Abstract
Purpose
Although fatigue is a common problem for men with prostate cancer undergoing androgen deprivation therapy (ADT), there has been little systematic research on this issue. The present study examined changes in fatigue among prostate cancer patients receiving ADT compared to controls and predictors of heightened fatigue in ADT patients.
Methods
Prostate cancer patients treated with ADT (ADT+ group, n=60) completed assessments of fatigue prior to or just after ADT initiation (baseline) and 6 and 12 months later. Prostate cancer patients treated with prostatectomy only (ADT− group, n=85), and men without cancer (CA− group, n=86) matched on age and education completed assessments at similar intervals.
Results
Group-by-time interactions for fatigue severity, interference, and duration were observed when comparing the ADT+ group to controls. Groups did not differ at baseline; however, the ADT+ group reported worse fatigue at 6 and 12 months. The same pattern was observed for changes in the prevalence of clinically meaningful fatigue and the extent of clinically meaningful change in fatigue. Within the ADT+ group, higher baseline comorbidity scores were associated with greater increases in fatigue interference and higher baseline Gleason scores were associated with greater increases in fatigue duration.
Conclusions
Prostate cancer patients receiving ADT demonstrate a trajectory of worsened fatigue during the first 12 months following treatment initiation relative to controls. Greater comorbidities and higher Gleason scores at baseline appear to be risk factors for heightened fatigue during the first year following ADT initiation. Results highlight important time points for implementation of interventions aimed at fatigue reduction.
Keywords: Fatigue, Quality of Life, Prostatic Neoplasms, Androgen Deprivation Therapy
Introduction
Androgen deprivation therapy (ADT) is a hormonal treatment administered to men with prostate cancer. ADT serves to stop or delay subsequent cancer growth and metastasis by depriving tumor cells the stimulating effects of androgens. Men receiving this treatment typically experience a variety of negative side effects, including hot flashes and loss of muscle mass [1–4].
Reports also suggest that fatigue commonly affects men with prostate cancer receiving ADT. Along these lines, studies consistently indicate that fatigue worsens over time following ADT initiation [5–7]. Moreover, as many as 43% of patients treated with ADT have been reported to experience clinically significant fatigue [8]. Although these studies provide useful information, they are characterized by a number of methodologic limitations that include small sample sizes, limited follow-up periods, lack of a non-cancer comparison group, and/or lack of a comparison group of prostate cancer patients not receiving ADT. In one of the most rigorous studies to date, Alibhai and colleagues [9] showed that ADT recipients experienced greater decrements in vitality over the course of 12 months compared to men with prostate cancer not receiving hormonal therapy and noncancer controls. There are reasons to believe, however, that reduced vitality is not equivalent to heightened fatigue and that fatigue is better assessed using measures designed specifically for that purpose [10].
The present study addresses these limitations by prospectively examining changes in fatigue during a one-year period following ADT initiation using a validated and multidimensional measure of fatigue. In addition, the present study included matched comparison groups consisting of men with prostate cancer not treated with ADT and men with no history of cancer. The study had three aims. First, we sought to build upon prior literature by investigating the trajectory of fatigue among men with prostate cancer during the 12 months following ADT initiation while simultaneously assessing fatigue among men in the two control groups. We hypothesized that men in the ADT group would report worsening fatigue over time compared to prostate cancer patients who did not receive ADT and men without cancer. The second aim was to examine changes in the prevalence of clinically meaningful fatigue and the extent of clinically meaningful changes in fatigue among men in the ADT group compared to men in the two control groups. We hypothesized that clinically meaningful fatigue would become more prevalent over time in men treated with ADT compared to men in the control groups and that men treated with ADT would be more like to experience clinically meaningful increases in fatigue over time. A third aim was to conduct exploratory analyses to investigate demographic and clinical predictors of changes in mean-level fatigue over time among men receiving ADT.
Methods
Participants
Three samples of participants were recruited as part of a larger, longitudinal study investigating cognitive side effects of ADT: men receiving ADT for the treatment of prostate cancer (ADT+ group), men receiving prostatectomy only for the treatment of prostate cancer (ADT− group), and men with no history of cancer (CA− group). Eligibility criteria required that all participants: be at least 18 years of age, be able to speak and read English, have at least an eighth grade education, have no history of stroke, and be free of cognitive impairment (Short Portable Mental Status Exam score < 3) [11]. Additional eligibility criteria for the ADT+ group were that they: be diagnosed with non-metastatic or asymptomatic metastatic prostate cancer, be scheduled to start or have started ADT in past month, be scheduled to received ADT for at least 6 months, have not received treatment for any other cancers in the 12 months prior to recruitment, have no history of brain cancer or previous treatment with cranial irradiation, and have not been treated with ADT in the 12 months prior to recruitment or an anti-androgen agent (e.g., bicalutamide) in the 6 months prior to recruitment. Additional eligibility criteria for the ADT− group were that they: be diagnosed with non-metastatic prostate cancer, have no history of other cancers except non-melanoma skin cancer, have undergone prostatectomy, have no history of recurrent disease since undergoing prostatectomy, have no history of other forms of prostate cancer treatment (e.g., radiotherapy), not be scheduled for additional prostate cancer treatment, and not be receiving testosterone supplementation. Additional eligibility criteria for CA− group were that they: have no history of any form of cancer except non-melanoma skin cancer, not be receiving testosterone supplementation, and have a working telephone number.
Procedures
ADT+ and ADT− participants were recruited from Moffitt Cancer Center. ADT+ participants were also recruited from the James A. Haley Veterans’ Hospital. CA− participants were identified through use of information obtained from Marketing Systems Group, Inc. (Fort Washington, PA) and were initially contacted by telephone; those eligible and interested were scheduled for an appointment at which written informed consent was obtained. Eligible ADT− patients were matched to ADT+ participants on age (within 5 years), educational level (≤12 years, 13–16 years, or ≥17 years), and time since prostate cancer diagnosis (within 6 months). Eligible CA− men were matched to ADT+ participants on age and education. Baseline assessments were completed by ADT+ participants before or within 21 days of starting ADT and 6 and 12 months later. ADT− and CA− participants were assessed at similar time intervals. See Supplementary Figures 1 – 3 for information about participant flow. The larger number of participants in the ADT− and CA− groups relative to the ADT+ group reflects that, for ADT+ participants whose ADT− or CA− matched control withdrew from the study, an additional matched control was recruited in order to obtain data at all three assessments. Data were collected between September 2008 and October 2013. Written informed consent was obtained prior to initiation of study procedures. Participants were paid $80 at each evaluation. This study was approved by the Institutional Review Board at the University of South Florida.
Measures
Demographic and Clinical Characteristics
Demographic information was collected at baseline via self-report. Medical comorbidities were assessed at baseline using a self-report version of the Charlson Comorbidity Index [12]. Clinical information for the ADT+ and ADT− groups was collected via medical record review.
Fatigue
Participants completed the 14-item Fatigue Symptom Inventory (FSI) at each time point. The FSI assesses 3 domains of fatigue: severity, interference, and duration [13]. Fatigue severity represented the average of 4 items assessing the most, least, average, and current level of fatigue experienced. Fatigue interference represented the average of 6 items assessing the extent to which fatigue interfered with the participant’s general level of activity, ability to bathe and dress, normal work activity, ability to concentrate, relations with other people, and enjoyment of life. The two items assessing fatigue duration (number of days fatigued, amount of time fatigued per day) were scored as separate outcomes for the purposes of this project. Consistent with National Comprehensive Cancer Network guidelines [14], the presence of clinically meaningful fatigue was defined as scores ≥ 4 for the average of the fatigue severity items. The FSI has been found to be a valid and reliable measure among cancer populations [15].
Statistical analyses
Chi-square and t-tests were used to determine if there were group differences on demographic or clinical factors. Variables found to differ between groups (p <.10) were included as covariates in all subsequent analyses that involved group comparisons. Mixed models analyses were conducted to test the hypothesis that the ADT+ group would experience greater increases in fatigue over time compared to the control group as evidenced by a significant group by time interaction. Significant interactions were followed up with examination of group differences at baseline, 6 months, and 12 months and additional mixed models examining within-group change over time. Generalized estimating equation models were conducted to examine differences in the prevalence of clinically meaningful fatigue over time between groups. Chi-squares were used to examine group differences in prevalence of clinically meaningful fatigue at baseline, 6 months, and 12 months. In a second approach to this issue, chi-squares were used to examine clinically meaningful changes in fatigue over time adopting as a criterion a 1 SD change in fatigue from baseline to the 6 and 12 month follow-ups. Finally, linear regression analyses were conducted to identify baseline demographic and clinical predictors of increases in fatigue over time in the ADT+ group. Baseline fatigue was entered first into each analysis to create a residualized change score, followed by demographic or clinical predictors. The 12 month fatigue value served as the dependent variable. A p value of .05 was considered statistically significant. Data analyses were performed using SAS Version 9.3 (Cary, NC).
Results
Demographic and Clinical Characteristics
Participants included 60 men in the ADT+ group, 85 men in the ADT− group, and 86 men in the CA− group. Demographic and clinical characteristics are shown in Table 1. As anticipated, Gleason scores were higher in the ADT+ group than the ADT− group (p < .001). The ADT+ group was less likely to be white (p values ≤ .03) and married (p values ≤ .04) than the ADT− or CA− groups. The ADT+ group was also less likely to have attended college than the ADT− group (p = .05) and reported more comorbidities than men in the ADT− group (p = .02). Therefore, race, marital status, education, and comorbidities were entered as control variables in all subsequent analyses.
Table 1.
Demographic and Medical Characteristics
| ADT+ (n = 60) |
ADT− (n = 85) |
CA−− (n = 86) |
ADT+ vs. ADT− | ADT+ vs. CA− | |
|---|---|---|---|---|---|
|
| |||||
| Characteristic | No. (%) | No. (%) | No. (%) | p | p |
| Age, years | .90 | .47 | |||
| Mean | 68.10 | 67.94 | 69.12 | ||
| SD | 8.75 | 7.37 | 8.03 | ||
| Race | .03 | .01 | |||
| White | 51 (85.0) | 81 (95.3) | 83 (96.5) | ||
| Nonwhite | 9 (14.0) | 4 (4.7) | 3 (3.5) | ||
| Marital Status | .04 | < 0.001 | |||
| Married | 37 (61.7) | 66 (77.7) | 75 (87.2) | ||
| Not married | 23 (38.3) | 19 (22.3) | 11 (12.8) | ||
| Education | .05 | 0.24 | |||
| High school or less | 40 (66.7) | 43 (50.6) | 49 (57.0) | ||
| College or more | 20 (33.3) | 42 (49.4) | 37 (43.0) | ||
| Time since | .26 | N/A | |||
| diagnosis, years | |||||
| Mean | 3.69 | 4.59 | – | ||
| SD | 4.87 | 4.48 | – | ||
| Comorbidity index | .02 | .45 | |||
| score | |||||
| Mean | 2.83 | 2.44 | 2.70 | ||
| SD | 1.04 | 0.89 | 1.08 | ||
| Gleason score | < .001 | N/A | |||
| 4–6 | 8 (13.3) | 38 (44.7) | – | ||
| 7 | 22 (36.7) | 39 (45.9) | – | ||
| 8 | 19 (31.7) | 2 (2.4) | – | ||
| 9–10 | 7 (11.7) | 0 (0.0) | – | ||
| Missing | 4 (6.7) | 6 (7.0) | – | ||
Note. N = 231. SD = standard deviation. p values calculated using χ2s for categorical variables and one-way analysis of variance for continuous variables. Missing levels were excluded from calculation of p values.
Consistent with previous studies [16–17], fatigue outcomes were first compared between the ADT− and CA− groups to determine whether they could be combined into a single control group, thereby increasing statistical power and decreasing the number of tests performed. The ADT− and CA− groups did not differ on any fatigue outcome at any time point (ps ≥ .37) or on change over time in any fatigue outcome (ps ≥ .43). Therefore, in all subsequent analyses the ADT− and CA− groups were combined into a single control group.
Changes in Fatigue Severity, Interference, and Duration
Figure 1a depicts adjusted group means for fatigue severity. Although the ADT− and CA− groups were combined for analytic purposes, their separate means are depicted for illustrative purposes. A group by time interaction was observed when comparing the ADT+ group to the control group (p = .02). No group differences in fatigue severity were evident at baseline (ADT+ group M = 2.18, SE = 0.25; CA− group M = 2.11, SE = 0.14; p = .93) or 6 months (ADT+ group M = 2.62, SE = 0.22; CA− group M = 2.20, SE = 0.12; p = .18); however, the ADT+ group reported greater fatigue severity than controls at 12 months (ADT+ group M = 3.06, SE = 0.26; CA−group M = 2.29, SE = 0.14; p = .02). Examination of within-group change indicated that fatigue severity worsened over time in the ADT+ group (p < .001), but did not change over time in the control group (p ≥ .15).
Fig 1.
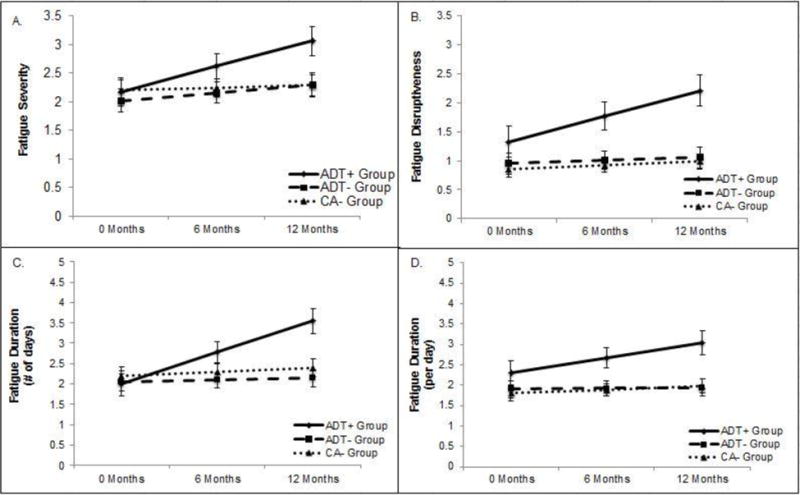
Adjusted mean estimates from mixed model analysis covarying for race, marital status, education, and comorbidities.
Figure 1b depicts adjusted group means for fatigue interference. A group by time interaction was observed when comparing the ADT+ group to the control group (p = .002). No group differences in fatigue interference were evident at baseline (ADT+ group M = 1.34, SE = 0.27; CA− group M = 0.90, SE = 0.11; p = .23); however, the ADT+ group reported greater fatigue interference at 6 (ADT+ group M = 1.77, SE = 0.24; CA− group M = 0.96, SE = 0.10; p = .004) and 12 months (ADT+ group M = 2.21, SE = 0.27; CA− group M = 1.02, SE = 0.11; p < .001) compared to controls. Examination of within-group change indicated that fatigue interference worsened over time in the ADT+ group (p < .001), but did not significantly change in the control group (p ≥ .20).
Figure 1c depicts adjusted group means for the number of days participants felt fatigued. A group by time interaction was observed when comparing the ADT+ group to the control group (p < .001). No group differences in number of days fatigued were evident at baseline (ADT+ M = 2.01, SE = 0.29; CA− group M = 2.12, SE = 0.15; p = .71); however, men in the ADT+ group reported a greater number of days fatigued at both 6 (ADT+ group M = 2.78, SE = 0.26; CA− group M = 2.19, SE = 0.13; p = .03) and 12 months (ADT+ group M = 3.54, SE = 0.30; CA− group M = 2.26, SE = 0.15; p < .001) compared to controls. Examination of within-group change indicated that the number of days participants felt fatigued worsened over time in the ADT+ group (p < .001), but did not significantly change in the control group (p ≥ .33).
Figure 1d depicts adjusted group means for the amount of time participants felt fatigued per day. A group by time interaction was observed (p = .05). No group differences in amount of time felt fatigued per day were evident at baseline (ADT+ group M = 2.30, SE = 0.29; CA− group M = 1.85, SE = 0.14; p = .21); however, men in the ADT+ group reported a greater amount of fatigue per day at both 6 (ADT+ group M = 2.67, SE = 0.25; CA− group M = 1.91, SE = 0.12; p = .01) and 12 months (ADT+ group M = 3.04, SE = 0.29; CA− group M = 1.96, SE = 0.14; p = .002) compared to controls. Examination of within-group change indicated that the amount of time participants felt fatigued worsened over time in the ADT+ group (p = .01), but did not change in the control group (p ≥ .41).
Changes in the Prevalence of Clinically Meaningful Fatigue and the Extent of Clinically Meaningful Change in Fatigue Over Time
Figure 2 depicts the prevalence of clinically meaningful fatigue by group over time. A group by time interaction was observed (p = .02) indicating that change over time in the prevalence of clinically meaningful fatigue differed by group status. At baseline, 12% of ADT+ patients reported clinically meaningful fatigue compared to 16% of men in the control group (χ2 = .60, p = .44). At 6 months, 33% of men in the ADT+ group reported clinically meaningful fatigue compared to 21% of men in the control group (χ2 = 3.74, p = .05). At 12 months, 32% of men in the ADT+ group reported clinically meaningful fatigue compared to 19% of the control group (χ2 = 4.19, p = .04).
Fig 2.
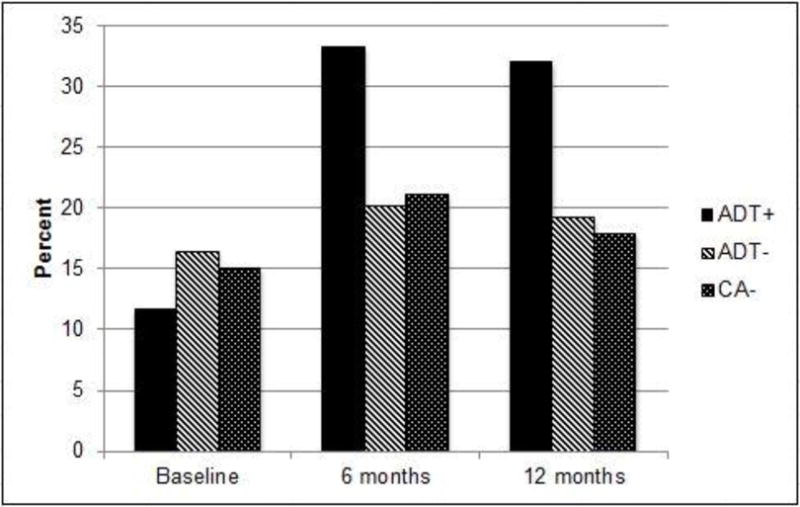
Percentage of patients with clinically meaningful fatigue, defined as scores ≥ 4 for the average of the fatigue severity items. The figure depicts raw percentages.
Figure 3 depicts the extent of clinically meaningful change in fatigue from baseline to 6 months and baseline to 12 months. The criterion for clinically meaningful change in fatigue over time was met among 30% of men in the ADT+ group versus 15% of men in the control group from baseline to 6 months, (χ2 = 4.87, p = .03). Similarly, 28% of men in the ADT+ group versus 14% of men in the control group met this criterion from baseline to 12 months, (χ2 = 4.54, p = .03).
Fig 3.
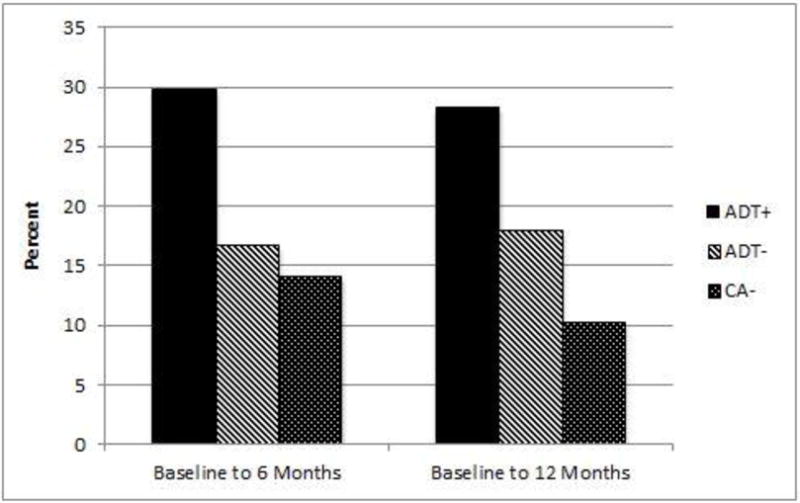
Percentage of patients with clinically meaningful change in fatigue, defined as 1SD change, from baseline to 6 months and from baseline to 12 months.
Demographic and Clinical Predictors of Changes in Fatigue
Based on the patterns of change in fatigue observed, analyses were conducted to explore baseline demographic and clinical predictors (shown in Table 1) of change over time in fatigue severity, interference, and duration (number of days fatigued, amount of fatigue per day) among men in the ADT+ group. None of the variables examined (see Table 1) predicted changes in fatigue severity over time (p values > .05). Increases in fatigue interference were predicted by greater baseline medical comorbidities (β = .233, p = .04). In addition, increases in the number of days fatigued were predicted by higher baseline Gleason scores (β = .293, p = .01).
Discussion
Results confirmed the hypothesis that prostate cancer patients receiving ADT would report worsening fatigue over time compared to prostate cancer patients not receiving ADT and men with no history of cancer. This pattern was evident on measures of fatigue severity, interference, and duration. Consistent with these changes, significant differences between ADT patients and controls were evident on measures of fatigue interference and duration at 6 months and remained significantly different at 12 months. Significant differences between ADT patients and controls were evident on fatigue severity solely at 12 months. This pattern of results is consistent with those obtained by Alibhai and colleagues [9] who observed a decrease in vitality among men receiving ADT, but not among a prostate cancer and healthy control group.
Findings also confirmed the hypothesis that prostate cancer patients receiving ADT would report greater increases over time in clinically meaningful fatigue compared to prostate cancer patients not receiving ADT and men with no history of cancer. Over the 12-month followup period, rates of clinically meaningful fatigue (i.e., scores ≥ 4) increased by 20% in ADT−treated patients versus 3% in controls. Consistent with these changes, a significantly higher percentage of ADT−treated patients than controls reported clinically meaningful fatigue at 6 months (33% vs 21%) and 12 months (32% vs. 19%). These figures are less than the 43% rate of clinically meaningful fatigue cases observed in a cross-sectional study of ADT−treated patients conducted by Storey and colleagues [8]. Discrepant findings may be partially explained by differences in methodology. For example, Storey et al. defined clinically meaningful fatigue as scores greater than 3 on a 0 to 10 scale, rather than scores greater than 4 as in our study, and sampled patients who had been receiving ADT treatments for longer (median 26 months) than in the present study (up to 12 months). Additional findings in the present study showed that clinically meaningful (i.e., 1 SD or greater) increases in fatigue between baseline and 6 months and baseline and 12 months were more likely to occur in the ADT−treated patients than controls.
Results of the exploratory analyses indicated that a greater number of comorbidities at baseline predict a worsening trajectory of fatigue interference among men receiving ADT. There is some prior evidence that clinically relevant fatigue may be more common among ADT recipients with greater concurrent co-morbidities [8]. The present study also identified an association between a higher baseline Gleason score and greater increases in the number of days fatigued raising the possibility that worsening fatigue may be due in part to advanced disease rather than ADT per se. To the best of our knowledge, this relationship has not been reported previously. Taken together, these findings highlight the need for additional research on risk factors for fatigue among ADT recipients and research on the mechanisms by which these risk factors influence fatigue among ADT recipients.
The current study is the first prospective, longitudinal study to investigate fatigue among men receiving ADT relative to both men with prostate cancer receiving prostatectomy only as well as men with no history of cancer. Moreover, we utilized a multidimensional measure of fatigue that allowed us to assess fatigue severity, the extent to which fatigue interfered with daily life, the duration of fatigue symptoms, and clinically significant fatigue. However, there were some limitations to the current project. First, this is an observational study and therefore we cannot determine with certainty that differences between groups in fatigue were due solely to ADT administration. Second, we acknowledge that other cut points may have been chosen to identify clinically meaningful fatigue; however, there is precedent for use of 4 as a cut point [14]. Third, patients were followed for only twelve months following ADT initiation. Future studies should follow patients over longer periods of time and should also examine the impact of ADT discontinuation on fatigue levels. Fourth, the present study examined only clinical and demographic variables as potential predictors of changes in fatigue over time in ADT−treated patients. Future studies should examine a broader range of potential predictors (e.g., psychosocial characteristics, genetic polymorphisms).
Studies investigating fatigue management among men receiving ADT have focused largely on physical activity interventions such as aerobic and resistance exercise. Many of these intervention studies have reported improvements in fatigue among participants receiving the intervention, suggesting that physical activity interventions are capable of reducing symptoms of fatigue among men receiving ADT [18–20]. While findings such as these are encouraging, other studies have failed to show improvements in fatigue [21–23] indicating that further research is needed to understand under what circumstances these interventions are effective. Additional behavioral interventions that have been shown to mitigate persistent fatigue in people with cancer and warrant further investigation include energy conservation [23] and cognitive-behavior therapy [24–25]. Moreover, pharmacologic interventions, such as methylphenidate or methylprednisolone for short-term use, among select patients may also be a viable option [26–27]. Despite the strategies employed, fatigue remains one of the most commonly reported symptoms among men receiving ADT. It is clear that further research is needed to determine effective strategies for fatigue management.
In summary, these findings suggest that prostate cancer patients receiving ADT experience high levels of fatigue that is clinically meaningful during the 12 months following ADT initiation. Patients appear to be particularly at risk for increases in fatigue between ADT initiation and 6 months post-initiation. In addition, several baseline risk factors have been identified, highlighting important time points for screening and implementation of cognitive-behavioral or pharmacological interventions aimed at fatigue reduction.
Acknowledgments
This work was supported by the following grants from the National Cancer Institute: R01 CA132803 (PI: Jacobsen) & R25 CA090314 (PI: Jacobsen).
Footnotes
None of the authors have any financial disclosures.
References
- 1.Irani J, Salomon L, Oba R, Bouchard P, Mottet N. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomized trial. Lancet Oncol. 2010;11:147–154. doi: 10.1016/S1470-2045(09)70338-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 3.Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40:655–671. doi: 10.1016/j.ecl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez BD, Jim HSL, Donovan KA, et al. Course and moderators of hot flash interference during androgen deprivation therapy for prostate cancer: A matched comparison. J Urol. 2015;194:690–695. doi: 10.1016/j.juro.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirl WF, Greer JA, Goode M, Smith MR. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psycho-Oncol. 2008;17:148–153. doi: 10.1002/pon.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for nonmetastatic prostate cancer. Psycho-Oncol. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–1141. doi: 10.1016/S0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 8.Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23:1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 9.Alibhai SMH, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 10.Deng N, Guyer R, Ware JE., Jr Energy, fatigue, or both? A bifactor modeling approach to the conceptualization and measurement of vitality. Qual Life Res. 2015;24:81–93. doi: 10.1007/s11136-014-0839-9. [DOI] [PubMed] [Google Scholar]
- 11.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue (version 2.2015) www.nccn.org.
- 15.Donovan KA, Jacobsen PB. The Fatigue Symptom Inventory: a systematic review of its psychometric properties. Support Care Cancer. 2011;19:169–185. doi: 10.1007/s00520-010-0989-4. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez BD, Jim HS, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen deprivation therapy: A controlled comparison. J Clin Oncol. 2015;33:2021–2027. doi: 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M, Jim HS, Fishman M, et al. Depressive symptomology in men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Psycho-Oncol. 2015;24:472–477. doi: 10.1002/pon.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 19.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 20.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 21.Cormie P, Newton RU, Spry N, et al. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16:328–335. doi: 10.1038/pcan.2013.22. [DOI] [PubMed] [Google Scholar]
- 22.Santa Mina D, Alibhai SMH, Matthew AG, et al. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J Aging Phys Act. 2013;21:455–478. doi: 10.1123/japa.21.4.455. [DOI] [PubMed] [Google Scholar]
- 23.Barsevick AM, Dudley W, Beck S, et al. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100:1302–1310. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery GH, Kangas M, David D, et al. Fatigue during breast cancer radiotherapy: An initial randomized study of cognitive-behavioral therapy plus hypnosis. Health Psychol. 2009;28:317–322. doi: 10.1037/a0013582. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen PB, Meade CD, Stein KD, et al. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. J Clin Oncol. 2002;20:2851–2862. doi: 10.1200/JCO.2002.08.301. [DOI] [PubMed] [Google Scholar]
- 26.Gong S, Sheng P, Jin H, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. 2014;9:e84391. doi: 10.1371/journal.pone.0084391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: A randomized, placebo-controlled, double-blind trial. J Clin Oncol. 2014;29:3221–3229. doi: 10.1200/JCO.2013.54.3926. [DOI] [PubMed] [Google Scholar]


