Abstract
How fisheries will be impacted by climate change is far from understood. While some fish populations may be able to escape global warming via range shifts, they cannot escape ocean acidification (OA), an inevitable consequence of the dissolution of anthropogenic carbon dioxide (CO2) emissions in marine waters. How ocean acidification affects population dynamics of commercially important fish species is critical for adapting management practices of exploited fish populations. Ocean acidification has been shown to impair fish larvae’s sensory abilities, affect the morphology of otoliths, cause tissue damage and cause behavioural changes. Here, we obtain first experimental mortality estimates for Atlantic cod larvae under OA and incorporate these effects into recruitment models. End-of-century levels of ocean acidification (~1100 μatm according to the IPCC RCP 8.5) resulted in a doubling of daily mortality rates compared to present-day CO2 concentrations during the first 25 days post hatching (dph), a critical phase for population recruitment. These results were consistent under different feeding regimes, stocking densities and in two cod populations (Western Baltic and Barents Sea stock). When mortality data were included into Ricker-type stock-recruitment models, recruitment was reduced to an average of 8 and 24% of current recruitment for the two populations, respectively. Our results highlight the importance of including vulnerable early life stages when addressing effects of climate change on fish stocks.
Introduction
The understanding of the effect of global change on fish populations is critical for sustainable exploitation and management of fisheries [1]. Ocean warming has already triggered poleward range shifts of many marine fish populations caused by their thermal tolerance [2–4]. However, higher latitudes provide no refuge with respect to the concomitant pH decline, caused by the dissolution of the major greenhouse gas CO2 in ocean waters. This “other CO2 problem”, also dubbed ocean acidification (OA) [5], is an inevitable consequence of anthropogenic release of CO2. The potential consequences of ocean acidification on commercially important fish populations are intensely debated [6,7], but currently unresolved since data on population-level processes, e.g. recruitment to the stock, are almost entirely lacking [8–10].
Adult fishes have been shown to tolerate extreme CO2 concentrations of up to 16,000 μatm [11], which led to the premature conclusion that fishes are less vulnerable to ocean acidification than for example calcifying organisms [12]. However, it is becoming increasingly evident that early life stages such as eggs and larvae are more susceptible to decreased ocean pH [7,13]. This is partly due to insufficient acid-base regulation prior to the formation of gills [14]. Recent studies have shown a diverse range of impacts of predicted future CO2 concentrations on larval fish, particularly on sensory abilities like olfaction [15], behaviour [16,17], otoliths [18–20], development, tissue and organ structure [13,21]. Studies also found effects on survival of eggs, more specifically hatching success [22], and survival of very early larval stages [7,23]. Other studies were not able to find an effect on survival [24,25].
Survival, however, is the most important parameter to assess recruitment, thus of paramount importance for stock management. Recruitment to an exploited fish stock is defined as that point of time when a year-class enters the fished population, i.e. at an age of 1 year in the case of Western Baltic cod, and at an age of 3 years in Barents Sea cod. Here we assess larval mortality as a key variable to predict population growth and size [26,27] in Atlantic cod (Gadus morhua, L.) under end-of-century CO2 concentrations. This is one of the most important species for commercial fisheries of the North Atlantic, It is of particular importance since landings of many cod stocks have decreased in the past decades with some stocks collapsing [28]. Any additional source of mortality, particularly one with a trend, should therefore be closely monitored and incorporated into management strategies.
We designed two experiments, in which the survival of cod larvae was quantified in direct response to increased pCO2 levels as predicted for the end of the century. Atmospheric CO2 concentrations have been continuously rising since the beginning of industrialisation and are currently exceeding 400 μatm. A third of the excess CO2 is absorbed by the world’s oceans, resulting in ocean acidification, leading to an estimated decrease in pH of 0.4 units (pCO2 ~ 1,000 μatm) by the end of the century [5,29,30]. Eggs and larvae from the Western Baltic cod stock, caught in the Øresund, and from the Arcto-Norwegian Barents Sea cod stock were kept under control (~400–500 μatm) and high CO2 (~1100 μatm) concentrations in two separate experiments until 25 and 22 days post-hatching (dph) respectively and survival was monitored closely.
Methods and Materials
For the Western Baltic experiment, adult cod were caught in the Øresund (55°58’N, 12°38’E) in March 2013 and strip-spawned. An equal volume of eggs was placed in 90 L rearing tanks at the Sven Lovén Centre, Kristineberg, Sweden. Three tanks were kept under ambient CO2 concentrations of 426 ± 47 μatm and three tanks were kept under increased CO2 conditions of 1033 ± 255 μatm. The temperature was kept constant at 7°C and the light regime was matched weekly to the ambient sun rise and sun set. After hatching the larvae were fed with natural plankton from the Gullmars Fjord under green water conditions with Nannochloropsis. (Food density estimates are given in Table A in S1 File). Survival was measured daily by collecting and counting all dead larvae from the bottom of the tanks. Initial number of larvae (on average ~800 larvae per tank) was then back-calculated to calculate survival in percentage. It was shown in separate experiments that dead larvae were easily found even after more than 24 hours post mortem in the tanks.
For the Barents Sea cod experiment adult fish were caught alive in the Barents Sea (70°15’N, 19°00’E) in March 2014 and transferred to the National Cod Breeding Centre, Tromsø. They were kept in large breeding tanks (25 m3) with flow-through from the fjord and at weekly matched ambient light regimes. All naturally produced eggs were collected using collectors behind the surface skimmer outflow. These were transferred to incubators with either ambient (503 ± 89 μatm CO2) or increased CO2 (1179 ± 87 μatm) concentrations. After peak hatch (more than 50% eggs hatched), 11,000 larvae were transferred into each of twelve 190 L rearing tanks with a constant flow-through of water from a common header tank. For the egg incubation and the start of the experiment the temperature was set to 6°C and was later raised to 10°C in all tanks at constant light conditions (24h). Larvae were fed with Nannochloropsis and Brachionus at different intervals for the high and the low food treatment (seven compared to three times daily), while the prey concentrations per feeding remained the same for both treatments. (For information on the feeding conditions, see Table B in S1 File). It should be noted, that even though the low food treatment only provided a fraction of the total amount of prey of the high food treatment, it is likely still higher than prey densities, which the larvae would experience in the field. However, this is difficult to compare, since we provided very high densities for short periods at the feeding times, which were then washed out of the tanks again. Therefore no steady density of prey was provided, but during feeding times prey densities were extremely high. This allowed for the exclusion of density and competition effects, which may have otherwise arisen due to different larval densities in the different treatments. Larvae in one tank in the ambient CO2 treatment were abruptly lost over night, due to an unknown factor, resulting in six replicates for the high CO2 treatment and five for the ambient treatment, each divided equally into the high and low food treatment. Starting on 8 dph survival was measured every four to six days by calculating the density of the larvae in the tanks. Five times 0.8 l of water was sampled from each tank over the whole water column using a pipe that could be closed at the bottom and the larvae contained in the pipe were subsequently counted in each sub sample. Prior to sampling an even distribution of larvae in the rearing tanks was achieved by increasing the aeration.
For both experiments the mean mortality coefficient was calculated after non-linear curve fitting of a negative exponential function for each replicate tank. Mean daily mortality rates (in percentage per day) were compared between treatments using a t-test (Western Baltic stock) and a two-way ANOVA (Barents Sea stock) after appropriate data transformation to achieve homogeneity of variances.
Ambient and increased CO2 levels were achieved by controlling the pH values in a header tank with pH sensors connected to an IKS computer system. If the values deviated from the set target pH a magnetic valve opened automatically, which allowed a pulse of CO2 from a CO2 bottle to be injected into the header tank. The volume of the header tank ensured a thorough mixing and equilibration of CO2 before the water entered the rearing tank thereby assuring constant conditions in the rearing tanks. The pH was furthermore manually checked every day in the rearing tanks with a separate pH sensor (WTW pH/Cond 340i/3320). Water chemistry, including DIC and alkalinity, was tested at the beginning and the end of the experiment for the Western Baltic cod experiment and weekly for the Barents Sea cod experiment based on the Best Practices Guide [31]. Further details regarding methods and carbon chemistry analysis are available in the Supporting Information.
All experiments were carried out in accordance to the national rules and regulations at the site of the experiments and all efforts where undertaken to minimize stress and suffering of the animals. Issues for work on vertebrate animals were obtained for each experiment and location. For the experiment in Kristineberg with the Western Baltic cod the ethics permit number is 332–2012 issued by the Swedish Board of Agriculture (Jordbruksverket). For the experiment in Tromsø on the Barents Sea cod the ethics permit number is FOTS ID 6382, issued by the Norwegian Animal Research Authority (Forsøksdyrutvalget). In accordance with these permits animals were euthanized after the experiment or whenever some were taken out for density measurements using Tricaine methanesulfonate (MS222). No endangered or protected species were used in these experiments and no other special permits were necessary.
Population level effects
Considering the potential impact of ocean acidification on fisheries requires scaling from physiological responses to population-level processes. A simple way is to consider how ocean acidification could modify the parameters of growth, mortality and reproduction in a single-species. Here we concentrate on the modification of the parameters of the stock-recruitment relationship in an age-structured fishery model.
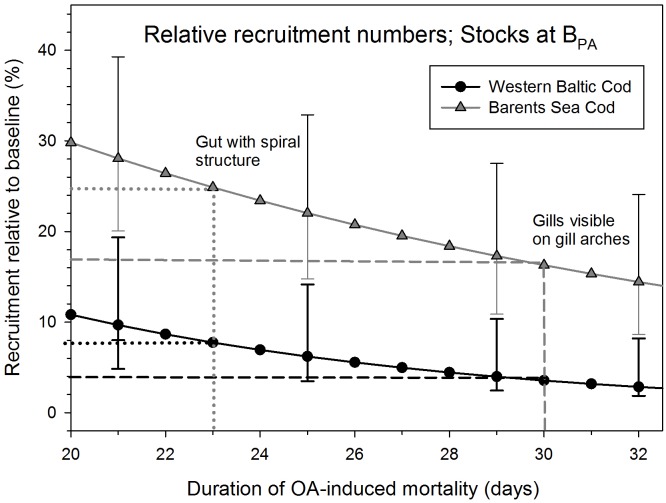
The effect of ocean acidification was assessed by modifying the density-independent parameter α of a Ricker type stock recruitment relationship. Ocean acidification causes a higher larval mortality rate. This leads to a density-independent mortality rate a caused by acidification. In the baseline scenario (no acidification) a = 0, while in the acidification scenarios, e-a is the fraction of larvae surviving the effect of acidification. We used our experimental data to quantify this effect, and to compare scenarios (See Supporting Information). We used ICES data for Western Baltic cod for the years 1970 to 2014 and for Arcto-Norwegian cod for the years 1946–2014 to estimate the stock-recruitment relationship for the baseline scenario. We assume log-normal auto-correlated errors, and estimated the model. (Further details regarding the recruitment models are available as Supporting Information.) Because the severity of ocean acidification induced mortality on recruitment depends on the duration of the additional mortality, two developmental stages were chosen as termination for the enhanced mortality [20]. Based on the experimental temperatures at day 23 days post hatching the larval gut has reached its typical spiral form (and potentially altered function) while at 30 dph gills become visible on the gill arches. These two time points were used to evaluate the effect of increased mortality on recruitment success assuming the same mortality estimates until 30 dph as shown in the experiments until 22 dph and 25 dph. Mortality during the recruitment process consists of both density-independent and density-dependent effects. For simplicity we assume that the effect of ocean acidification on the survival will only influence the density-independent mortality during the recruitment phase potentially biasing the data to be on the conservative side.
Results
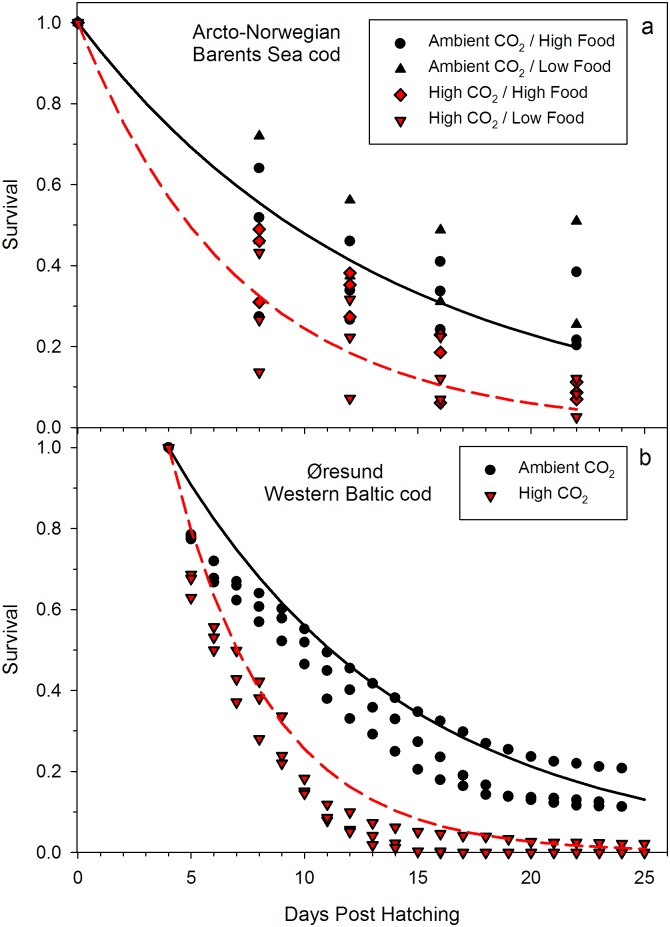
The effect of CO2 was consistent among stocks and experimental conditions, i.e. different feeding conditions. At increased CO2 concentrations the daily mortality rates had approximately doubled in both experiments, from 7 to 13% in the Barents Sea stock (Fig 1a) and from 9.2 to 20.4% in the Western Baltic Sea stock (Fig 1b) (Western Baltic experiment, T-test, t = -3.749, df = 2.41, p = 0.024; Barents Sea experiment Two-way ANOVA F = 8.434, df = 1, p = 0.023). In the Barents Sea experiment the food density had no detectable effect on mortality rate, neither as main effect nor in interaction with the CO2-treatment (for additional statistics, see Tables C and D in S1 File). Cod larvae therefore appear to be negatively affected by ocean acidification even when ad libitum prey densities should ensure that energy is available for potential acid-base regulation mechanisms.
Fig 1. Effect of increased CO2 on early life survival of Gadus morhua from (a) Barents Sea cod (b) Western Baltic cod.
Each symbol represents the value of one replicate tank. Lines depict the number of survivors according to the fitted negative exponential function.
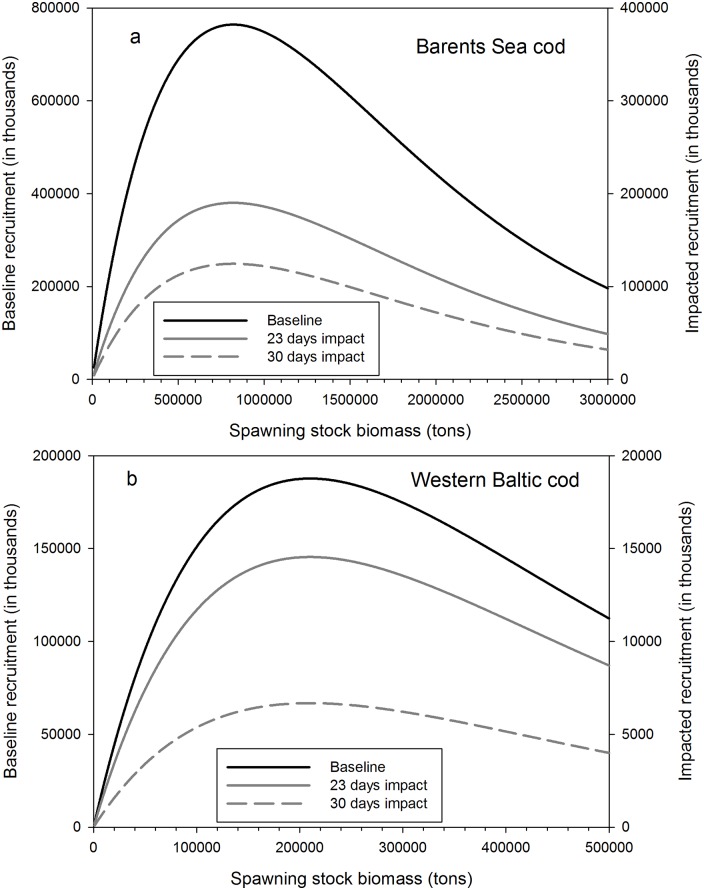
Next, the experimentally assessed larval mortality rates were incorporated into a Ricker-type stock-recruitment model that was parametrized for the two studied cod populations. We concentrated on altering the larval mortality in order to evaluate the overall stock-recruitment relationship to assess their effects on population dynamics (for details see Supporting Information). The model results show that for both mortality scenarios increased larval mortality due to ocean acidification will reduce recruitment substantially. Recruitment levels will be reduced on average to only 8% of the baseline scenario in the case of Western Baltic cod for ocean acidification-induced mortality periods of 23 days (and 4% for a mortality period of 30 days), and to 24.5% (and 17% respectively) in Arcto-Norwegian cod (Figs 2 and 3).
Fig 2. Recruitment functions under baseline and under ocean acidification scenarios for (a) the Barents Sea cod and (b) the Baltic Sea cod.
The baseline scenario is based on no OA and spawning stock biomass at ICES precautionary biomass levels (BPA) in dependence of the duration of OA-induced mortality. For better visualization a different scaling on the second y-axes was chosen for the impacted recruitment.
Fig 3. Population recruitment under ocean acidification (OA) for Western Baltic cod (black line and symbols) and Barents Sea cod (grey line and symbols).
Recruitment is given relative to a baseline scenario of no OA and spawning stock biomass at ICES precautionary biomass levels (BPA) in dependence of the duration of OA-induced mortality. Two important points in larval development are highlighted. Standard deviations displayed only for selected days to improve readability.
Discussion
Under realistic scenarios of end-of-century ocean acidification, early larval survival of cod was significantly reduced in two separate experiments with two different Atlantic cod stocks. Results were consistent under different feeding regimes and strongly suggest that there is a severe effect of ocean acidification on Atlantic cod larvae and recruitment.
Mass spawning fishes such as cod have many offspring with low survival probability in nature. The salient question is whether our experimental conditions provide appropriate controls with reasonable natural mortality levels. Larval survival rates are naturally low even under ambient CO2 concentrations and optimal feeding conditions. The mortality is mainly caused by the difficulty in a successful first feeding once the yolk sac is absorbed [27]. Other studies find similar mortality rates as our control values in the two experiments during early larval development [32,33]. Survival of larvae in our experiment from the Western Baltic stock was lower than for the Barents Sea stock, since they were fed with natural plankton in concentrations as provided by the fjord, while the larvae from the Barents Sea stock were kept under aquaculture conditions aiming for the production of the highest numbers of fingerlings for stocking of industrial scale production net pens.
Larval fish survival under ocean acidification has so far been shown in only one other study by Baumann et al. (2012) [7], albeit in a non-commercial fish species, the Atlantic silverside (Menidia menidia). In their study reduced larval survival was observed at 1100 ppm, a level of ocean acidification, which is predicted to occur globally at the start of the next century under the IPCC RCP 8.5, during the first week post hatch. Chambers et al. (2013) [22] found a decreased hatching success (reflecting embryonic development) of the summer flounder by 50% under 1860 ppm. This is a realistic ocean acidification level for the environment of this species within this century, even though values on a global average are predicted to be lower. Munday et al. (2015) [25] found no effect on the survival of yellowtail kingfish larvae. Other studies, like Munday et al. (2009b) [24]; Franke & Clemmesen (2011) [34]; Frommel et al. (2013) [35]; Hurst et al. (2013, 2015) [36,37], have addressed hatching success and have not seen any effects of ocean acidification. We are confident that this does not necessarily indicate that these species will not be affected or that our results present a contradiction. It is well known that early life stages of marine fish go through several bottlenecks with high mortalities during development and that different populations of the same species can react differently to CO2 stress [35]. Our results show that the first days and weeks after hatching are a vulnerable phase to ocean acidification. So far studies on tropical fish have not seen an ocean acidification effect on survival [38]. This is not surprising, since early development in the studied species is very different from temperate fish and newly hatched larvae are further developed and physiologically more competent thus less vulnerable to physiological stressors. Furthermore the study by Munday et al. (2011), and other studies like Hurst et al. (2013), only quantified survival at a single day, which may not have been the final day of any additional mortality. Additionally, even if this was an end-point measurement, it does not allow for calculations of mortality rates.
One factor that this study is not taking into account is possibility that parental exposure to the high CO2 environment could limit the adverse effects of ocean acidification. This kind of transgenerational adaptation has been shown to mediate negative growth effects of OA in tropical reef fish [39]. However since most commercially important fish species are quite large and temperate fish species reach sexual maturity late, it will be difficult to perform experiments with long parental exposure time. Furthermore it cannot be ruled out, that ocean acidification might also have an additional negative effect on gonadal development in adult fishes, which might further reduce recruitment potential.
Range shifts are responses of many fish populations to track the poleward movement of their thermal range [2]. Unfortunately, this may exacerbate direct CO2 effects identified here, since oceanic waters in higher latitudes will take up more CO2 due to higher solubility and experience lower carbonate saturation [40]. Previously, ocean acidification has been shown to affect marine fish larvae’s sensory abilities, morphology of the otoliths, cause tissue damage and behavioural differences [13,17,18,19,21].
Here we give the first demographic estimates for Atlantic cod under realistic end-of-century ocean acidification levels which are urgently needed to estimate whether these exploited fish populations could potentially expect population declines as a direct consequence of ocean acidification. The estimated recruitment declines shown are severe, of similar magnitude as population collapses due to overfishing [41] and have highly significant implications for the governance of exploited fish populations. We show that indeed, increased mortality will affect recruitment at the population level, demonstrating that any future management of exploitation must directly consider effects induced by global change.
Supporting Information
(DOCX)
Acknowledgments
Funding was provided through the BIOACID project (BIOlogical Impacts of Ocean ACIDification) funded by the German Ministry for Education and Research (BMBF), the AquaExcel transnational access grant for aquaculture infrastructures and the Bonus Baltic Sea research and development programme (Art 185) BIO-C3 project, funded jointly by the EU and the BMBF (Grant No. 03F0682A). The Western Baltic cod experiment was conducted at the Sven Lovén Centre, Kristineberg, Sweden. The Barents Sea cod experiment was conducted at the Norwegian Cod Breeding Centre, NOFIMA, Tromsø, Norway. The authors are grateful to all scientists and staff at both research stations.
Data Availability
All data files are available from the pangea database under https://doi.pangaea.de/10.1594/PANGAEA.858616.
Funding Statement
Funding was provided through the BIOACID project (BIOlogical Impacts of Ocean ACIDification) funded by the German Ministry for Education and Research (BMBF), http://www.bioacid.de/; the AquaExcel transnational access grant for aquaculture infrastructures, http://www.aquaexcel.eu/; and the Bonus Baltic Sea research and development programme (Art 185) BIO-C3 project, funded jointly by the EU and the BMBF (Grant No. 03F0682A), https://www.bio-c3.eu/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.MacNeil MA, Graham NAJ, Cinner JE, Dulvy NK, Loring PA, Jennings S, et al. Transitional states in marine fisheries: adapting to predicted global change. Phil Trans R Soc B. 2010;365:3753–63. 10.1098/rstb.2010.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry AL, Low PJ, Ellis JR, Reynolds JD. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–5. [DOI] [PubMed] [Google Scholar]
- 3.Poloczanska ES, Brown CJ, Sydeman WJ, Kiessling W, Schoeman DS, Moore PJ, et al. Global imprint of climate change on marine life. Nat Clim Chang. 2013;3(10):919–25. [Google Scholar]
- 4.Pörtner H-O. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol. 2010;213(6):881–93. 10.1242/jeb.037523 [DOI] [PubMed] [Google Scholar]
- 5.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean Acidification: The Other CO2 Problem. Annu Rev Mar Sci. 2009;1(1):169–92. [DOI] [PubMed] [Google Scholar]
- 6.Lam VWY, Cheung WWL, Sumaila UR. Marine capture fisheries in the Arctic: winners or losers under climate change and ocean acidification? Fish Fish. 2014; [Google Scholar]
- 7.Baumann H, Talmage SC, Gobler CJ. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Chang. 2012;2(1):38–41. [Google Scholar]
- 8.Haigh R, Ianson D, Holt CA, Neate HE, Edwards AM. Effects of Ocean Acidification on Temperate Coastal Marine Ecosystems and Fisheries in the Northeast Pacific. PLoS One. 2015;10(2):e0117533 10.1371/journal.pone.0117533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung WWL, Pinnegar J, Merino G, Jones MC, Barange M. Review of climate change impacts on marine fisheries in the UK and Ireland. Aquatic Conserv: Mar Freshw Ecosyst. 2012;22(3):368–88. [Google Scholar]
- 10.Denman K, Christian JR, Steiner N, Pörtner H-O, Nojiri Y. Potential impacts of future ocean acidification on marine ecosystems and fisheries: current knowledge and recommendations for future research. ICES J Mar Sci. 2011;68(6):1019–29. [Google Scholar]
- 11.Ishimatsu A, Hayashi M, Kikkawa T. Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser. 2008;373(1):295–302. [Google Scholar]
- 12.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, et al. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob Chang Biol. 2013;19(6):1884–96. 10.1111/gcb.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, et al. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Chang. 2012;2(1):42–6. [Google Scholar]
- 14.Falk-Petersen IB. Comparative organ differentiation during early life stages of marine fish. Fish Shellfish Immun. 2005; 19:397–412. [DOI] [PubMed] [Google Scholar]
- 15.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA. 2009;106(6):1848–52. 10.1073/pnas.0809996106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixson DL, Pratchett MS, Munday PL. Reef fishes innately distinguish predators based on olfactory cues associated with recent prey items rather than individual species. Anim Behav. 2012;84(1):45–51. [Google Scholar]
- 17.Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP. Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA. 2010;107:12930–4. 10.1073/pnas.1004519107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. Elevated CO2 enhances otolith growth in young fish. Science. 2009. June 26;324:1683 10.1126/science.1169806 [DOI] [PubMed] [Google Scholar]
- 19.Bignami S, Enochs IC, Manzello DP, Sponaugle S, Cowen RK. Ocean acidification alters the otoliths of a pantropical fish species with implications for sensory function. Proc Natl Acad Sci USA. 2013;110(18):7366–70. 10.1073/pnas.1301365110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maneja RH, Frommel AY, Geffen AJ, Folkvord A, Piatkowski U, Chang M, et al. Effects of ocean acidification on the calcification of otoliths of larval Atlantic cod Gadus morhua. Mar Ecol Prog Ser. 2013;477:251–8. [Google Scholar]
- 21.Frommel AY, Maneja R, Lowe D, Pascoe CK, Geffen AJ, Folkvord A, et al. Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol Appl. 2014;24(5):1131–43. [DOI] [PubMed] [Google Scholar]
- 22.Chambers RC, Candelmo AC, Habeck EA, Poach ME, Wieczorek D, Cooper KR, et al. Ocean acidification effects in the early life-stages of summer flounder, Paralichthys dentatus. Biogeosciences Discuss. 2013;10:13897–929. [Google Scholar]
- 23.Bromhead D, Scholey V, Nicol S, Margulies D, Wexler J, Stein M, et al. The potential impact of ocean acidification upon eggs and larvae of yellowfin tuna (Thunnus albacares). Deep Sea Res Part II. 2015. March;113:268–79. [Google Scholar]
- 24.Munday PL, Donelson JM, Dixson DL, Endo GGK. Effects of ocean acidification on the early life history of a tropical marine fish. Proc R Soc B. 2009;276:3275–83. 10.1098/rspb.2009.0784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munday PL, Watson S-A, Parsons DM, King A, Barr NG, Mcleod IM, et al. Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J Mar Sci. 2016;73(3):641–649 [Google Scholar]
- 26.Houde ED. Emerging from Hjort’s Shadow. J Northw Atl Fish Sci. 2008;41:53–70. [Google Scholar]
- 27.Llopiz JK, Cowen RK, Hauff MJ, Ji R, Munday PL, Muhling BA, et al. Early Life History and Fisheries Oceanography: New Questions in a Changing World. Oceanography. 2014;27(4):26–41. [Google Scholar]
- 28.Pauly D, Christensen V, Guénette S, Pitcher TJ, Sumaila UR, Walters CJ, et al. Towards sustainability in world fisheries. Nature. 2002;418:689–95. [DOI] [PubMed] [Google Scholar]
- 29.Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365 [DOI] [PubMed] [Google Scholar]
- 30.IPCC. Climate change 2007: The Physical Science Basis Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al. , editors. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 31.Riebesell U, Fabry VJ, Hansson L, Gattuso J-P (Eds.). Guide to Best Practices in Ocean Acidification Research and Data Reporting. Luxembourg: Publications Office of the European Union; 2010. 260 p. [Google Scholar]
- 32.Puvanendran V, Brown JA. Foraging, growth and survival of Atlantic cod larvae reared in different prey concentrations. Aquaculture. 1999;175:77–92. [Google Scholar]
- 33.Van der Meeren T, Mangor-Jensen A, Pickova J. The effect of green water and light intensity on survival, growth and lipid composition in Atlantic cod (Gadus morhua) during intensive larval rearing. Aquaculture. 2007;265:206–17. [Google Scholar]
- 34.Franke A, Clemmesen C. Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences. 2011;8:3697–707. [Google Scholar]
- 35.Frommel AY, Schubert A, Piatkowski U, Clemmesen C. Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol. 2013;160:1825–34. [Google Scholar]
- 36.Hurst TP, Fernandez ER, Mathis JT. Effects of ocean acidification on hatch size and larval growth of walleye pollock (Theragra chalcogramma). ICES J Mar Sci. 2013;70(4):812–22. [Google Scholar]
- 37.Hurst TP, Laurel BJ, Mathis JT, Tobosa LR. Effects of elevated CO2 levels on eggs and larvae of a North Pacific flatfish. ICES J Mar Sci. 2015;71:982–90. [Google Scholar]
- 38.Munday PL, Gagliano M, Donelson JM, Dixson DL, Thorrold SR. Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol Prog Ser. 2011;423:211–221 [Google Scholar]
- 39.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Chang. 2012;2(12):858–61. [Google Scholar]
- 40.Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–6. [DOI] [PubMed] [Google Scholar]
- 41.Pinsky ML, Jensen OP, Ricard D, Palumbi SR. Unexpected patterns of fisheries collapse in the world’s oceans. Proc Natl Acad Sci USA. 2011;108(20):8317–22. 10.1073/pnas.1015313108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All data files are available from the pangea database under https://doi.pangaea.de/10.1594/PANGAEA.858616.