Laviolette M, Borgeat P. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am J Respir Crit Care Med. 2000;161:S88–S94. doi: 10.1164/ajrccm.161.supplement_1.ltta-18. [DOI] [PubMed] [Google Scholar]
- 45.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 46.Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-κB p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKKβ. J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kase Y, Obata T, Okamoto Y, Iwai K, Saito K, Yokoyama K, Takinami M, Tanifuji Y. Removal of 2-arachidonylglycerol by direct hemoperfusion therapy with polymyxin B immobilized fibers benefits patients with septic shock. Ther Apher Dial. 2008;12:374–380. doi: 10.1111/j.1744-9987.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 48.Knapp HR, Melly MA. Bactericidal effects of polyunsaturated fatty acids. J Infect Dis. 1986;154:84–94. doi: 10.1093/infdis/154.1.84. [DOI] [PubMed] [Google Scholar]
- 49.Leu GZ, Lin TY, Hsu JT. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem Biophys Res Commun. 2004;318:275–280. doi: 10.1016/j.bbrc.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 50.Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, Malli R, Graier WF. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- 53.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 55.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 56.Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, Katsumata Y, Yamamura H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–12918. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- 57.Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, Kostenis E, Brown AJ, Heinemann A, Waldhoer M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011;21:1452–1469. doi: 10.1038/cr.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 59.Small-Howard AL, Shimoda LM, Adra CN, Turner H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem J. 2005;388:465–473. doi: 10.1042/BJ20041682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surette ME, Krump E, Picard S, Borgeat P. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A2A receptor agonists and crucial role of autocrine activation by leukotriene B4. Mol Pharmacol. 1999;56:1055–1062. doi: 10.1124/mol.56.5.1055. [DOI] [PubMed] [Google Scholar]
- 61.Serezani CH, Aronoff DM, Jancar S, Peters-Golden M. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J Leukoc Biol. 2005;78:976–984. doi: 10.1189/jlb.1004587. [DOI] [PubMed] [Google Scholar]
- 62.Thomas A, Hopfgartner G, Giroud C, Staub C. Quantitative and qualitative profiling of endocannabinoids in human plasma using a triple quadrupole linear ion trap mass spectrometer with liquid chromatography. Rapid Commun Mass Spectrom. 2009;23:629–638. doi: 10.1002/rcm.3918. [DOI] [PubMed] [Google Scholar]
- 63.Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di MV, Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, Mach F, Schindler TH. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J. 2011;32:1369–1378. doi: 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- 64.Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J. 2001;15:2171–2178. doi: 10.1096/fj.01-0181com. [DOI] [PubMed] [Google Scholar]
- 65.Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 66.Pestonjamasp VK, Burstein SH. Anandamide synthesis is induced by arachidonate mobilizing agonists in cells of the immune system. Biochim Biophys Acta. 1998;1394:249–260. doi: 10.1016/s0005-2760(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 67.Rouzer CA, Marnett LJ. Glycerylprostaglandin synthesis by resident peritoneal macrophages in response to a zymosan stimulus. J Biol Chem. 2005;280:26690–26700. doi: 10.1074/jbc.M501021200. [DOI] [PubMed] [Google Scholar]
- 68.Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Murikinati S, Juttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 70.Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007;21:1788–1800. doi: 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tüting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 72.Csóka B, Németh ZH, Mukhopadhyay P, Spolarics Z, Rajesh M, Federici S, Deitch EA, Bátkai S, Pacher P, György H. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS ONE. 2009;4:e6409. doi: 10.1371/journal.pone.0006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, Ishima Y, Sugiura T. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- 74.Tschop J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschop MH, Caldwell CC. The cannabinoid receptor 2 is critical for the host response to sepsis. J Immunol. 2009;183:499–505. doi: 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraft B, Wintersberger W, Kress HG. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP 55,940, but not by anandamide. Life Sci. 2004;75:969–977. doi: 10.1016/j.lfs.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Kraft B, Kress HG. Indirect CB2 receptor and mediator-dependent stimulation of human whole-blood neutrophils by exogenous and endogenous cannabinoids. J Pharmacol Exp Ther. 2005;315:641–647. doi: 10.1124/jpet.105.084269. [DOI] [PubMed] [Google Scholar]
- 77.McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- 78.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 79.Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I, Inoue K. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- 80.Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry. 2001;40:861–866. doi: 10.1021/bi002303b. [DOI] [PubMed] [Google Scholar]
- 81.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor α agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 82.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 83.Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–79. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 84.Palmantier R, Rocheleau H, Laviolette M, Mancini J, Borgeat P. Characteristics of leukotriene biosynthesis by human granulocytes in presence of plasma. Biochim Biophys Acta. 1998;1389:187–196. doi: 10.1016/s0005-2760(97)00149-5. [DOI] [PubMed] [Google Scholar]
- 85.Ferland C, Guilbert M, Davoine F, Flamand N, Chakir J, Laviolette M. Eotaxin promotes eosinophil transmigration via the activation of the plasminogen-plasmin system. J Leukoc Biol. 2001;69:772–778. [PubMed] [Google Scholar]
- 86.Steinhilber D, Radmark O, Samuelsson B. A heat stable serum factor upregulates 5-lipoxygenase activity in HL-60 cells, modulation by TNF α or GM-CSF. Biochem Biophys Res Commun. 1993;193:1083–1090. doi: 10.1006/bbrc.1993.1736. [DOI] [PubMed] [Google Scholar]
- 87.Prohaszka Z, Nemet K, Csermely P, Hudecz F, Mezo G, Fust G. Defensins purified from human granulocytes bind C1q and activate the classical complement pathway like the transmembrane glycoprotein gp41 of HIV-1. Mol Immunol. 1997;34:809–816. doi: 10.1016/s0161-5890(97)00097-7. [DOI] [PubMed] [Google Scholar]