Abstract
Purpose
To evaluate costs of panretinal photocoagulation (PRP) vs. intravitreal ranibizumab (IVR) for proliferative diabetic retinopathy (PDR).
Design
A Markov-style model of cost-effectiveness and cost utility.
Participants
There were no participants.
Methods
Based on results from Diabetic Retinopathy Clinical Research (DRCR) Network Protocol S, we performed a Markov-style analysis to generate the total 2-year costs for each treatment arm. The cost per line-year saved and cost utility were calculated based on the estimated life years remaining. Both treatment arms were assumed to result in 9 lines of vision saved in 20% of patients. Medicare reimbursement data were acquired to determine costs, which were then separately calculated for practice settings of a hospital-based facility as the highest end of the cost range and a nonfacility in the same geographic area as the lowest end. Cost parameters for a prototypical patient's life expectancy also were modeled and calculated.
Main Outcome Measures
Inputed cost of therapy, cost per line saved, cost per line-year saved, and cost per quality-adjusted life years (QALY).
Results
When PRP was the primary treatment, the 2-year cost in the facility setting was $13 053, with cost per line saved $7252, cost per line-year $240, and cost per QALY $7988. In the nonfacility setting costs were approximately 21% lower. When IVR was the primary treatment, the 2-year cost in the facility setting was $30 328, cost per line saved was $16 849, cost per line-year $575, and cost per QALY $19 150. In the nonfacility setting costs were approximately 15% lower. Extrapolation to lifetime therapy yielded the cost per QALY with PRP treatment of $14 219 to $24 005 and with IVR of $138 852 to $164 360. Cost utility for PRP would be 85% lower than IVR in the facility setting and 90% lower than IVR in the nonfacility setting.
Conclusions
PRP compared with IVR as primary treatment for PDR is less expensive over 2 years, but both fall well below the accepted cost per QALY upper limit. However, over an average lifetime, the cost differential between PRP and IVR increases, and IVR therapy may exceed the typical accepted limit of cost per QALY.
Diabetic retinopathy is a prevalent disease affecting more than 8 million Americans, with a predicted increase of 35% by 2032.1 In the United States, the economic impact of retinopathy-associated morbidity is estimated at more than $620 million per year.2 The Diabetic Retinopathy Study (DRS) data demonstrated a 50% reduction in severe visual loss, defined as visual acuity (VA) <5/200 at 2 or more consecutively completed visits at 4-month intervals, in eyes that received photocoagulation.3
The first-line treatment for diabetic retinopathy is principally preventive—dmaximal control of the metabolic dysfunction of the disease. In contrast, proliferative diabetic retinopathy (PDR) warrants panretinal photocoagulation (PRP) not only to prevent future progression but also to effect regression of existing proliferative disease, with the goal of maximizing visual function. Panretinal photocoagulation was established as the standard of care after the DRS results more than 30 years ago.4 Its principal mechanism of action seems to occur via reducing vascular endothelial growth factor (VEGF).5,6 Indeed, intravitreal anti-VEGF therapy has become recognized as a feasible alternative to treatment of PDR during the past 10 years.7,8
Ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA) is a recombinant, humanized, monoclonal antibody fragment that binds and inactivates all active iso-forms of human VEGF-A, which has been implicated in promoting neovascularization.9,10 Treatment with intravitreal ranibizumab (IVR) has been shown to have a synergistic effect with PRP and most recently has been shown to be noninferior to PRP in regard to improvement in VA.11,12 In addition, anti-VEGF agents reduce the risk of worsening diabetic retinopathy in patients with diabetic macular edema (DME) as well,13,14 although the utility of that potential benefit has not been delineated. Furthermore, DME often coincides with PDR and is subject to anti-VEGF treatment. The obvious concerns with the paradigm shift in treating PDR are that chronic anti-VEGF therapy is expensive and may have deleterious side effects.
The purpose of this study was to calculate the parameters of cost-effectiveness using a Markov-style decision-tree analysis for treatment of PDR with PRP and IVR.
Methods
This cost-utility analysis incorporated published use and outcomes data from the Diabetic Retinopathy Clinical Research (DRCR) Network Protocol S, a randomized clinical trial comparing PRP with IVR as the primary treatment for PDR.12 The basis of the current study was to calculate costs of 2 different scenarios using a Markov-style decision analysis (Fig 1). Institutional review board approval was not required because there were no research participants or medical records reviewed. Our model used the Protocol S results as an index study to quantitate cost parameters using the mean number of treatments of PRP and IVR, as well as focal laser and pars plana vitrectomy (PPV), for a patient with PDR.
Figure 1.
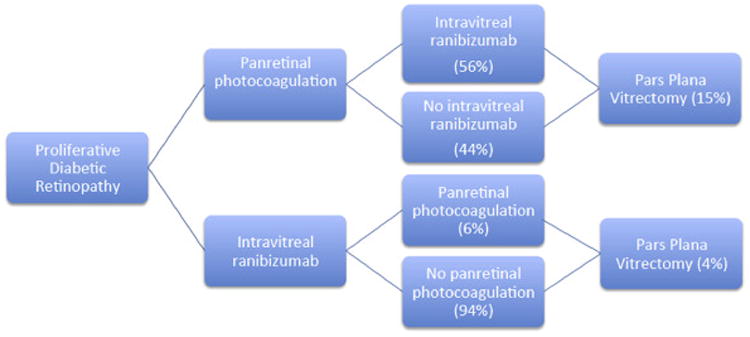
Decision model used for Markov-style analysis. Focal laser not shown.
Medicare fee data from 2016 were acquired from the Centers for Medicare and Medicaid Services (CMS) to ascertain the allowable cost (in US dollars) associated with each procedure, injection, study, or office visit.15—17 Costs were calculated for both a hospital-based facility (with any necessary PPV surgery performed in a hospital operating room) and a nonfacility (i.e., office-based clinical services with vitrectomy performed in an ambulatory surgery center) in the same geographic area to demonstrate the range of potential reimbursement settings. Professional fees (including the use of an anesthesiologist in the operating room for the fraction needing PPV) and facility fees were included in the calculations.
The dollars conversion factor per relative value unit (RVU) used was $35.82, the established rate for 2016.18 The equation used to calculate the cost for a given provider service included work RVUs in the form of professional fees, practice expense RVUs, and malpractice RVUs. Each of these factors was subject to geographic modifiers; the rates for New York, New York, were used in this analysis.15
On the basis of the index study,12 a Markov-style decision analysis was used to generate a total cost for each treatment arm.19 The primary analysis incorporated the costs over 2 years, as encountered in the index study, and assumed no other costs would be incurred and that the VA would remain stable beyond that interval until the end of the patient's life expectancy. In scenario 1, the initial treatment was PRP, whereas in scenario 2 the initial treatment was IVR. Costs for patients who received secondary injections of ranibizumab in the PRP arm were accounted for, as well as those who received secondary PRP in the IVR arm.
The Current Procedural Terminology (CPT) codes used for the procedures were as follows: 67228 for PRP, 67028 for intravitreal injection, 67210 for focal laser, and 67040 for PPV with laser (Table 1). A comprehensive eye code (CPT 92004) was included as the initial visit, with subsequent follow-up visits calculated using the intermediate eye code (CPT 92012). The Healthcare Common Procedure Coding System code used for ranibizumab was J2778. The reimbursement schedules for procedures were based on the CMS terminology for procedures performed in the facility and nonfacility settings.
Table 1. Medicare Allowable Costs For Panretinal Photocoagulation/Intravitreal Ranibizumab And Associated Treatments.
| Procedure | CPT Code | Facility | Nonfacility | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Professional Fee | Facility Fee | Anesthesia | Total | Professional Fee | Facility Fee | Anesthesia | Total | ||
| PRP | 67228 | $354 | $440 | $0 | $794 | $393 | $0 | $0 | $393 |
| Intravitreal injection | 67028 | $116 | $280 | $0 | $396 | $117 | $0 | $0 | $117 |
| OCT | 92134 | $51 | $56 | $0 | $107 | $51 | $0 | $0 | $51 |
| Fundus photography | 92250 | $91 | $91 | $0 | $182 | $91 | $0 | $0 | $91 |
| Focal laser | 67210 | $578 | $440 | $0 | $1018 | $598 | $0 | $0 | $598 |
| PPV with PRP | 67040 | $1203 | $3381 | $250 | $4834 | $1204 | $1711 | $250 | $3165 |
| New comprehensive eye examination | 92004 | $111 | $102 | $0 | $213 | $168 | $0 | $0 | $168 |
| Intermediate follow-up examination | 92012 | $59 | $102 | $0 | $162 | $97 | $0 | $0 | $97 |
CPT = Current Procedural Terminology; OCT = optical coherence tomography; PPV = pars plana vitrectomy; PRP = panretinal photocoagulation.
Reimbursement per 0.1-mg unit of ranibizumab is $388 according to the 2016 CMS reimbursement rates.20 A drug maintenance cost of 6% of the medication cost was applied to the overall injection cost in each group. As used in this study, a dose of ranibizumab 0.5 mg would cost $1938, and the 6% stocking fee would be $116. In the facility setting, the cost was considered to be both the professional and hospital fees; in the nonfacility setting, the cost was limited to the professional fee because there were no facility charges for laser or injections. It was assumed that all injections were done in the office setting, so no anesthesia professional fees were applied.
The DRS defined severe visual loss in untreated eyes as visual acuity of 5/200 or worse, so the number of lines saved would be 9 if we were to assume that 20/100 vision would be otherwise maintained.4,21 Data from this study showed a 20 percentage point differential benefit: 0.2×9 lines = 1.8 lines saved.4 An average age of 51 years in the PRP group and 52 years in the IVR group was used, with 43% and 45% female patients within those groups, respectively.12 The years of life expectancy were obtained from the actuarial tables of the Social Security Administration and weighted by gender.22 Quality-adjusted life year (QALY) data were adapted from previously published articles; a conversion of 0.03 QALY per line-year of vision saved was applied.23 In all cases, the VA of the treated eye was assumed to be equal to or worse than that of the fellow eye.
Whereas the cost analysis of this study assumes there were no additional costs after 2 years, more “real-world” application prompted estimates of additional treatment beyond this period; calculations assumed a 5% re-treatment rate per year for 2 years for additional PRP and an average of 2 yearly injections carried out over the years remaining. With the likelihood of decreasing injection burden for subsequent years and without knowing what the lasting effects of IVR injections are over a patient's lifetime, 2 injections per year for the years of life remaining is a conservative estimate. Beyond 2 years, the PPV rates were assumed to be 5% per year for 2 additional years in the PRP group and 2.5% per year for 2 additional years in the IVR group. These future costs per QALY factored in a standard 3% inflation rate per year. Calculations and analyses were performed using Microsoft Excel (Microsoft Corp., Seattle, WA).
Results
The assumed costs were calculated for each procedure, including the facility and professional fees (Table 1). Estimated frequency of resource use was based on the average number of treatments over 2 years in the PRP or IVR arm (Table 2), because this was the duration of Protocol S follow-up. These results yielded cost-utility measures (Table 3).
Table 2. Estimated Use of Resources Based on Initial Procedure over 2 Years.
| Procedure | CPT Code | Scenario 1, PRP | Scenario 2, IVR |
|---|---|---|---|
| New comprehensive eye examination | 92004 | 1 | 1 |
| Intermediate follow-up examination | 92012 | 5.5 | 15.25 |
| PRP | 67228 | 1.5 | 0.06 |
| Intravitreal injection | 67028 | 3.7 | 10.8 |
| OCT | 92134 | 3 | 3 |
| Fundus photography | 92250 | 3 | 3 |
| Focal laser | 67210 | 0.1 | 0.08 |
| PPV with PRP | 67040 | 0.15 | 0.04 |
CPT = Current Procedural Terminology; IVR = intravitreal injection ranibizumab; OCT = optical coherence tomography; PPV = pars plana vitrectomy; PRP = panretinal photocoagulation.
Table 3. Cost per Line-Years Saved and Cost per Quality-Adjusted Life Year (Intravitreal Injection Ranibizumab 0.5 mg).
| Primary Procedure | Lines Saved | Mean Age | Years Remaining (Average) | Facility Billing | Nonfacility Billing | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Cost per Line Saved | Cost per Line-Year Saved | Cost per QALY | Cost per Line Saved | Cost per Line-Year Saved | Cost per QALY | ||||
| PRP | 9 | 51 | 30.26 | $7252 | $240 | $7988 | $5717 | $189 | $6297 |
| IVR | 9 | 52 | 29.33 | $16 849 | $575 | $19 150 | $14 287 | $487 | $16 238 |
IVR = intravitreal ranibizumab; PRP = panretinal photocoagulation; QALY = quality-adjusted life years.
Scenario 1, Panretinal Photocoagulation as Primary Treatment
For facility billing with hospital surgery, the imputed 2-year cost for treatment was $13 053. The cost per line of vision saved was $7252, whereas the cost per line-year saved was $240. The cost per QALY was $7988.
In the nonfacility setting, the imputed 2-year cost for the same duration of treatment was $10 290. The cost per line of vision saved was $5717. The cost per line-year saved was $189, and the cost per QALY was $6297.
Scenario 2, Intravitreal Ranibizumab as Primary Treatment
In the facility setting with hospital surgery, primary treatment with IVR yielded an imputed 2-year cost of $30 328. The cost per line saved was $16 849, the cost per line-year saved was $575, and the cost per QALY was $19 150.
In the nonfacility setting, the imputed 2-year cost for ranibizumab was $25 716. The cost per line was $14 287, the cost per line-year saved was $487, and the cost per QALY was $16 238.
This cost analysis used the Food and Drug Administration–approved ranibizumab dosage of 0.5 mg, which was different from the 0.3 mg labeled for use in PDR associated with DME, but follows the protocol used in Protocol S.12 Because providers can only give ranibizumab at the 0.3-mg dose in the United States, cost utilities for this dose also were calculated (Table 4). A dose of ranibizumab 0.3 mg would cost $1163 with the 6% stocking fee of $70. Using these numbers, the cost difference between 0.5 mg and 0.3 mg would be a reduction of $1860 per QALY in the PRP treatment arm (decrease of 23% in facility and 30% in nonfacility) and a reduction of $5602 per QALY in the IVR treatment arm (decrease of 29% in facility and 34% in nonfacility).
Table 4. Cost per Line-Years Saved and Cost per Quality-Adjusted Life Year (Intravitreal Injection Ranibizumab 0.3 mg).
| Primary Procedure | Lines Saved | Mean Age (Yrs) | Years Remaining (Average) | Facility Billing | Nonfacility Billing | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Cost per Line Saved | Cost per Line-Year Saved | Cost per QALY | Cost per Line Saved | Cost per Line-Year Saved | Cost per QALY | ||||
| PRP | 9 | 51 | 30.26 | $5563 | $184 | $6128 | $4028 | $133 | $4437 |
| IVR | 9 | 52 | 29.33 | $11 920 | $406 | $13 548 | $9358 | $319 | $10 636 |
IVR = intravitreal ranibizumab; PRP = panretinal photocoagulation; QALY = quality-adjusted life years.
An analysis substituting intravitreal bevacizumab (IVB), assuming a cost of $100 per 1.25-mg dose and assuming the same number of injections and same effects, also was calculated. This yielded a 2-year cost per QALY of $5864 in the facility setting, a 69% decrease compared with IVR. In the nonfacility setting, the cost per QALY of IVB over 2 years was $2952, an 82% decrease compared with IVR.
The “real-world” extended PRP treatment beyond the initial 2-year period yielded an additional annual cost of $861 over the second 2 years (including 5% annual PRP re-treatment rate and 5% annual PPV rate), whereas office visits and imaging would add $579 yearly after in the facility setting; over the average number of life years remaining, this yields a total cost per QALY of $24 005. In the nonfacility setting, the additional annual cost of PRP would be $462 over the second 2 years and $284 yearly after, yielding a lifetime cost per QALY of $14 219. If primary treatment with IVR was to require 2 average injections per year after the initial 2-year follow-up period, this would cost an additional $5600 per year for the second 2 years (including 2.5% annual PPV rate) and $5479 yearly thereafter in the facility setting, resulting in a cost per QALY of $164 360 over the average number of life years remaining. In the nonfacility setting, treatment with IVR would cost an additional $4706 for the second 2 years and $4627 per year annually thereafter, yielding a lifetime cost per QALY of $138 852 (Fig 2).
Figure 2.
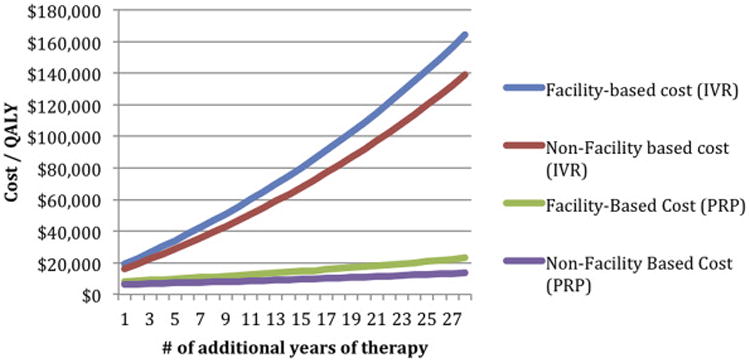
Estimated cost utility of additional therapy beyond 2 years (panretinal photocoagulation [PRP] and intravitreal injection ranibizumab [IVR]). QALY = quality-adjusted life year.
For treatment with IVB beyond 2 years, assuming the identical number of treatments needed as in IVR, cost per QALY over the average number of life years remaining in the facility setting would be $47 915, whereas in the nonfacility setting this would be $22 407. This would make treatment with IVB 71% to 84% less than IVR over a lifetime, and primary treatment with PRP would be 37% to 50% less than IVB.
Discussion
This study used Markov-style modeling of the costs of primary treatment of PDR and found a lower cost utility using PRP as the initial treatment in both the facility and nonfacility settings, yielding a cost per QALY 58% to 61% lower than that for IVR as the primary treatment over the first 2 years. This suggests that based on cost-based measures, PRP is still the less-expensive therapy for patients with PDR regardless of the practice setting. However, both PRP ($6297–$7988) and IVR ($16 238–$19 150) initial treatments are well below the QALY value considered by health policymakers ($50 000–$100 000) to be acceptable.24 Furthermore, because the baseline and final visual acuities were approximately 5 lines better (median VA of ∼20/32) in the DRCR study 12 compared with the DRS, the cost outcome parameters might be approximately 30% lower. The cost utility of intravitreal therapy in this study compares favorably to that in studies that evaluated the use of intravitreal therapy in DME, because PDR therapy prevents a higher magnitude of vision loss and requires fewer injections.25,26
However, the primary analysis included only 2 years of treatment and assumes a durable lifetime treatment effect without additional costs. Considering the more likely application of anti-VEGF therapy for the patient's lifetime, the cost increases, and more so than for PRP-based treatment. The current analysis assumed diabetic patients to have a life expectancy (and need for future treatment) equivalent to that of the general population. Although this assumption may not be far from the truth, one study estimated a 10% to 20% shorter life expectancy, so costs per QALY would be higher in the 2-year model and beyond for both therapies.27,28
The practical and cost advantages of PRP are that fewer visits are required, which may also lessen the impact of visit noncompliance possible with IVR. Once PRP is complete and PDR is stabilized, additional treatments for PDR generally are not needed, whereas the duration of treatment necessary with IVR is unknown and likely indefinite.29,30 Continued follow-up needs for PRP-treated patients are less extensive (and being lost to follow-up less consequential with PRP compared with IVR treatment).
A disadvantage of PRP is that it is a destructive treatment by nature, which is manifested by peripheral visual field loss, noted in Protocol S and the DRS, in which eyes treated with xenon had more severe visual field loss compared with eyes treated with argon laser.12,31 The possibility and utility implications of induced glaucoma or PRP-induced visual field loss were beyond the scope of this study, but we would estimate that it would likely play a small factor.32–34 In addition, more vitrectomies were performed in the PRP compared with the IVR group,12 although the 15% rate seems higher than in our experience.
A factor that Protocol S introduced was the coexistence of PDR and DME, which has a confounding effect on evaluating these treatment benefits because IVR has the dual advantage of being a useful treatment of DME and PDR. Substantial numbers of patients had coexisting baseline DME in the DRCR study (23% in the PRP group and 22% in the IVR group).12 The concurrent treatment of DME with anti-VEGF therapy in both groups explains why the cost differential between the 2 treatments is less disparate than one might intuitively presume. Intravitreal ranibizumab can improve VA and vision-related quality of life in a substantial fraction of patients, and coincident use may even prevent (as opposed to simply treating existing) some DME.35 Considering costs, VEGF inhibitors with or without laser treatment for DME compared more favorably with laser or triamcinolone alone.36 Thus, using IVR to treat both DME and PDR furthers its benefit. Although more than half of patients being treated with PRP eventually require intravitreal injections (presumably as they develop DME), only 6% of subjects in the IVR treatment arm from Protocol S needed PRP.12 Retinopathy grade regressed in the IVR group more than in the PRP group, but the clinical value awaits quantitation.
Estimates extending treatment beyond the 2-year period of the DRCR study demonstrate the substantial additional cost of chronic injection-based therapy, which decreases the cost utility; PRP yields an 85% decrease (facility) and a 90% decrease (nonfacility) in the average lifetime cost per QALY compared with IVR. Treatment with IVR would take 18 to 21 years after the initial 2-year treatment period to exceed the $100 000 cost-utility threshold. This might suggest targeting younger patients with PRP and older patients with IVR from a cost-utility standpoint. Although there were no significant safety concerns identified in the IVR group, experience with larger studies of IVR (or other anti-VEGF injections) suggests a finite adverse event rate (i.e., endophthalmitis, lens damage) that may be higher than PRP, including events in the Medical Dictionary for Regulatory Activities system organ classes of cardiac, endocrine, infections/infestations, respiratory, skin, and surgical disorders.12 Although PRP carries no risk of endophthalmitis or systemic exposure to anti-VEGF therapy, it has been suggested that long-term treatment with anti-VEGF agents may increase the possibility of death and cerebrovascular accidents,37 although this has not yet been fully substantiated.
An alternative strategy that might reduce costs for intravitreal anti-VEGF therapy could be to substitute off-label use of IVB for IVR (69% facility and 82% non-facility reduction of costs per QALY of IVB over IVR), which also has been reported to be cost-effective in the treatment of DME.38 Cost estimates for lifetime IVB therapy compared more favorably to PRP, staying well below the $100 000 cost-utility threshold. Yet the use of formulated, off-label use has been met with implementation hurdles constructed in the spirit of enhancing patient safety.
Study Limitations
The limitations of this type of financial modeling are substantial. Although the primary model carries limitations primarily propagated from time and protocol limitations of the DRCR study, transferability of any study results to the general population is not exact, but likely would lead to a lower cost due to lower resource use. In addition, the current model was based on Medicare costs in New York; a rough estimate is that if New York represents the high end of allowable costs, the lowest end introduces only approximately a 10% cost reduction and would be congruous between the 2 groups.
Moreover, the analysis of full-term treatment is contingent on the accuracy of the current study's assumptions, most importantly regarding the need and duration of ongoing injections, which this study could only estimate at 2 per year. The management of diabetic patients is multifaceted; thus, the true cost utility of treatment for PDR may not be completely reflected. The DRCR study population as a group seems to have better sight than the DRS patients, which, as noted earlier, would underestimate the utility of both treatment options, reducing cost per QALY estimates even more. In addition, type 1 and type 2 diabetic patients were not stratified; the differential cost utility of PRP for patients with onset of disease at a younger age would be expected to be greater because of longer treatment durations.
The results of this study should be seen as providing some insight into the cost arm of the “triple aim” of health care goals, assuming the validity of the conclusion of the Protocol S report that both treatment options are equivalent in their health improvement ramifications.39 However, considering patient satisfaction with, for example, sustained injections is beyond the scope of this study. However, it has been demonstrated that the utility of maintaining vision decreases cost use due to other medical conditions, and preserving more patients in the workforce avoids other public assistance costs. These considerations were not incorporated in the study model. Also, information from qualified clinical data registry reporting, specifically the Intelligent Research in Sight Registry of the American Academy of Ophthalmology, may be used to guide future Markov-style models in assessing cost utility of treatments.
This study model presumes the same QALY for both IVR and PRP because there may be functional differences in quality of life between the 2 treatments. The use of 0.03 QALY per line year may be a more generous estimate when the range of severe visual loss is considered; the true QALY benefit in the treatment of PDR may be slightly lower. Thus, there are clinical and cost considerations that may not be captured by this model.
This study demonstrates the short-term cost utility of both PRP and IVR in the treatment of PDR, with overall cost utility of PRP 58% to 61% lower than that of IVR in the first 2 years. Because they are both initially under the accepted cost per QALY, this model reveals that the costs of both treatments are not as significantly different as one might expect, giving the physician flexibility in determining which treatment should be started first. The principal finding of Protocol S was that both approaches yielded similar clinical results and thus need not form a constraining algorithm of care but rather cogent and possibly customizable options for the patient with PDR and the physician. However, full life expectancy treatment estimates yield a cost per QALY differential of 85% to 90% less for PRP compared with IVR and challenges the acceptable cost-utility figures for IVR treatment. Perhaps implementation strategies including targeting certain higher-risk groups or substituting lower-cost anti-VEGF treatments may be developed to enhance cost utility.
Acknowledgments
Supported by National Institutes of Health core grant 5P30EY019007, the Gerstner Family Foundation, and unrestricted funds from Research to Prevent Blindness to Columbia University, New York, NY.
Abbreviations and Acronyms
- CMS
Centers for Medicare and Medicaid Services
- CPT
Current Procedural Terminology
- DME
diabetic macular edema
- DRCR
Diabetic Retinopathy Clinical Research
- DRS
Diabetic Retinopathy Study
- IVB
intravitreal bevacizumab
- IVR
intravitreal ranibizumab
- PDR
proliferative diabetic retinopathy
- PPV
pars plana vitrectomy
- PRP
panretinal photocoagulation
- QALY
quality-adjusted life year
- RVU
relative value unit
- VA
visual acuity
- VEGF
vascular endothelial growth factor
Footnotes
Financial Disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Author Contributions: Conception and design: Lin, Chang, Smiddy
Data collection: Lin, Chang, Smiddy
Analysis and interpretation: Lin, Chang, Smiddy
Obtained funding: Not applicable
Overall responsibility: Lin, Chang, Smiddy
References
- 1.Prevent Blindness Predicts Diabetic Eye Disease to Rise. [Accessed March 1, 2016]; Available at: https://ohsonline.com/articles/2015/10/23/prevent-blindness-predicts-diabetic-eye-disease-to-rise.aspx.
- 2.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–41. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 3.Preliminary report on effects of photocoagulation therapy. The Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1976;81:383–96. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 4.Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 5.Bressler NM, Beck RW, Ferris FL., 3rd Panretinal photocoagulation for proliferative diabetic retinopathy. N Engl J Med. 2011;365:1520–6. doi: 10.1056/NEJMct0908432. [DOI] [PubMed] [Google Scholar]
- 6.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 7.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–4. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 11.Filho JA, Messias A, Almeida FP, et al. Panretinal photocoagulation (PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol. 2011;89:e567–72. doi: 10.1111/j.1755-3768.2011.02184.x. [DOI] [PubMed] [Google Scholar]
- 12.Writing Committee for the Diabetic Retinopathy Clinical Research N. Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314:2137–46. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetic Retinopathy Clinical Research N. Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122:367–74. doi: 10.1016/j.ophtha.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Physician Fee Schedule. [Accessed January 5, 2016]; Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/physicianfeesched/
- 16.Centers for Medicare and Medicaid Services. Ambulatory Surgical Center (ASC) Payment. [Accessed January 5, 2016]; Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/ASC-Regulations-and-Notices-Items/CMS-1613-FC.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending.
- 17.Centers for Medicare and Medicaid Services. Addendum B. Final OPPS Payment by HCPCS Code for CY 2016, January 2016. [Accessed January 5, 2016]; Available at: https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/HospitalOutpatientpps/Addendum-A-and-Addendum-B-Updates.html.
- 18.2016 Medicare Physician Fee Schedule Final Rule Released. [Accessed March 16, 2016]; Available at: http://www.ascrs.org/node/23095.
- 19.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. 2016 ASP Drug Pricing Files. [Accessed February 6, 2016]; Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2016ASPFiles.html.
- 21.Smiddy WE. Relative cost of a line of vision in age-related macular degeneration. Ophthalmology. 2007;114:847–54. doi: 10.1016/j.ophtha.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Social Security. Actuarial Life Table. Period Life Table, 2011. [Accessed January 10, 2016]; Available at: https://www.ssa.gov/oact/STATS/table4c6.html.
- 23.Brown MM, Brown GC, Lieske HB, Lieske PA. Preference-based comparative effectiveness and cost-effectiveness: a review and relevance of value-based medicine for vitreoretinal interventions. Curr Opin Ophthalmol. 2012;23:163–74. doi: 10.1097/ICU.0b013e3283523fc1. [DOI] [PubMed] [Google Scholar]
- 24.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48:204–23. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 25.Smiddy WE. Clinical applications of cost analysis of diabetic macular edema treatments. Ophthalmology. 2012;119:2558–62. doi: 10.1016/j.ophtha.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Brown GC, Brown MM, Turpcu A, Rajput Y. The cost-effectiveness of ranibizumab for the treatment of diabetic macular edema. Ophthalmology. 2015;122:1416–25. doi: 10.1016/j.ophtha.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30:834–9. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gollamudi SR, Smiddy WE, Schachat AP, et al. Long-term survival rate after vitreous surgery for complications of diabetic retinopathy. Ophthalmology. 1991;98:18–22. doi: 10.1016/s0161-6420(91)32349-2. [DOI] [PubMed] [Google Scholar]
- 29.Blankenship GW. Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology. 1991;98:125–8. doi: 10.1016/s0161-6420(91)32326-1. [DOI] [PubMed] [Google Scholar]
- 30.Dogru M, Nakamura M, Inoue M, Yamamoto M. Long-term visual outcome in proliferative diabetic retinopathy patients after panretinal photocoagulation. Jpn J Ophthalmol. 1999;43:217–24. doi: 10.1016/s0021-5155(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 31.Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- 32.Hoang QV, Tsuang AJ, Gelman R, et al. Clinical predictors of sustained intraocular pressure elevation due to intravitreal anti-vascular endothelial growth factor therapy. Retina. 2013;33:179–87. doi: 10.1097/IAE.0b013e318261a6f7. [DOI] [PubMed] [Google Scholar]
- 33.Subash M, Comyn O, Samy A, et al. The effect of multispot laser panretinal photocoagulation on retinal sensitivity and driving eligibility in patients with diabetic retinopathy. JAMA Ophthalmol. 2016;134:666–72. doi: 10.1001/jamaophthalmol.2016.0629. [DOI] [PubMed] [Google Scholar]
- 34.Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116:823–32. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 35.Turkoglu EB, Celik E, Aksoy N, et al. Changes in vision related quality of life in patients with diabetic macular edema: ranibizumab or laser treatment? J Diabetes Complications. 2015;29:540–3. doi: 10.1016/j.jdiacomp.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Pershing S, Enns EA, Matesic B, et al. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160:18–29. doi: 10.7326/M13-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery RL, Gordon GM. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol. 2016;134:21–9. doi: 10.1001/jamaophthalmol.2015.4070. [DOI] [PubMed] [Google Scholar]
- 38.Stein JD, Newman-Casey PA, Kim DD, et al. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology. 2013;120:1835–42. doi: 10.1016/j.ophtha.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27:759–69. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]


