Abstract
PURPOSE
To determine (1) incidence, (2) risk factors, (3) risk period, and (4) characteristics of recurrent retinopathy of prematurity (ROP) treated by intravitreal bevacizumab (IVB) monotherapy.
DESIGN
Retrospective case series.
PARTICIPANTS
Premature infants developing Type 1 ROP [subdivided into ROP stage 3+ and aggressive posterior ROP (APROP)] in zone I or zone II posterior, receiving IVB monotherapy, and followed for at least 65 weeks adjusted age (AA).
METHODS
Retrospective review of infants who developed recurrence of Type 1 ROP following IVB monotherapy was performed including examination of RetCam fundus photographs and fluorescein angiograms.
MAIN OUTCOMES MEASURES
Incidence, risk factors, risk period, and characteristics of recurrent ROP.
RESULTS
IVB monotherapy in 241 infants (471 eyes) was reviewed. (1) Recurrence incidence was 8.3% (20/241) for infants and 7.2% (34/471) for eyes. (2) Recurrence risk factors of greatest significance were appearance of neovascularization as APROP (P=0.006), extended duration of hospitalization (P=0.01), and lower birth weight (P=0.024). (3) Recurrence risk period was between ≈45 and ≈55 weeks AA [90.0% (18/20) for infants and 94.1% (32/34) for eyes] with mean recurrence of 51.2 weeks AA (±4.6; range 45.7 to 64.9), and mean interval of 16.2 weeks (±4.4) between treatments. (4) Recurrence characteristics included plus disease, (20/20 infants; 100%) and neovascularization appeared at the following sites: (i) ROP stage 3+ with confluent neovascularization recurred both at the advancing edge and at the initial ridge and extra-retinal fibrovascular proliferative complex (12/14 infants; 85.7%). However, (ii) APROP (6/6 infants; 100%) [and ROP stage 3+ with non-confluent neovascularization (2/14 infants; 14.3%)] recurred only at the advancing edge. Also, the anterior extent of retinal vascularization was decreased (mean 1.76 versus 4.48 disc diameters [DD]), and the rate of retinal vascularization was delayed (mean 0.11 versus 0.23 DD/week) in those with versus without recurrence, respectively. Following retreatment with IVB, plus disease persisted, neovascularization regressed, and retinal vascularization proceeded minimally and slowly.
CONCLUSIONS
Premature children developing severe ROP are being treated successfully with IVB monotherapy. However, recurrence is not uncommon, so vigilant follow-up is necessary to ensure timely re-treatment when needed. Knowledge of recurrence incidence, risk factors, risk period, and characteristics allows tailored clinical management.
Retinopathy of prematurity (ROP) is a vascular proliferative disease of the developing retina in premature infants and currently is the most common ocular cause of childhood blindness worldwide with more than 30,000 affected annually.1 Historically, there have been two treatment modalities (first cryotherapy, then laser therapy)2, 3 that reduce the risk of blindness by ablating the peripheral retina. As early as 1978,4 an angiogenic factor was suggested to initiate the pathogenesis of ROP with later demonstration of angiogenic activity in ocular tissues.5 In 1984,6 electron microscopy was utilized to localize an unknown angiogenic factor to the peripheral retina in ROP. Vascular endothelial growth factor (VEGF) was discovered to be an important angiogenic factor in 19897 and was later related to retinal neovascularization.8 Studies utilizing anti-VEGF substances, especially intravitreal bevacizumab (IVB) (Avastin, Genentech/Roche, San Francisco, CA), in the rat animal model of ROP have provided further insight into the mechanisms affecting retinal neovascularization.9, 10 IVB therapy for ROP in preterm infants was first published in 2007,11 and multiple case reports and small case series of IVB use for ROP appeared thereafter. Since 2011, utilization of IVB monotherapy for severe ROP has gained popularity, following publication of IVB efficacy in a prospective, stratified, randomized, controlled, multi-center, clinical trial, Bevacizumab Eliminates the Angiogenic Threat of ROP (BEAT-ROP).12
Some practitioners are implementing IVB as part of a dual therapy with laser, rather than as monotherapy; however, this combination may have ramifications. Laser may cause more recurrences due to breakdown of the blood-retinal barrier perhaps allowing more escape of IVB from the eye. Especially in zone 1 cases and when near confluent laser therapy is performed, unavoidable consequences frequently occur with clinical implications including loss of visual field,13 increased myopia,14 and development of late angle closure glaucoma.15,16 Other potential complications of laser therapy include posterior synechiae, irregular pupils with inability to completely dilate, cataracts, development of early angle closure glaucoma, phthisis, leakage from choroidal vessels, and retinal detachment. Most of these consequences and complications (especially the increased myopia17–20 related to altered anterior segment development)19, 20 may be decreased by IVB monotherapy.21
Cryotherapy for Retinopathy of Prematurity (CRYO-ROP)2 and Early Treatment for Retinopathy of Prematurity (ETROP)3 both suggest that recurrence following peripheral retinal ablation for Type 1 ROP generally occurs before 54 weeks adjusted age (AA). However, IVB causes a delay in retinal vascularization, which is especially concerning if recurrence develops.22 The appropriate time to perform and to suspend follow-up examinations to allow timely diagnosis and treatment of recurrence following IVB monotherapy must be properly structured and extended until retinal vascularization is “complete”,22 which may not necessarily reach the ora serrata in the most immature infants.23, 24
Cases of late recurrence following anti-VEGF monotherapy or combined with laser therapy have been reported in the literature with seemingly minimal lapses in follow-up. Thus, the apprehension of not knowing how frequently recurrence occurs (incidence of recurrence), which infants are at greatest risk for recurrence (risk factors), when the period of risk for recurrence finally ends (risk period), and where in the retina to concentrate follow-up examinations (clinical characteristics), causes many physicians to be very hesitant to treat ROP with IVB monotherapy.
Participants and Methods
Two cohorts were reviewed in this large retrospective case series: 1) Seventy-five infants treated with IVB in the BEAT-ROP clinical trial (Clinicaltrials.gov trial registration number NCT00622726) from March 13, 2008 to August 4, 2010;12 and, 2) 218 infants treated consecutively with IVB (by HMH) following the BEAT-ROP clinical trial, who were treated and followed at the Memorial Hermann Hospital System in Houston, TX, and at the hospitals in Webster, Corpus Christi and El Paso, TX from August 5, 2010 to December 31, 2014. The Committee for the Protection of Human Subjects (the Institutional Review Board at The University of Texas Health Science Center) approved the study. All research adhered to the tenants of the Declaration of Helsinki and was HIPAA compliant.
All infants received IVB monotherapy for Type 1 ROP in zone I or zone II posterior with plus disease (published in ETROP3 in 2003) {subdivided in this study into ROP stage 3+ and aggressive posterior ROP (APROP) (included in the International Classification of ROP revisited (ICROP-R)25, 26 in 2005). Stage 3+ (defined as “extraretinal fibrovascular proliferation [EFP] or neovascularization that went through stages 1 and 2 to 3 that extends from the ridge into the vitreous”); and APROP (defined as an aggressive form of ROP typically seen in the posterior pole vessels that “show increased dilation and tortuosity in all 4 quadrants that is out of proportion to the peripheral retinopathy”…and…“may appear as only a flat network of neovascularization [FNV] at the deceptively featureless junction between vascularized and nonvascularized retina”) (as defined by ICROP-R).25, 26 All infants included in the study and its analysis had follow-up examinations extending to 65 weeks. All infants were treated with IVB monotherapy using the standard protocol as described in the BEAT-ROP clinical trial.12 Both the first (initial treatment for Type 1 ROP) and the second (treatment for recurrence of Type 1 ROP) IVB injections given to patients were the same dose (0.625 mg; 0.025 mL). All bilateral treatments and retreatments were performed on the same day, rather than as a staged procedure. RetCam fundus photography (Clarity Medical Systems, Inc., Pleasanton, CA) was used prior to the first IVB injection in all infants (241) to document Type 1 ROP, prior to the second IVB injection in all infants (20) with recurrent ROP to document recurrence characteristics, and in follow-up as needed for clinical management. Fluorescein angiography was performed on infants (9) at the time of recurrence and, when possible, to document the final extent of retinal vascularization in infants with and without recurrence. The final extent of retinal vascularization was measured, and the rate of retinal vascularization was calculated in eyes (25) without and (25) with recurrence. The non-recurrent eyes were selected from the 104 non-recurrent eyes of the BEAT-ROP clinical trial because these had been photographed (as per protocol) at the time of initial treatment and at approximately 54 weeks.(12) Zone I ROP cases were selected to be measured so that final extent was clear. The final extent of zone II ROP cases was not always clear and measurable. The recurrent eyes were selected from 34 eyes (recurrences from both cohorts (the six recurrent eyes of BEAT-ROP clinical trial and the 28 recurrent eyes following the end of that clinical trial treated with bevacizumab monotherapy). Of these 34 eyes, 18 had ROP in zone I and16 had ROP zone II, initially. The zone I and II eyes with the clearest final extent were selected to be measured. The selected photographs were printed and assembled from the optic nerve through the macula to the advancing edge. The disc diameter was measured. This measurement was used to calculate the extent of progression of retinal vascularization from the initial treatment site to the final extent of the retina in disc diameters (non-recurrent eyes) or from the initial to the second treatment sites in the retina in disc diameters (recurrent eyes). Dates of the photographs were used to calculate the rate of progression of retinal vascularization.
The study unit for all data analyses was infant, not eye, thus any unilateral recurrence was considered a recurrence. Any infants with asymmetric zones of ROP between the two eyes were classified as the smallest of the two zones. Demographic and baseline clinical data were summarized by mean (± standard deviation) for continuous variables or frequency (percentage) for discrete variables. Univariate analysis, two-sample t-test or the Fisher Exact test was used to compare each demographic, health status, and characteristic of initial treatment variable between infants without and with recurrence. Due to the correlation among the potential risk factors, a multivariate analysis with bidirectional stepwise logistic regression was used to identify and assess any potential risk factors for recurrence of ROP, including birth weight (BW), gestational age (GA), APGAR score at 1 and 5 minutes, initial location of ROP (zone I or zone II posterior), initial appearance of Type 1 ROP (subdivided into ROP stage 3+ or APROP), duration of hospital stay, and other systemic conditions including necrotizing enterocolitis (NEC) requiring surgery, sepsis proven by positive blood culture, patent ductus arteriosus (PDA) requiring surgery, and intraventricular hemorrhage (IVH). The odds ratio and associated P value (with a P value of <0.05 indicating significance) for each risk factor were determined. All statistical analyses were performed using SAS for Windows v9.4 (SAS, Inc., Cary, NC).
The characteristics of ROP were recorded at the first (for initial Type 1 ROP) and the second (for recurrent ROP) treatment (whether unilateral or bilateral) as follows:
At first (initial) treatment: (a) Type 1 ROP: (ROP stage 3+ or APROP); (b) the location (zone) of the ROP; and (c) the timing of the development of ROP (as postnatal age [PNA] and as adjusted age [AA] = GA + PNA) were recorded.
At second (recurrent) treatment: (a) the site of re-appearance of neovascularization (NV) at the advancing edge and at the original site of the ridge and EFP complex [R+EFP] with return of plus disease; (b) the location (zone: now distance from original location of ROP) of the advancing edge of retinal vascularization; and (c) the timing (PNA, and AA) of the recurrence of ROP, and interval between treatments (weeks) were recorded.
The number of weeks from the initial treatment to the last follow-up examination and AA at the last follow-up examination for all infants (without or with recurrence) were also recorded.
-
Fundus photographs and fluorescein angiograms were reviewed to observe the location of neovascular changes at first treatment (for initial Type 1 ROP), at second treatment (for recurrent ROP), and after stabilization of these changes:
In a sample of eyes either with or without recurrence, the extent of retinal vascularization at the first treatment with IVB was measured in number of disc diameters (DD, measured from the temporal edge of the optic nerve through the fovea to the advancing edge) (MMG).
In those eyes with recurrence, the extent of vascularization at the location of the second treatment for recurrence was measured in number of DD (MMG). The difference between the first and second IVB treatments (exact dates used) was calculated as the extent of progression of retinal vascularization.
In those eyes without recurrence, the extent of vascularization at their location at 54 weeks AA was measured in number of DD as was part of the initial BEAT-ROP protocol12 (MMG). The difference between IVB treatment and the extent of vascularization at 54 weeks AA (exact dates used) was calculated as the extent of progression of retinal vascularization.
The rate of vascular progression for eyes with recurrence was then calculated by the number of DD radial progression between the first and second IVB treatments divided by number of weeks between the IVB treatments (exact dates used).
The rate of vascular progression for eyes without recurrence was then calculated by the number of DD radial progression between the IVB treatment and 54 weeks AA divided by number of weeks between the IVB treatment and 54 weeks AA (exact dates used).
Any difference in extent of vascular progression (in number of DD) or in rate of vascular progression (in number of DD/week) between eyes with or without recurrence was evaluated by a mixed effect model. In the model, the subject unit was infant, the correlation structure between eyes was compound symmetric, and the fixed effect was without and with recurrence.
Results
A total of 241 infants (471 eyes, i.e., 11 unilateral cases) treated with IVB monotherapy for ROP in zone I or zone II posterior were eligible for review in this study (55 infants from the BEAT-ROP clinical trial and 186 infants treated following the BEAT-ROP clinical trial). Thirty-one infants without follow-up examinations extending to 65 weeks were excluded from analysis, as well as 21 infants who had died prior to 65 weeks.
The demographics, infant characteristics, and initial treatment information are summarized for all infants and subdivided between those without and with recurrence (Table 1). The recurrences were further subdivided between those with ROP stage 3+ and APROP at initial treatment (Table 1). Of all 241 study infants, the average BW was 714 grams (± 200 confidence interval [CI], ranging from 340 to 1590), the average GA was 24.7 weeks (±1.7 CI, ranging from 22 to 32), 131 (54.4%) were male, and 136 (56.4%) were Hispanic. Most of the infants (230 [95.4%]) had bilateral ROP; 222 (92.1%) had ROP stage 3+, and 181 (75.1%) had ROP in zone II posterior (all ROP stage 3+), while 60 (24.9%) had ROP in Zone I (41 with ROP stage 3+ and 19 with APROP). The average AA at the initial treatment was 35.9 weeks (± 2.6 CI, ranging from 31 to 49). These 241 infants were followed after the initial treatment for an average of 132.6 weeks (± 90.2 CI, ranging from 19 to 384) with the last follow-up examination at an average of 168.5 weeks AA (± 89.9 CI, ranging from 66 to 417). For those without recurrent ROP, the average follow-up was for 131.2 weeks (± 89.7 CI, ranging from 19 to 370), while for those infants with recurrent ROP, the average follow-up was for 148.4 weeks (±95.9 CI, ranging from 39 to 384).
Table 1.
Summary: Demographics, infant characteristics, and initial treatment by recurrent status (N=241 Infants, 471 Eyes)
| Demographics (N=241 Infants, 471 Eyes) | |||||
|---|---|---|---|---|---|
| Variable | All ROP | Non-Recurrent ROP | Recurrent ROP | ||
| All Recurrence | Initial Type 1 ROP Appearance | ||||
| Stage 3+ | APROP | ||||
| Number of Infants (%) | 241 | 221 (91.7%) | 20 (8.3%) | 14 | 6 |
| Number of Eyes (%) | 471 | 437 (92.8%) | 34 (7.2%) | 26 | 8 |
| Treated Eye at the Initial (1st) Treatment | |||||
| One Eye Only (%) | 11 (4.6%) | 10 (4.5%) | 1 (5.0%) | 1 | 0 |
| Both Eyes (%) | 230 (95.4%) | 211 (95.5%) | 19 (95.0%) | 13 | 6 |
| Infant Characteristics (N=241 Infants) | |||||
| Birth Weight (grams, mean ± SD, [min, max]) P<0.001 |
714 (± 200) [340 to 1590] | 724 (± 203) [340 to 1590] | 600 (± 113) [450 to 885] | 638 (± 107) [470 to 885] P=0.017 |
512 (± 73) [450 to 640] |
| Gestational Age (weeks, mean ± SD, [min, max]) P<0.001 |
24.7 (± 1.7) [22 to 32] | 24.8 (± 1.8) [22 to 32] | 23.9 (± 0.9) [22 to 26] | 24.1 (± 0.8) [23 to 26] | 23.3 (± 0.8) [22 to 24] |
| Sex (Male, %) | 131 (54.4%) | 120 (54.3%) | 11 (55.0%) | 7 | 4 |
| Race (%) | |||||
| White | 56 (23.2%) | 54 (24.4%) | 2 (10.0%) | 1 | 1 |
| Black | 35 (14.5%) | 30 (13.6%) | 5 (25.0%) | 5 | 0 |
| Hispanics | 136 (56.4%) | 124 (56.1%) | 12 (60.0%) | 8 | 4 |
| Others | 14 (5.8%) | 13 (5.9%) | 1 (5.0%) | 0 | 1 |
| APGAR Score (Missing 2 - born at home) | |||||
| At One Minute (score, mean ± SD, [min, max]) | 4.9 (± 2.4) [0 to 9] | 5.0 (± 2.4) [0 to 9] | 4.5 (± 2.0) [1 to 7] | 4.5 (± 2.0) [2 to 7] | 4.5 (± 2.3) [1 to 7] |
| At Five Minutes (score, mean ± SD, [min, max]) | 6.8 (± 1.8) [1 to 10] | 6.8 (± 1.8) [1 to 10] | 6.7 (± 1.9) [1 to 9] | 7.0 (± 1.4) [4 to 9] | 6.0 (± 2.7) [1 to 8] |
| Intra Ventricular Hemorrhage (%) | |||||
| 0 | 152 (63.1%) | 143 (64.7%) | 9 (45.0%) | 8 | 1 |
| 1 | 30 (12.5%) | 267 (12.2%) | 3 (15.0%) | 1 | 2 |
| 2 | 18 (7.5%) | 16 (7.2%) | 2 (10.0%) | 2 | 0 |
| 3 | 12 (5.0%) | 11 (5.0%) | 1 (5.0%) | 0 | 1 |
| 4 | 29 (12.0%) | 24 (10.9%) | 5 (25.0%) | 3 | 2 |
| Respiratory Distress Syndrome (%) | 258 (100%) | 238 (100%) | 20 (100%) | 14 | 6 |
| Patent Ductus Arteriosus (%) | 96 (439.8%) | 87 (39.4%) | 9 (45.0%) | 5 | 4 |
| Sepsis (%) | 65 (27.0%) | 59 (26.7%) | 6 (30.0%) | 3 | 3 |
| Necrotizing Enterocolitis (%) P=0.035 |
48 (19.9%) | 40 (18.1%) | 8 (40.0%) | 6 | 2 |
| Hospital Duration (weeks, mean ± SD, [min, max]) P<0.001 |
20.2 (11.9) [4 to 118] | 19.2 (± 11.6) [4 to 118] | 29.2 (± 13.5) [14 to 67] | 29.9 (± 15.6) [14 to 67) | 27.4 (± 7.4) [17 to 37] |
| Initial Treatment (N=241 Infants) | |||||
| Initial Type 1 ROP Appearance P=0.002 | |||||
| Stage 3+ (%) | 222 (92.1%) | 208 (94.1%) | 14 (70.0%) | N/A | |
| APROP (%) | 19 (7.9%) | 13 (5.9%) | 6 (30.0%) | ||
| Zone P=0.003 | |||||
| I (%) | 60 (24.9%) | 49 (22.2%) | 11 (55.0%) | 5 | 6 |
| Posterior II (%) | 181 (75.1%) | 172 (77.8%) | 9 (45.0%) | 9 | 0 |
| Adjusted Age at the Initial Treatment (weeks, mean ± SD, [min, max]) | 35.9 (± 2.6) [31 to 49] | 36.0 (± 2.6) [31 to 49] | 35.0 (± 2.1) [32 to 39] | 35.5 (± 2.2) [32 to 39] | 33.8 (± 1.4) [32 to 35] |
| Weeks of Follow-up (Initial Treatment to Last Follow-up Visit (weeks, mean ± SD, [min, max]) | 132.6 (± 90.2) [19 to 384] | 131.2 (± 89.7) [19 to 370] | 148.4 (± 95.9) [39 to 384] | 149.3 (± 88.7) [39 to 357] | 146.3 (± 120.2) [59 to 384] |
| Adjusted Age at Last Follow-up Visit (weeks, mean ± SD, [min, max]) | 168.5 (± 89.9) [66 to 417] | 167.1 (± 89.5) [66 to 405] | 183.4 (± 95.2) [77 to 417] | 184.8 (± 88.0) [77 to 391] | 180.1 (± 119.4) [94 to 417] |
Incidence of Recurrence
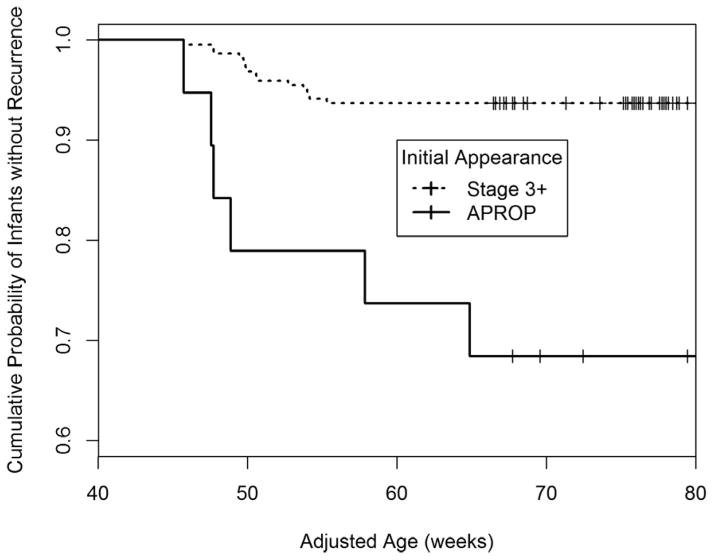
Twenty of 241 infants (8.3%) with 34 of 471 eyes (7.2%) had recurrent ROP (see Table 1). Figure 1 shows the Kaplan-Meier survival curve subdivided by initial appearance (ROP stage 3+ compared to APROP). Infants treated for APROP had significantly greater recurrences following initial treatment (31.6% recurrence incidence in those with APROP, versus 6.3% recurrence incidence in those with ROP stage 3+, P<0.001). Details of recurrence for each infant are given in Table 2.
Figure 1.
The Kaplan-Meier survival curve for the probability for NO RECURRENCE with Type 1 ROP was found for infants Stage 3+ to be 93.7% (208/222) and for infants with APROP to be 68.4% (13/19).
Table 2.
Details of ROP patients with recurrence following intravitreal bevacizumab monotherapy (N=20 Infants, 34 Eyes)
| Patient | BW | GA | INITIAL (1ST) TREATMENT | RECURRENCE (2ND) TREATMENT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eyes | Type 1 | Zone | PNA | AA | Eyes | NV Site(s) | Zone | PNA | AA | Interval | OUTCOME | |||
| 1 | 450 | 23 | OU | APROP | 1 | 8.7 | 31.7 | OU | AE | 2 | 22.7 | 45.7 | 14.0 | Regressed |
| 2 | 450 | 23 | OU | APROP | 1 | 11.0 | 34.0 | OU | AE | 2 | 25.9 | 48.9 | 14.9 | Regressed |
| 3 | 490 | 24 | OU | APROP | 1 | 10.6 | 34.6 | OD | AE | 2 | 23.7 | 47.7 | 13.1 | Regressed |
| 4 | 490 | 24 | OU | APROP | 1 | 10.3 | 34.3 | OS | AE | 2 | 40.9 | 64.9 | 30.6 | Regressed |
| 5 | 550 | 22 | OU | APROP | 1 | 10.6 | 32.6 | OD | AE | 2 | 35.9 | 57.9 | 25.3 | OD 4A |
| 6 | 640 | 24 | OU | APROP | 1 | 11.4 | 35.4 | OS | AE | 2 | 23.6 | 47.6 | 12.3 | Regressed |
| Mean (APROP) | 512 | 23.3 | 10.4 | 33.8 | 28.8 | 52.1 | 18.4 | |||||||
| 7 | 605 | 24 | OU | 3+* | 1 | 9.6 | 33.6 | OU | AE | 2 | 25.4 | 49.4 | 15.9 | Regressed |
| 8 | 750 | 24 | OU | 3+* | 2 | 8.1 | 32.1 | OU | AE | 2 | 23.7 | 47.7 | 15.6 | Regressed |
| 9 | 470 | 24 | OU | 3+ | 1 | 11.1 | 35.1 | OU | AE & R+EFP | 2 | 28.7 | 52.7 | 17.6 | Regressed |
| 10 | 515 | 24 | OU | 3+ | 2 | 10.4 | 34.4 | OU | AE & R+EFP3 | 2 | 25.9 | 49.9 | 15.4 | Regressed |
| 11 | 550 | 23 | OU | 3+ | 1 | 15.9 | 38.9 | OU | AE & R+EFP3 | 2 | 30.7 | 53.7 | 14.9 | Regressed |
| 12 | 580 | 24 | OU | 3+ | 2 | 13.4 | 37.4 | OU | AE & R+EFP3 | 2 | 26.6 | 50.6 | 13.1 | Regressed |
| 13 | 590 | 24 | OU | 3+ | 2 | 11.4 | 35.4 | OU | AE & R+EFP3 | 2 | 26.6 | 50.6 | 15.1 | OD 5/OS 4B |
| 14 | 590 | 23 | OU | 3+ | 1 | 9.4 | 32.4 | OU | AE & R+EFP3 | 2 | 22.7 | 45.7 | 13.3 | Regressed |
| 15 | 650 | 24 | OU | 3+ | 1 | 9.7 | 33.7 | OU | AE & R+EFP3 | 2 | 26.0 | 50.0 | 16.3 | Regressed |
| 16 | 660 | 25 | OU | 3+ | 2 | 12.1 | 37.1 | OS | AE & R+EFP3 | 2 | 24.8 | 49.8 | 12.7 | Regressed |
| 17 | 660 | 24 | OU | 3+ | 2 | 11.0 | 34.0 | OU | AE & R+EFP3 | 2 | 24.7 | 47.7 | 13.7 | Regressed |
| 18 | 700 | 26 | OU | 3+ | 2 | 11.1 | 37.1 | OU | AE & R+EFP3 | 2 | 28.0 | 54.0 | 16.9 | Regressed |
| 19 | 730 | 24 | OU | 3+ | 2 | 14.1 | 38.1 | OU | AE & R+EFP3 | 2 | 20.1 | 54.1 | 16.0 | Regressed |
| 20 | 885 | 25 | OD | 3+ | 2 | 12.1 | 37.1 | OD | AE & R+EFP3 | 2 | 30.3 | 55.3 | 18.1 | Regressed |
| Mean (Stage 3+) | 638 | 24.1 | 11.4 | 35.5 | 26.0 | 50.8 | 15.3 | |||||||
| Overall Mean | 600 | 23.9 | 11.1 | 35.0 | 26.8 | 51.2 | 16.2 | |||||||
BW = Birth Weight (grams); GA = Gestational Age (weeks); PNA = Postnatal Age (weeks); AA = Adjusted Age (weeks); NV = Neovascularization: [1] AE = Advancing Edge; [2] R+EFP = Initial Ridge + Extraretinal Fibrovascular Proliferative Complex; Interval between 1ST and 2ND treatments (weeks);
non-confluent EFP Initially; 4A = partial retinal detachment, macula on; 4B = partial retinal detachment, macula off; 5 = total retinal detachment.
Risk Factors for Recurrence
Six baseline characteristics, including immaturity factors (BW [P<0.001], GA [P<0.001], zone [P=0.003]); initial treatment appearance (APROP [P=0.002]); and health risk factors (single risk factor of NEC [P=0.035] and cumulative health issues noted by duration of hospitalization [P<0.001]), were significantly different between infants without and with recurrence of ROP (see Table 1).
Infants with ROP recurrence were significantly more immature at birth than those without ROP recurrence. Average BW of recurrent ROP infants (600 grams [±113]) was significantly lower than the non-recurrent ROP infants (724 grams [±203]), P<0.001. Average GA was significantly lower in recurrent ROP group (23.9 weeks [±0.9]) compared to non-recurrent ROP infants (24.8 weeks [±1.8]), P<0.001. Eleven of 60 zone I infants recurred (18.3%), while nine of 181 zone II posterior infants (5.0%) recurred, P=0.003. Additionally, in the recurrent ROP infants, the BW of APROP infants (512 grams [±73]) was significantly lower than the ROP stage 3+ infants (638 grams [±107]), P=0.017.
The results of stepwise logistic regression analysis with bidirectional elimination of all clinical and demographic data identified three risk factors for developing recurrent ROP: 1) the initial Type1 ROP appearance of APROP (P=0.006); 2) increased duration of hospital stay (P=0.010); and 3) decreased BW (P=0.024). Infants initially treated for APROP were found to have a 5-fold increased risk of developing recurrent disease than those infants treated for ROP stage 3+ (95% CI, ranging from 1.59 to 16.4). An average of one week increase in hospital stay increased the risk of recurrence by a factor of 1.04 (95% CI, ranging from 1.01 to 1.07). Every 100 grams of BW decrease was associated with an increased risk of recurrence by a factor of 1.56 (95% CI, ranging from 1.06 to 2.30).
Risk Period of Recurrence
Recurrent ROP was seen in 20 infants between 45.7 and 64.9 weeks AA (mean 51.2 weeks ±4.6). Eighteen of 20 infants (90.0%) and 32 of 34 eyes (94.1%) developed recurrence within a 10-week window from ≈45 to ≈55 weeks AA. There were 2 infants treated initially for APROP who developed recurrence at 57.9 weeks AA and 64.9 weeks AA (see Figure 1 and Table 2).
Patient # 5 developed ROP stage 4A temporal retinal detachment in the right eye and received a second IVB injection with final outcome of macular dragging, but resolution of the ROP stage 4A detachment. Patient #13 returned for follow up with ROP stage 5 right eye and ROP stage 4B left eye and was referred for vitrectomies, which were unsuccessful, and did not receive a second injection of IVB (Table 2). Both of these patients were previously detailed in the BEAT-ROP clinical trial.12)
Clinical Characteristics of Recurrence
Clinical characteristics of recurrence included return of plus disease (in 20/20 (100%) infants [and 34/34 (100%) eyes] with recurrence) and neovascularization (seen clinically, but best seen by fluorescein angiography, which was performed on 9 infants at the time of recurrence) at one or two specific sites in 12/14 (85.7%) infants [and 22/26 (84.6%) eyes] with ROP stage 3+ recurred at both the initial ridge and extraretinal fibrovascular proliferative complex (R+EFP) as well as at the advancing edge. The only exceptions to this were 2/14 (14.3%) infants [and 4/26 (15.4%) eyes], which had non-confluent neovascularization initially and recurred only at the advancing edge (see Table 2). However, 6/6 (100%) infants [and 8/8 (100%) eyes] with APROP recurred only at the advancing edge. Following the second IVB treatment, plus disease did not decrease, but neovascularization resolved in all cases.
A third characteristic of recurrence was a decreased extent of retinal vascularization as evidenced by progression of the advancing edge only a mean of 1.76 DD (±1.0 CI; range 0.48 to 4.75DD; n=25 eyes) from its location at time of initial treatment to that at the time of recurrence. This was significantly less (P=0.024) than the extent of progression of retinal vascularization seen in eyes without recurrence mean of 4.48 DD (±1.46 CI, range 1.41 to 7.60 DD; n=25 eyes). Similarly, there was also a significantly decreased rate of retinal vascularization in eyes with ROP recurrence mean of 0.11 DD/week (±0.05 CI, range 0.03 to 0.20; n=25 eyes) versus in those without ROP recurrence mean of 0.23 DD/week (±0.07 CI, range 0.07 to 0.36; n=25 eyes; P=0.032).
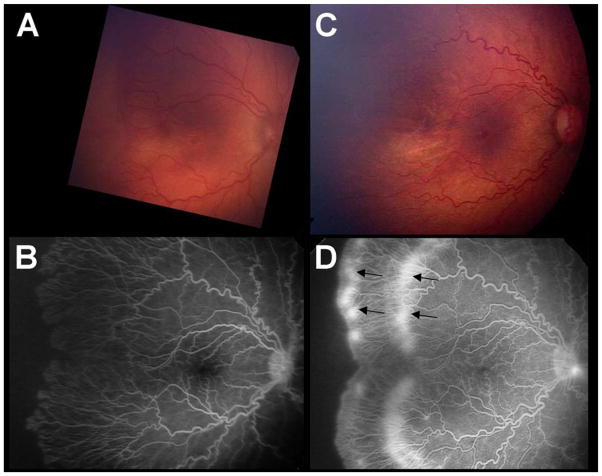
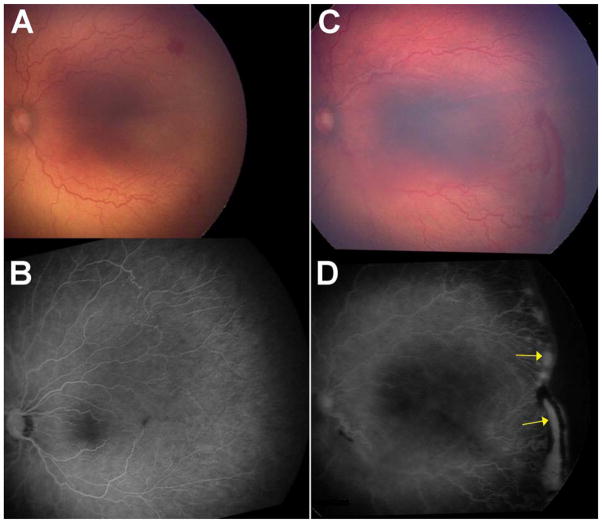
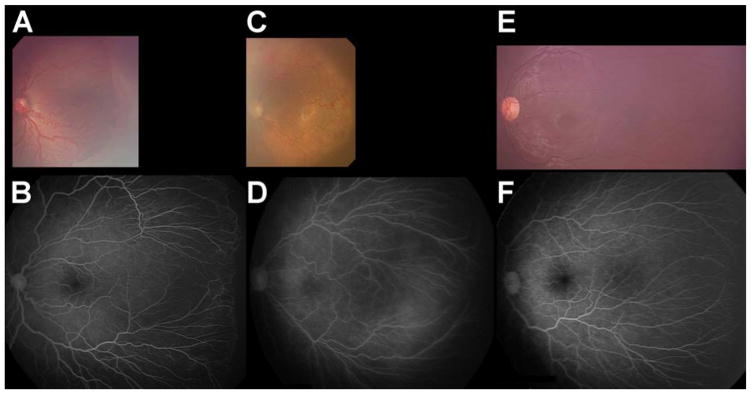
Figures 2C and 2D show an eye that recurred and required a second injection to induce regression for ROP stage 3+ with 2 recurrence sites. Figure 3C and 3D show an eye that recurred and required a second injection for APROP with 1 recurrence site. Figures 4A and 4B show an eye that required only one injection to induce regression for ROP stage 3+. Figures 4C and 4D show an eye that required only one injection to induce regression for APROP. Figures 4E and 4F show an eye that required no injection (for ROP stage 2) and spontaneously regressed, for comparison. These five infants (in Figures 2 to 4) were selected because they had similar BW (BW ranging from 450 to 510 grams) and GA (GA ranging from 23 to 24 weeks).
Figure 2.
The right eye of Patient 9 had ROP stage 3+ requiring 2 injections of bevacizumab for initial and recurrent disease (470 grams, 24 weeks at birth). A. Photograph just prior to first injection for ROP stage 3+ ROP (35.1 weeks AA). B. Fluorescein angiogram of same eye (A) after completion of retinal vascularization (77.9 weeks AA). C. Photograph and D. Fluorescein angiogram just prior to second injection for recurrent ROP at two sites (52.7 weeks AA). (Arrows).
Figure 3.
The left eye of Patient 2 had APROP requiring 2 injections of bevacizumab for initial and recurrent disease (450 grams, 23 weeks at birth). A. Photograph just prior to first injection for APROP (34.0 weeks AA). B. Fluorescein angiogram of same eye (A) after completion of retinal vascularization (96.0 weeks AA). C. Photograph and D. Fluorescein angiogram just prior to second injection for recurrent ROP at a single site (48.9 weeks AA). (Arrows).
Figure 4.
The eyes of infants matched for BW (490 to 510 grams) and GA (23 to 24 weeks) at time of initial successful injection of bevacizumab (A) for ROP stage 3+ or (C) for APROP, and (E) for no treatment for spontaneously regressed ROP compared to the same eyes when retinal vascularization reached the fullest extent possible based on GA21 in B, D, and F, respectively. A. Photograph of right eye treated for ROP Stage 3+ (495 grams, 23 weeks at birth) at 35.6 weeks AA. B. Fluorescein angiogram of same eye (A) after completion of retinal vascularization (79.6 weeks AA). C. Photograph of right eye treated for APROP (490 grams, 24 weeks at birth) at 34.6 weeks AA. D. Fluorescein angiogram of same eye (C) after completion of retinal vascularization (131.1 weeks AA). E. Photograph of right eye untreated (spontaneously regressed ROP stage 2) (510 grams, 23 weeks at birth) at 113.4 weeks AA. F. Fluorescein angiogram of same eye (E) after completion of retinal vascularization (113.4 weeks AA).
Discussion
There have been several case reports of late recurrence following treatment of ROP stage 3+ or APROP with IVB monotherapy27, 28 and following combined treatment utilizing laser therapy with intravitreal ranibizumab29 or with IVB.27, 30–32 In each of these reports, close follow-up (every 1 or 3 weeks) was not performed beyond 55 weeks AA; however, standard follow-up recommendations have not been established by ophthalmologic studies reviewing prospective data. Also, not surprisingly, there are reports of failure following use of IVB monotherapy for ROP stages 4B and 5 because treatment was performed too late and membrane formation was too extensive.33–35
Of greatest interest in the literature is a case series of 9 patients (17 eyes) treated initially with IVB monotherapy for ROP stage 3+ or APROP at a mean of 34.1 weeks AA with recurrences developing at mean of 49.3 weeks AA. Thus, they found an interval between initial IVB monotherapy and recurrence with a mean of 15.2 weeks.22 Authors of this case series discussed the return of plus disease and the re-appearance of neovascularization at one or two sites: at the R+EFP and/or at the advancing edge of retinal vascularization. This is identical to our findings.
From these previous reports of recurrence and the results of this study, several clinical management issues can be recognized: IVB monotherapy is a very effective recent addition to the treatment armamentarium for ROP with <8% incidence of recurrence in infants or eyes requiring a second IVB treatment. There is only one case series from Turkey, which reports need of a third injection of IVB;36 however, there are no published cases of infants requiring a third administration of IVB in developed nations, to our knowledge. If an infant has identified risk factors (APROP at the time of initial treatment, extended duration of hospitalization due to multiple clinical risk factors, or very low BW) this should cause clinicians to be more vigilant and to examine more frequently and possibly longer.
Clinical characteristics of return of plus disease and re-appearance of neovascularization [at either the original site of the ridge and extraretinal fibrovascular proliferative complex ± at the advancing edge of retinal vascularization22 (± at other currently unknown sites)] are required to diagnose the need for re-treatment of recurrent Type 1 ROP following IVB monotherapy. Additionally, a small extent and a slow rate of advancement of retinal vascularization is noted (see Figures 2 and 3), which is especially noticeable in zone I cases, and should be considered a warning sign of possible imminent recurrence. When an infant was initially treated for ROP stage 3+ with confluent EFP, recurrence was observed and documented at two distinct sites: at the original R+EFP and at the advancing edge of retinal vascularization (see Figure 2). In comparison, when an infant was initially treated for APROP or ROP stage 3+ with only nonconfluent EFP, recurrence was observed and documented at only one site: at the advancing edge of retinal vascularization (see Figure 3). While the location of the neovascularization could be seen with the indirect ophthalmoscope with a 20 or 28 Diopter lens and with fundus photography, it was best documented by fluorescein angiography with the RetCam imaging system (see Figure 2C and 2D and Figure 3C and 3D). Although we were unable to perform fluorescein angiography in all cases of recurrence, based on the photographs taken in all recurrences and the available fluorescein angiograms (9 infants), it is our impression that Figures 2 and 3 are representative of the two common patterns of recurrence.
Since there is a virtual cessation of retinal vessel advancement following the second IVB injection, there is often a large avascular peripheral area of retina that remains undeveloped, especially in former zone I and APROP cases. Successful IVB treatment (inducing regression of ROP) is usually recognized before 65 weeks AA by a rapid advancement of retinal vascularization from the original R+EFP in ROP stage 3+ (see Figures 4A and 4B) or from the original FNV in APROP (see Figures 4C and 4D), toward, but not necessarily to, the ora serrata in the most immature infant eyes.23, 24 Anecdotally, spontaneous regression of ROP is also accompanied by rapid advancement of retinal vascularization before 65 weeks AA (see Figures 4E and 4F) toward, but not necessarily to, the ora serrata in the most immature infant eyes.23, 24 In all cases of regressed ROP, whether following two, one, or no IVB injections, it is our experience and belief that unless extensive tractional elements are present, no further intervention, such as laser ablation, is indicated for the remaining permanently undifferentiated, peripheral, avascular retina.
Timing of the administration of IVB monotherapy is critical
IVB is an ideal treatment when ocular (and blood) VEGF levels are high with sufficient neovascularization (Phase 2) and rather than when VEGF levels are low with minimal neovascularization (before Phase 2).37 IVB can inhibit normal development of the retina if administered in Phase 1, and thus, IVB cannot be utilized as prophylactic treatment.37 Similarly, IVB is ideally administered prior to the development of advancing retinal detachment (after Phase 2) as has been demonstrated by reports of accelerated retinal detachment following IVB treatment for ROP stages 4B and 5,33–35 and therefore, IVB cannot be utilized as salvage therapy. This is the clinical experience in diabetic patients who develop accelerated retinal detachment associated with IVB administration when tractional membranes are extensive.38
Appropriate follow-up examinations are mandatory for successful clinical management. Recurrence of ROP following IVB monotherapy should now be more easily and promptly identifiable by treating ophthalmologists. Since the incidence of recurrence is <8%, most infants will demonstrate regression with rapid progression of retinal vessels toward the ora serrata. The risk factors for recurrence are known to be immaturity (lower BW and GA, and zone 1 ROP), APROP, and cumulative health issues (i.e. duration of hospital stay and NEC requiring surgery), which should heighten vigilance during follow-up examinations. The risk period of recurrence of ROP following IVB, although more delayed, is predictable with a critical 10-week recurrence window from ≈45 to ≈55 weeks AA, with recurrence developing at a mean of 51.2 weeks AA, and infrequently, as late as 65 weeks AA, as shown by this series of recurrences. We believe collaborative consensus groups should consider amending the current guidelines of weekly examinations until 70 weeks AA39 in light of the knowledge of this report to better reflect the available evidence. We believe clinicians should be more vigilant in their examinations for recurrence during the 45 to 55 week AA “recurrence window” and should perform more frequent examinations during this time. Before and after this critical period of time, we believe examinations can be less frequent until retinal vascularization is completed. However, we believe those initially treated for APROP are at increased risk for recurrence and should be monitored closely until at least 65 weeks AA (as was the case of the delayed recurrences in this series).
There are several appearances of regressing ROP in the first few weeks following initial treatment with IVB, with the retinal vessels ranging from the appearance of vascular projections from the former R+EFP without a defined new ridge to an advancing edge with a defined ridge that may thicken. However, clinicians can easily distinguish these normal regression patterns from recurrence of ROP by the continued resolution of plus disease and the lack of continued progression of formation of dilated, hyperemic vessels (neovascularization) in the former R+EFP. Also, the appearance of these regression patterns is often much earlier than the time period in which recurrence usually occurs (≈45 to ≈55 weeks AA). Furthermore, it is our clinical impression from anecdotal experience that after treatment with IVB the plus disease resolves rather quickly, i.e., within a couple of days, while the R+EFP can take several weeks to resolve, depending on how extensive the neovascularization was prior to treatment and/or retreatment. However, it is our clinical impression that this delayed resolution of R+EFP can be discerned from early recurrence by the appearance of closed gray to white vessels at the site of the former R+EFP.
The strengths of this study are that the large sample size with all infants following the same examination, documentation, and treatment protocols utilized in the BEAT-ROP clinical trial, and that the same clinical information is available for all of these infants who are all documented photographically, with last mean follow-up at age 148.1 weeks AA, over a period of almost 7 years.
The limitations of this study are that much of what we now recognize about recurrence has been learned after the initial documentation protocol was designed. Thus, patterns of recurrence were not known from the initial time that IVB monotherapy began to be utilized for treatment warranted ROP. Further, fluorescein angiography was not available at some of the centers, and the importance of doing fluorescein angiography at the time of recurrence was not known. Thus, fluorescein leakage at the 2 specific sites with recurrence was not documented for all infants. Fortunately, the changes in the critical sites of recurrence in the retina were observed by indirect ophthalmoscopy and were documented by fundus photography for all recurrences. Further, it would have been ideal to sample prospectively each infant’s blood VEGF level before each IVB injection and the bevacizumab blood level for a few months after each IVB injection to establish any relationship to severity of initial disease and to the development of recurrence. Additionally, perhaps the screening and treatment of this study’s population with more Hispanic patients than those of other published ROP clinical trials may have influenced the clinical findings to some degree.
As with any treatment involving injection of a drug not FDA approved for intravitreal use in premature infants for ROP, further investigation to determine the long-term safety concerns (both ocular and systemic) of IVB monotherapy should be systematically performed. More investigation into the most efficient drug and dose of an intravitreal anti-VEGF drug for ROP is also needed. Ultimately, a higher dose of an “ideal” anti-VEGF drug may be appropriate for those infants with higher risk of recurrence of ROP following anti-VEGF therapy. Conversely, a lower dose of an “ideal” anti-VEGF drug may be appropriate for those infants who have lower risk of recurrence of ROP following anti-VEGF therapy.
In summary, IVB is a very effective treatment modality for ROP. Recurrence is very important to recognize promptly, and infants should be followed closely until they are beyond the time period during which recurrence has been reported. We hope this report can help clinicians feel more comfortable treating with IVB monotherapy and following patients for recurrence with knowledge that (1) the incidence of recurrence is low (8.3% in this study); (2) some infants at increased risk for recurrence are identifiable (initial treatment for APROP, multiple ROP risk factors requiring prolonged hospitalization, especially if NEC requiring surgery has occurred, and extremely low BW); (3) recurrence risk period is limited, although extended compared to laser therapy, with the vast majority of cases recurring during a critical time period (≈45 to ≈55 weeks AA) and before 65 weeks AA; and 4) with the return of plus disease and lack of progression of retinal vascularization, there are two specific locations to re-examine for recurrent neovascularization. With this knowledge, we believe collaborative consensus groups may be able to amend the current screening guidelines to help the clinician more safely and efficiently monitor for recurrence following treatment with IVB.
Acknowledgments
Sources of Funding: Supported by National Eye Institute Vision Core Grant P30EY010608 (Bethesda, MD), a Challenge Grant from Research to Prevent Blindness (New York, NY) to The University of Texas Health Science Center at Houston (UTHealth) McGovern Medical School, National Center for Research Resources Grant UL1 RR024148, Alfred W. Lasher III Research Funds to The University of Texas Health Science Center at Houston (UTHealth) McGovern Medical School, and the Hermann Eye Fund (Houston, TX). The funding organizations had no role in the design or conduct of this research.
Abbreviations
- ROP
retinopathy of prematurity
- APROP
aggressive posterior retinopathy of prematurity
- CRYO-ROP
cryotherapy for retinopathy of prematurity
- ETROP
early treatment for retinopathy of prematurity
- BEAT-ROP
bevacizumab eliminates angiogenic threat of retinopathy of prematurity
- VEGF
vascular endothelial growth factor
- ICROP-R
International Classification for Retinopathy of Prematurity-Revisited
- FDA
federal drug administration
- R+EFP
ridge and extraretinal fibrovascular proliferative complex
- FNV
flat neovascularization
- BW
birth weight
- GA
gestational age
- PNA
postnatal age
- AA
adjusted age
- APGAR
backronym for Dr. Virginia Apgar’s score: appearance, pulse, grimace, activity, respiration
- CI
confidence interval
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- PDA
patent ductus arteriosus
- DD
disc diameters
Footnotes
Conflicts of Interest: None of the authors has any conflicts of interest to disclose.
Meeting Presentation: Previously presented in part at the JJ Kanski Medal Lecture at the 3rd Congress of the World Society of Pediatric Ophthalmology and Strabismus, Barcelona, Spain, September 6, 2015.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blencowe H, Lawn JE, Vasquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(1):35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106(4):471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 4.Henkind P. Ocular neovascularization. The Krill memorial lecture. Am J Ophthalmol. 1978;85(3):287–301. [PubMed] [Google Scholar]
- 5.Glaser BM, D’Amore PA, Michels RG, et al. The demonstration of angiogenic activity from ocular tissues. Preliminary report. Ophthalmology. 1980;87(5):440–446. doi: 10.1016/s0161-6420(80)35224-x. [DOI] [PubMed] [Google Scholar]
- 6.Kretzer FL, Mehta RS, Johnson AT, Hunter DG, Brown ES, Hittner HM. Vitamin E protects against retinopathy of prematurity through action on spindle cells. Nature. 1984;309(5971):793–795. doi: 10.1038/309793a0. [DOI] [PubMed] [Google Scholar]
- 7.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. New Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 9.Geisen P, Peterson LJ, Mariniuk D, Uppal A, Saito Y, Hartnett ME. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Molecular Vision. 2008;14(11):345–357. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Yang Z, Jiang Y, et al. Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014;55(2):737–744. doi: 10.1167/iovs.13-13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55(1):75–76. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- 12.Mintz-Hittner HA, Kennedy KA, Chuang AZ the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity: preliminary results of the BEAT-ROP clinical trial. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment for Retinopathy of Prematurity Cooperative Group. Quinn GE, Dobson V, et al. Visual field extent at 6 years of age in children who had high-risk prethreshold retinopathy of prematurity. Arch Ophthalmol. 2011;129(2):127–132. doi: 10.1001/archophthalmol.2010.360. [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment for Retinopathy of Prematurity Cooperative Group. Quinn GE, Dobson V, et al. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: findings at 4 to 6 years of age. J AAPOS. 2013;17(2):124–128. doi: 10.1016/j.jaapos.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hittner HM, Rhodes LM, McPherson AR. Anterior segment abnormalities in cicatricial retinopathy of prematurity. Ophthalmology. 1979;86(5):803–816. doi: 10.1016/s0161-6420(79)35437-9. [DOI] [PubMed] [Google Scholar]
- 16.Trigler L, Weaver RG, Jr, O’Neil JW, Barondes MJ, Freedman SF. Case series of angle-closure glaucoma after laser treatment for retinopathy of prematurity. J AAPOS. 2005;9(1):17–21. doi: 10.1016/j.jaapos.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Harder BC, Schlichtenbrede FC, von Baltz S, et al. Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol. 2013;155(6):1119–1124. doi: 10.1016/j.ajo.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Geloneck MM, Chuang AZ, Clark WL, et al. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132(11):1327–1333. doi: 10.1001/jamaophthalmol.2014.2772. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-H, Chen S-N, Lien RI, et al. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye. 2014;28:1080–1087. doi: 10.1038/eye.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015;122(5):1008–1015. doi: 10.1016/j.ophtha.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mintz-Hittner HA. Bevacizumab as monotherapy for retinopathy of prematurity. J AAPOS. 2010;14(1):2–3. doi: 10.1016/j.jaapos.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso J, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130(8):1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 23.Mintz-Hittner HA, Kretzer FL. Postnatal retinal vascularization in former preterm infants with retinopathy of prematurity. Ophthalmology. 1994;101(3):548–558. doi: 10.1016/s0161-6420(94)31301-7. [DOI] [PubMed] [Google Scholar]
- 24.Blair MP, Shapiro MJ, Hartnett ME. Fluorescein angiography to estimate normal peripheral retinal nonperfusion in children. J AAPOS. 2012;16(3):234–237. doi: 10.1016/j.jaapos.2011.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(11):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 26.Capone A, Jr, Ells AR, Fielder AR, et al. Standard image of plus disease in retinopathy of prematurity. Arch Ophthalmol. 2006;124(11):1669–1670. doi: 10.1001/archopht.124.11.1669-c. [DOI] [PubMed] [Google Scholar]
- 27.Lee BJ, Kim JH, Heo H, Yu YS. Delayed onset atypical vitreoretinal traction band formation after an intravitreal injection of bevacizumab in stage 3 retinopathy of prematurity. Eye. 2012;26(7):903–909. doi: 10.1038/eye.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel RD, Blair MP, Shapiro MJ, Lichtenstein SJ. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012;130(6):801–802. doi: 10.1001/archophthalmol.2011.1802. [DOI] [PubMed] [Google Scholar]
- 29.Jang S, Choi K, Lee SJ. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J AAPOS. 2010;14(5):457–459. doi: 10.1016/j.jaapos.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Zepeda-Romero LC, Liera-Garcia JA, Gutierrez-Padilla JA, Valtierra-Santiago CI, Avila-Gomez CD. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye. 2010;24(1):202. doi: 10.1038/eye.2009.156. [DOI] [PubMed] [Google Scholar]
- 31.Ittiara S, Blair MP, Shapiro MJ, Lichtenstein SJ. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J AAPOS. 2014;17(3):323–325. doi: 10.1016/j.jaapos.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Binenbaum G, Karp K, et al. Late recurrence of retinopathy of prematurity after treatment with both intravitreal bevacizumab and laser. J AAPOS. 2014;18(4):402–404. doi: 10.1016/j.jaapos.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246(7):1061–1063. doi: 10.1007/s00417-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 34.Kondo H, Arita N, Osato M, Hayashi H, Oshima K, Uchio E. Late recurrence of retinal detachment following successful vitreous surgery for stages 4B and 5 retinopathy of prematurity. Am J Ophthalmol. 2009;147(4):661–666. doi: 10.1016/j.ajo.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Suk KK, Berrocal AM, Murray TG, et al. Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. doi: 10.3928/01913913-20101217-06. Published online: 22 December 2010. [DOI] [PubMed] [Google Scholar]
- 36.Yetik H, Gunay M, Sirop S, Salihoglu Z. Intravitreal bevacizumab monotherapy for type-1 prethreshold, threshold, and aggressive posterior retinopathy-27 month follow-up results from Turkey. Graefes Arch Clin Exp Ophthalmol. doi: 10.1007/s00417-014-2867-0. Published online: 14 December 2014. [DOI] [PubMed] [Google Scholar]
- 37.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. NEJM. 2012;367(26):2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitral bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(10):213–216. 2526. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 39.AAP, AAO, AAPOS, and AAOCO. Screening examination of prematurity infants for retinopathy of prematurity. Pediatrics. 2013;131:189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]