Abstract
Background
A major goal of treatments for cocaine addiction is to reduce relapse-associated cravings, which are typically induced by environmental stimuli associated with cocaine use and related to changes in dopamine neurotransmission.
Methods
The present study utilized an animal model of cocaine seeking to determine functional consequences of cue exposure using [18F]fluoro-deoxyglucose (FDG) and PET and to relate findings to juvenile levels of dopamine transporter and D2-like receptor availabilities determined prior to any drug exposure. Adult male rhesus monkeys (N=11) self-administered cocaine (0.2 mg/kg/injection) under a second-order schedule of reinforcement, in which responding was maintained by conditioned reinforcers. PET scans assessing glucose utilization, a marker of functional activation, were conducted during cocaine-cue responding and food-reinforced responding in a context where cocaine was never available.
Results
Compared to the non-cocaine condition, we found significant functional activation in the medial prefrontal cortex, anterior cingulate, precuneus region of the parietal cortex, and striatum; findings similar to those reported in human cocaine abusers. Furthermore, these functional activations in the prefrontal, cingulate and parietal cortex measured during cocaine-cue responding were significantly correlated with juvenile measures of DAT availability, while no significant relationship with prior D2-like receptor availability was observed in any brain region.
Conclusions
The similarity between the present findings and those in human cocaine users supports the use of this model for examination of factors that impact the development and intensity of cue-induced drug seeking, and provide evidence for potential biomarkers for the evaluation of potential treatments (behavioral and pharmacological) for cocaine abuse.
Keywords: craving, cocaine, self-administration, PET imaging, dopamine transporters, rhesus monkeys
INTRODUCTION
A major obstacle for successful treatment of cocaine dependence is the intense cravings manifested by emotional, cognitive, and physiological signs and symptoms. These cravings can be brought on by stress, or often by exposure to stimuli that have been associated with cocaine use. Such cue reactivity is thought to play an important role in maintaining ongoing drug use as well as in precipitating relapse, even after prolonged periods of abstinence. Therefore, understanding the neurobiological basis of cue reactivity is critical for developing effective treatments for substance abuse.
It is not surprising then, that there have been extensive investigations focused on understanding the underlying behavioral, motivational, and cognitive factors that modulate cue reactivity. A number of recent reviews have identified such factors as addiction severity, withdrawal status, abstinence duration, polydrug use, and the type of environmental stimuli (1–5) as determinants of the magnitude of responses to drug-associated cues. Brain imaging studies, with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have shown patterns of functional activation in regions such as the dorsal and ventral striatum, anterior cingulate, amygdala, and prefrontal and parietal cortex in response to cues associated with cocaine use and availability (6–12). The role of these circuits can be difficult to study systematically in addict populations given the complex confounds, including poor self-report of drug histories, presence of co-morbid psychiatric illness, pre-existing differences in cognitive ability and education, and variability of duration of drug use and abstinence attempts.
One way to obviate these issues is to use preclinical models in which carefully controlled experiments can isolate the influence of individual variables. Preclinical animal models can provide accurate regulation of drug exposure in terms of duration of exposure, total intake, and use of other drugs, and can control for the impact of differences in experiences with the environment. Therefore, a goal of this study was to utilize a preclinical model of cue reactivity in cocaine self-administering nonhuman primates that would closely parallel studies in human addicts. Monkeys self-administered cocaine under a second-order schedule of reinforcement, in which cocaine seeking was maintained by conditioned stimuli, until the monkey received a cocaine injection that ended the session. We used [18F]fluoro-deoxyglucose (FDG) in combination with PET to measure patterns of metabolic activity in response to the presentation of cues associated with cocaine self-administration, and compared those to patterns obtained in response to cues associated with an environment in which the same animals responded for food reinforcement, but never received cocaine.
Another goal was to evaluate potential factors that may influence cue reactivity. We first considered the impact of two cocaine-related factors: total lifetime drug intake, and response rates under the second-order schedule. Secondly, since dopamine transporter (DAT) and dopamine D2-like receptor availabilities had been assessed previously with PET in each of these monkeys as juveniles, and prior to any drug exposure (13), we considered the relationship between DAT and D2-like receptor availabilities and the metabolic responses to cocaine-related cues as measured with FDG. These pre-existing markers may provide an index of vulnerability to drug-associated cue reactivity. We report a pattern of neural activation that accompanies the presentation of cocaine-associated cues and environmental context in nonhuman primates similar to that observed in human cocaine addicts. Furthermore, we show significant relationships between this pattern and pre-drug exposure DAT availability.
METHODS AND MATERIALS
Subjects and Apparatus
Eleven individually housed adult male rhesus monkeys (Macaca mulatta), ~6 years old, surgically implanted with indwelling intravenous catheters and subcutaneous vascular access ports (VAP; Access Technologies, Skokie, IL), with histories of cocaine and methylphenidate exposure as previously reported (13, 14), served as subjects (see Table 1). As described previously (13), five days per week, each monkey was seated in a primate chair and placed into a ventilated, sound-attenuating operant chamber (1.5 x 0.74 x 0.76 m; Med Associates, St. Albans, VT). Monkeys were weighed weekly and fed enough food daily (Purina Monkey Chow and fresh fruit and vegetables) to maintain body weights at ~98% of free-feeding levels. Water was available ad libitum. Animal housing, handling, and experimental procedures were performed in accordance with the 2011 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Institutional Animal Care and Use Committee (IACUC) of Wake Forest University. Environmental enrichment was provided as outlined in the Wake Forest University IACUC Non-Human Primate Environmental Enrichment Plan.
Table 1.
Mean [± SD] response rates for cocaine (resp/sec), cocaine intake (mg/kg), and previous methylphenidate intake (mg/kg) for each monkey
| FR1 | FR2 | FR3 | FR4 | FR5 | Mean Resp. Rate¶ | Lifetime Intake (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|
| Coc | MPH | |||||||
| R-1545 | 0.04 [0.01] | 0.03 [0.01] | 0.06 [0.02] | 0.10 [0.06] | 0.10 [0.08] | 0.07 [0.03] | 154.20 | 64.75 |
| R-1547 | 0.27 [0.13] | 0.30 [0.20] | 0.41 [0.18] | 0.37 [0.18] | 0.16 [0.20] | 0.30 [0.10] | 139.55 | 15.59 |
| R-1551 | 0.54 [0.34] | 0.74 [0.37] | 0.64 [0.28] | 0.80 [0.17] | 0.74 [0.39] | 0.69 [0.10] | 148.90 | 9.28 |
| R-1617 | 0.51 [0.01] | 0.45 [0.12] | 0.46 [0.02] | 0.52 [0.04] | 0.50 [0.10] | 0.49 [0.03] | 122.90 | 13.70 |
| R-1618 | 0.08 [0.04] | 0.08 [0.09] | 0.20 [0.09] | 0.26 [0.02] | 0.27 [0.06] | 0.18 [0.09] | 145.48 | 13.20 |
| R-1619 | 0.08 [0.02] | 0.08 [0.08] | 0.16 [0.09] | 0.30 [0.08] | 0.29 [0.04] | 0.18 [0.11] | 113.31 | 5.25 |
| R-1621 | 0.05 [0.02] | 0.20 [0.10] | 0.33 [0.16] | 0.36 [0.16] | 0.45 [0.25] | 0.28 [0.16] | 136.36 | 19.62 |
| R-1623 | 0.07 [0.02] | 0.05 [0.03] | 0.06 [0.06] | 0.05 [0.03] | 0.06 [0.01] | 0.06 [0.01] | 174.91 | 4.17 |
| R-1622 | 0.05 [0.02] | 0.31 [0.04] | 0.67 [0.16] | 0.58 [0.11] | 0.55 [0.11] | 0.43 [0.25] | 123.40 | 11.62 |
| R-1616 | 0.03 [0.00] | 0.06 [0.04] | 0.13 [0.05] | 0.10 [0.04] | 0.25 [0.09] | 0.12 [0.09] | 136.00 | 15.74 |
| R-1620 | 0.11 [0.05] | 0.14 [0.05] | 0.12 [0.06] | 0.19 [0.06] | 0.11 [0.06] | 0.13 [0.03] | 113.43 | 10.20 |
|
| ||||||||
| Mean SEM | 0.17 [0.06] | 0.22 [0.07] | 0.30 [0.07] | 0.33 [0.07] | 0.32 [0.07] | 0.27 [0.02] | 137.13 [5.88] | 17.42 [4.02] |
Mean rate of responding across the entire session (total responses/total session time)
Second-order schedule training
Sessions began with illumination of the houselight and white light above a photo-optic switch (finger poke, either the right or left; counterbalanced between monkeys). During the 10-s cocaine injection, the white light above the switch was extinguished, and the red light was illuminated. All monkeys had previously been trained to respond under a fixed-ratio (FR) 30 schedule of reinforcement (13). For the present study, the schedule was changed to a fixed-interval (FI) 300-s schedule of cocaine presentation, in which the first response after 300 s resulted in a 10-s cocaine injection. Monkeys were exposed to gradually increasing FI values, beginning with FI 30-s, over approximately 8–12 weeks until the final FI 300-s was obtained. The cocaine dose was initially 0.03 mg/kg/injection and sessions lasted 2 hr or until 20 injections had been obtained. After ~15 sessions, the cocaine dose was increased to 0.1 mg/kg/injection for approximately 30 sessions. Finally, as the monkey approached a total intake of 100 mg/kg, the dose was increased to 0.2 mg/kg/injection and the session length was lowered to 1 hr or 10 injections, whichever occurred first. Monkeys continued to respond under the FI 300-s schedule of 0.2 mg/kg cocaine until they had received a total cocaine intake under the FI contingency of approximately 100 mg/kg (including training with the lower doses). This total dose was chosen because we have previously shown that this intake produces robust changes in DA measures (15, 16).
Next, monkeys began training under the second-order schedule. First, the conditions were changed to a second-order FR 2 (FI 300-s: S) schedule of reinforcement such that the first response after 300-s resulted in extinction of the white lights and a 2-sec illumination of the red (“S”) lights (FR 1), followed by a return to the FI 300-s schedule, with the next FI completion (FR 2) resulting in a 10 sec 0.2 mg/kg cocaine injection (with illumination of the red lights and extinction of the white lights for 10 sec). Under these conditions, sessions ended after 5 cocaine injections or 1 hr. After 5–10 sessions, the schedule was changed to FR 3 (FI 300-s: S) and sessions ended after 4 injections or 1 hr and then to an FR 4 (FI 300-s: S) schedule with sessions ending after 3 injections or 1 hr. Under the final schedule parameters [FR 5 (FI 300-s: S)] sessions ended after 1 injection or 30 min. The primary dependent variable was response rates per FR. Mean data were analyzed with a one-way repeated-measures ANOVA and Newman-Keuls post-hoc tests analysis; P< 0.05 was considered significant.
Non-Cocaine Condition
During this same training period, all animals were trained to respond for 190 mg sucrose pellets in another room. During these non-cocaine conditions, monkeys were placed in a room and sound attenuating chambers distinct from their cocaine self-administration chambers. Monkeys were trained to touch a screen (0.3 x 0.23 m) using the Cambridge Neuropsychological Test Automated Battery (CANTAB; Lafayette Instruments, Lafayette, IN) apparatus. These CANTAB stations (0.38 x 0.56 x 0.31 m) were located in sound-attenuating, ventilated chambers (0.8 x 0.8 x 1.32 m) that included a non-retractable response lever, and a pellet receptacle located to the right side of each panel. For this condition, monkeys responded on a touch screen, when a purple box was presented, for the delivery of a pellet (FR 1); the purple box filled most of the screen. Sessions lasted 25 minutes and occurred 1–2 times per week for the duration of the study. Second-order responding was studied on the same days as CANTAB responding, but occurred at least 3 hrs after the end of the touch screen session. The primary dependent variable was number of reinforcers earned.
FDG Studies
Measurements of rates of cerebral glucose metabolism (CMRglc) were carried out in all eleven monkeys under two conditions: non-cocaine condition, involving the touch screen, and cocaine cue condition in the chambers in which they self-administered cocaine. All animals were acclimated to PET conditions so that behavior was not influenced by these procedures. A minimum of 10 sessions under the FR 5 (FI 300-s: S) schedule occurred before the first FDG PET study. At least 7 days separated non-cocaine and cocaine cue FDG studies. Scans were counter-balanced.
Because 40 min were required for incorporation of FDG into the brain, on the day of an FDG scan, the second-order schedule was changed to an FR 8 (FI 300-s: S) schedule of reinforcement, such that the monkey responded during that entire session solely for the cocaine-related cue (red light) and did not self-administer cocaine in that session. That is, the session was terminated by the investigator at 40 min (before a cocaine injection could have been obtained), and the monkey was sedated with ketamine while still in the chamber and transported to the PET Center. Likewise, for the non-cocaine condition FDG study, session length was 40 minutes. Monkeys were allowed to receive 150 mg sucrose pellets while in the non-cocaine condition and earned, on average, 35 pellets.
On the day of the scan, animals were placed in their chambers and a baseline blood glucose sample was obtained through the VAP. This was followed 2 minutes later by a 30 s injection of 5–7 mCi of [18F]-FDG through the VAP followed by a 5 ml flush of sterile saline. All procedures were carried out with the chamber door closed to prevent disturbing the monkey. At the end of 40 minutes monkeys were rapidly anesthetized with ketamine (10 mg/kg, i.v.) and a blood sample was obtained via the saphenous vein for glucose determination. Sedated animals were transported to the PET center where scanning began approximately 60 minutes after FDG injection. During the scan, anesthesia was maintained by isoflurane and core body temperature, blood pressure and O2 levels were monitored.
PET scans were carried out on a GE 16-slice PET/CT Discovery ST Scanner with 24 detector rings that provide 47 contiguous image planes over a maximum 70 cm transaxial field of view with CT attenuation correction. Axial spatial resolution of this scanner is 2.97 mm at the center of the gantry. Scans consisted of a 5 min transmission scan acquired in 2D mode, followed by a 10 min emission scan acquired in 3D mode. The image reconstruction of the 3D data used the 3D-reprojection method with full quantitative corrections and was smoothed using a 3.0 mm Gaussian filter transaxially and then segmented. Magnetic resonance imaging (MRI) scans were acquired earlier (13) and used for co-registration.
Image Processing and Analysis
PET data were analyzed using Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB (MathWorks, Natick, MA). Reconstructed images for each scan were co-registered to corresponding structural T1 weighted MRI using automated image registration; they were normalized to standard atlas space with an FDG template constructed in our laboratory based on procedures of Maldjian et al. (17). All images were processed by applying plasma glucose and radioactivity values measured during uptake and scanning using the MRGlu option of the FDG Autorad module in PMOD 3.4 (PMOD Technologies Ltd. Zurich, SW). Resultant images were spatially smoothed using a 2 mm isotropic Gaussian kernel with a voxel size of 1 × 1 × 1 mm.
Effects at each voxel were estimated according to the general linear model using the multi-subject conditions and covariates option in SPM with a minimum threshold of p < 0.005. Correction for multiple comparisons was accomplished by correction at the cluster level. In accordance with previous studies of FDG in nonhuman primates (18–21), contrasts across non-cocaine and cocaine-cue conditions were accomplished using paired t-tests. Proportional scaling was utilized to account for variations in global signal between sessions. Color-coded maps of statistical significance were projected onto the MRI template.
Correlations
As part of an earlier study, DAT and D2-like receptor availabilities were measured using PET prior to any cocaine or methylphenidate exposure when animals were between 24 and 30 months of age (13). To assess whether D2-like receptor or DAT availability was predictive of the response to the presentation of cocaine-associated cues, voxel-wise correlations between distribution volume ratio (DVR) values in the striatum and normalized CMRglc values were measured using SPM8. Significance was set at P<0.01 (uncorrected > 100 voxels). Pearson product moment correlations were calculated between DVRs and relative regional metabolic measures calculated in regions of interest based on significant clusters in the regression analysis. A similar strategy was used to calculate relationships between the metabolic response to cocaine-cues and total cocaine intake and average self-administration response rates.
RESULTS
Behavioral measures
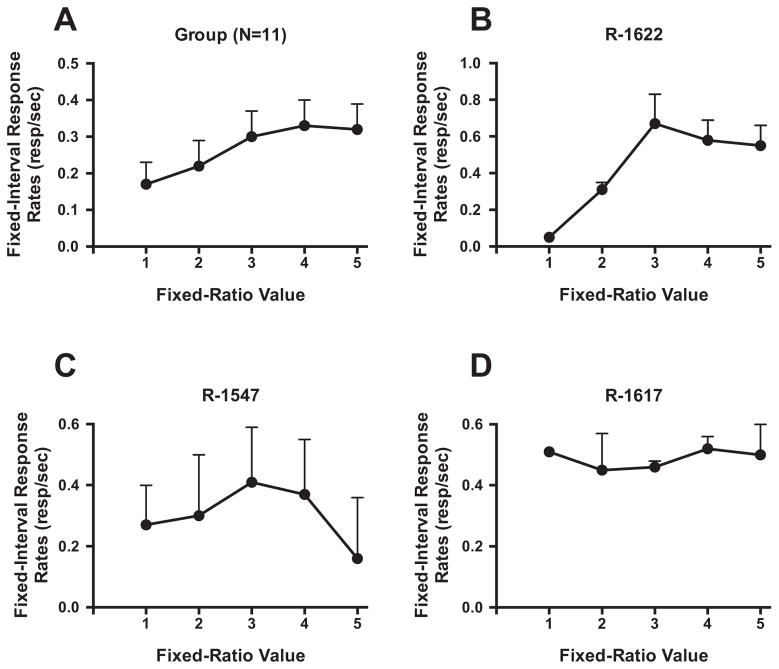
Response rates varied under the FR5 (FI 300-s: S) schedule of 0.2 mg/kg cocaine for the 11 monkeys (range 0.03–0.74 resp/sec; Table 1). For the group (Fig. 1A), response rates within each FI 300-s varied as a function of the FR value [F(4,10) = 6.23, P<0.005], with lower response rates associated with responding during the first FR compared to later components [FR 3, FR 4 and FR 5, all P<0.05]. However, there was individual-subject variability associated with patterns of responding within the second-order schedule. Representative examples of varying patterns of responding are shown in Fig. 1. Six monkeys responded at very low rates in the early FR components, with increases in responding later in the session (Fig. 1B), one monkey showed high rates early and lower rates later in the session, resembling an inverted-U shaped function (Fig. 1C), and four monkeys showed stable responding across each FR (Fig. 1D). Irrespective of the pattern, all monkeys completed each FR and received 0.2 mg/kg cocaine to end the session. The mean total lifetime cocaine intake prior to the first FDG study was 137.13 (± 5.9) mg/kg and the range was 113–175 mg/kg (Table 1). During the FDG study, the schedule was changed to FR8 (FI 300-s: S) in order to assure that monkeys did not receive Porrino, L.J 10 a cocaine injection during the FDG incorporation. Overall response rates under this schedule were not significantly different from mean baseline response rates (data not shown).
Figure 1.
Rate of responding (responses/second) in each component (fixed-ratio, FR) of a second-order FR 5 (fixed-interval 300-s: S) schedule of cocaine (0.2 mg/kg) reinforcement. A: Mean (± SEM) data; N=11. B-D: Representative data from individual monkeys demonstrating different patterns of responding across the session.
Effects of cocaine cues on glucose utilization measured with PET
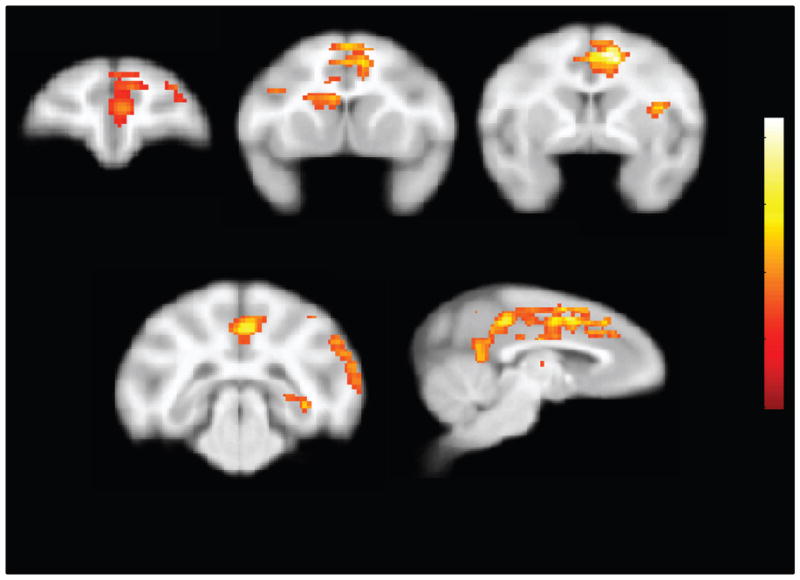
The results of the whole brain SPM analysis of the effects of the presentation of cocaine-associated cues as compared to the presentation of non-cocaine (CANTAB chamber) cues are shown in Table 2 and Fig. 2. SPM analysis showed increases in 4 clusters (threshold P < 0.005, false-discovery rate corrected at the cluster level, P < 0.05). These included the anterior cingulate, precuneus/cuneus, medial prefrontal cortex and the caudate region of the striatum. There were no significant clusters for the comparison Non-cocaine > Cocaine Cue even when the threshold was lowered to P < 0.05.
Table 2.
Significant clusters of activation during presentation of cocaine-associated cues (Cocaine vs Non-Cocaine).
| Analysis | Area | Hemisphere | Cluster | Coordinates | Maximum voxel t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cocaine > Non-Cocaine | Anterior cingulate | L, R | 1572 | 3 | 23 | 31 | 7.12 |
| Precuneus/cuneus | L, R | 909 | 1 | 7 | 30 | 6.33 | |
| Medial prefrontal cortex | L, R | 258 | 1 | 32 | 28 | 4.49 | |
| Caudate | L | 299 | −4 | 27 | 21 | 4.70 | |
| Non-Cocaine > Cocaine | No significant clusters | ||||||
Threshold, P < 0.005; corrected for multiple comparisons at the cluster level (false discovery rate, P < 0.05)
Figure 2.
Areas where regional relative rates of cerebral metabolism are significantly greater during exposure to cocaine-associated cues as compared to exposure to an environment never associated with drug (Cocaine > Non-Cocaine). Data were analyzed in SPM8 with images thresholded at P < 0.005 corrected for multiple comparisons at the cluster level (false discovery rate, P < 0.05). Areas of activation in medial prefrontal cortex, dorsal striatum, anterior cingulate cortex and precuneus are shown on T1 weighted MR structural images.
Effects of cocaine cues and correlation with behavioral measures
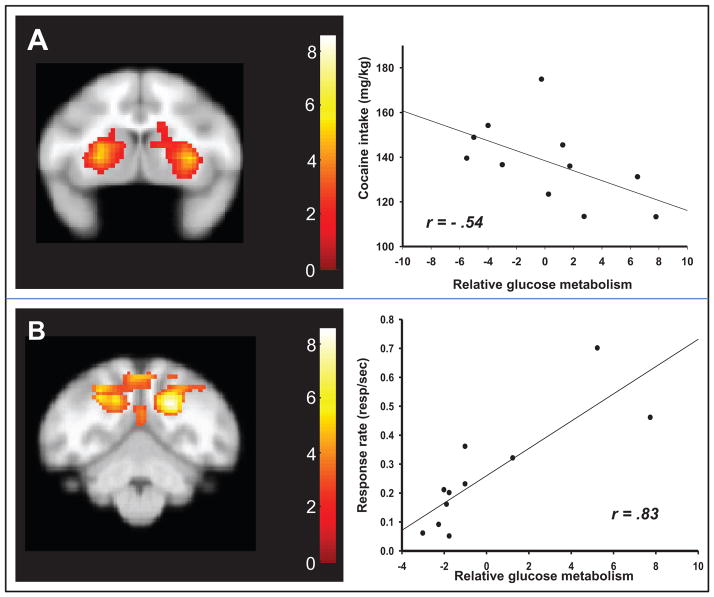
Secondary analyses were conducted to evaluate the relationship between some potential factors that have been identified as contributors to the intensity of craving in human studies (1, 2, 4, 22) and patterns of cue-associated brain activation. The correlation analyses (Fig. 3A) showed that there was a negative relationship between total lifetime cocaine intake and relative glucose utilization in response to cue presentation in a large cluster that encompassed the dorsolateral prefrontal cortex and striatum (r = −0.59; P = 0.032; k = 2328). There was also a significant positive relationship between average response rates over the session and relative glucose utilization in a cluster that spanned the cuneus and precuneus areas (r = 0.83; P = 0.043; k = 2474) (Fig. 3B). Measures of responding during non-cocaine (CANTAB) sessions did not correlate with patterns of brain activation during cocaine-cue or non-cocaine conditions.
Figure 3.
SPM results for voxelwise correlations between behavioral variables and rates of relative glucose metabolism during exposure to cocaine-associated stimuli. A. Rates of relative glucose metabolism in the dorsal striatum were negatively correlated with total lifetime cocaine intake. B. Rates of relative glucose metabolism in the inferior parietal/precuneus area were positively associated with average response rates during second order schedule cocaine sessions.
Effects of cocaine cues and correlation with pre-drug exposure dopamine measures
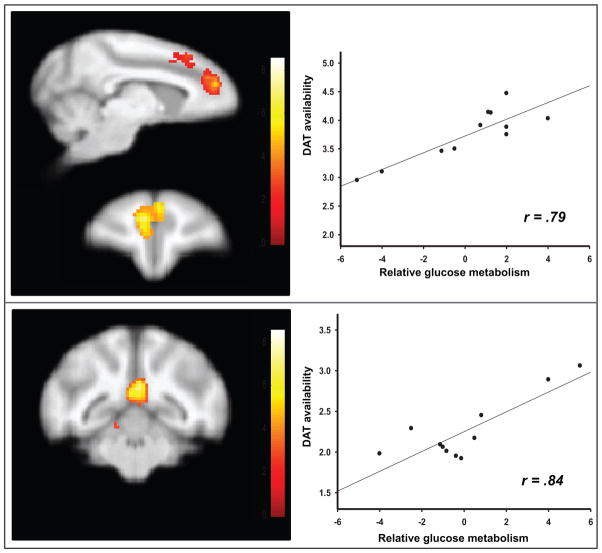
The correlation analyses also showed a significant positive association (r = 0.79; P = 0.050; k = 1601) between relative glucose utilization in a cluster that encompassed the anterior cingulate gyrus and the medial prefrontal cortex and previously obtained measures of DAT availability in the dorsal striatum (Fig. 4A). In addition, there was a significant positive correlation (r = 0.84; P = 0.008; k = 2059) between DAT availability in the ventral striatum and glucose utilization changes in a cluster that included the precuneus and posterior cingulate (Fig. 4B). There were no significant negative correlations. In contrast, the availability of D2-like receptors, as reflected by DVRs, did not correlate with responses to cues in any brain region. Neither DAT nor D2-like availability correlated with patterns of brain activation during non-cocaine conditions.
Figure 4.
SPM results for voxelwise correlations between dopamine transporter (DAT) availability measured prior to any drug exposure and rates of relative glucose metabolism during exposure to cocaine-associated stimuli. A. Rates of relative glucose metabolism in a cluster encompassing the medial prefrontal cortex and anterior cingulate were correlated with DAT availability in the dorsal striatum. B. Rates of relative glucose metabolism in the precuneus area were positively correlated with DAT availability in the ventral striatum.
DISCUSSION
The present study examined the effects of cocaine-associated cue exposure on functional brain activity, in monkeys self-administering cocaine, as measured with FDG and PET. Similar to what has been reported in human cocaine addicts, cue exposure elicited functional activation in a network of brain regions that included the medial prefrontal cortex, anterior cingulate cortex, precuneus, and striatum (6–12). The results of the present study also revealed significant relationships between cocaine history and the expression of cue-elicited functional activation in prefrontal cortex and striatum, as well as response rates and precuneus activity. The data further demonstrate a positive correlation between measures of DAT, but not D2-like receptor availability (assessed as juveniles prior to any drug exposure or experimental procedures) and metabolic activity elicited by cocaine cues. These correlations may provide new insights into the variables that play an important role in the predisposition to, and the expression of, reactivity to cocaine-associated environmental cues.
Studies in cocaine users employ a variety of approaches to engender cue-elicited craving. Videos of drug-related scenes or actual drug paraphernalia are often shown to subjects, or personalized scripts of drug experiences have been used and compared to various “neutral” conditions. Given the broad range of paradigms and differences among subjects, it would be expected that many circuits and brain regions have been associated with reactivity to cocaine cues including the anterior cingulate, striatum, prefrontal cortex, amygdala, insula, and posterior cingulate/inferior parietal cortex. There has been some distinction between the effects of cue exposure in treatment seekers vs. non-treatment seekers, with the amygdala activated in treatment seekers and dorsolateral and orbital prefrontal cortex, posterior cingulate/precuneus, and occipital areas showing stronger activations in non-treatment seekers (7, 10, 23). Despite the differences in species and paradigms, the network of structures identified here is highly consistent with those identified in cocaine users, but especially in non-treatment seekers, substantiating this network as key to the neural response to drug cues.
In the current study average cue-maintained response rates during second-order sessions were used as an indicator of cocaine motivation and were shown to be related to cue-elicited functional activity in the inferior parietal/precuneus region. It is important to note that these measurements were made in the environment in which the animals received drug. Although different from many human studies, where assessments are made in a laboratory setting quite distinct from where users typically experience cocaine, there is again much overlap in the areas related to craving intensity. Recent meta-analyses have identified consistent relationships in regions that include the inferior parietal cortex, visual cortical regions, and precuneus (1, 2, 5). The role of the precuneus in integrating such high-order tasks as attention to visual cues and preparation for goal-directed actions (24) is consistent with the recruitment of attention mechanisms in response to cocaine cues, as well as with studies of attentional bias to cocaine cues (25) where such bias has been shown to correlate with activity in neural networks including the precuneus (26). While this area is not generally thought to be a component of the reward circuit, activation here could serve as an important biomarker for treatment.
A role for the precuneus is further emphasized by the fact that this area, along with the prefrontal cortex and anterior cingulate, was among those regions in which the intensity of the response to cocaine was greatest in monkeys with the highest levels of pre-drug striatal DAT availability. Indeed, the relationship of striatal DAT availability to metabolic activity in the precuneus region following cocaine cues mirrored the relationship of metabolic activity and response rates (cocaine seeking) in this region. These data suggest that cocaine cue-induced responding, although coded in the ventral striatum, may be heavily influenced by attentional processes.
The importance of the DAT has also been suggested by studies that have shown that rats showing greater responsiveness to cocaine have higher DAT (27, 28). In the current study, because DAT measurements were made as juveniles, prior to any drug exposure, this could be considered akin to studies of the relationship of genetic variation in the DAT to drug-related cue responding in substance abusers. Moeller and colleagues (29) have demonstrated that, among current cocaine users, carriers of the DAT1 9-R allele showed an increased responsiveness to drug cues. In this study, there was a significant interaction between cocaine cues and DAT allele type within the prefrontal cortex, one of the regions in which a significant correlation was seen in the current study. These authors interpreted their findings to suggest that higher DAT availability in the DAT1 9-R carriers (30, 31) resulted in reduced tonic DA levels. This could magnify the decreases in DA produced by prolonged cocaine use (32, 33), further accentuating the effects of cocaine exposure. Although there remain differences in the interpretation of the consequences of the DAT1 9-R genetic variation (29–31, 34–36), taken together with the preclinical findings, there are clear indications of significant contributions of the DAT to the addiction process and further suggest the DAT as an important target for medication.
Although the role of D2-like receptors in vulnerability to cocaine abuse, as well as in the consequences of chronic cocaine abuse, has been well-established in both clinical and preclinical studies (see, for example, 16, 32, 37, 38–41), D2-like receptor availability did not predict functional activation in response to cocaine-cue exposure in the current study. These results may seem surprising in view of the effects of D2-like receptor antagonists on reinstatement in preclinical models (42–44), however, in contrast to reinstatement studies in which subjects (by definition) must have a cocaine history of self-administration, the D2-like receptor measures used in the present study were obtained prior to any cocaine or other drug history. Using within-subjects designs, PET measures have shown changes in D2-like receptor availability (16), but not DAT availability (45) following cocaine exposure, so the baseline measures do not necessarily represent current availability, but rather reflect basal levels unaffected by drug exposure. Future studies should examine the relationship between D2-like receptor availability in monkeys with more extensive cocaine histories and metabolic responsiveness in the presence of cocaine cues.
Each of these animals had a prior history of methylphenidate self-administration (14). It is difficult to compare the two drugs due to differences in the paradigms and the limited opportunities for methylphenidate consumption. This may account for the absence of any correlations between cocaine and methylphenidate intakes, as well as the absence of any relationship to dopamine markers (data not shown). A subset of animals (n=4) had been treated with extended release methylphenidate (13, 14). As demonstrated previously, this exposure did not alter cocaine or methylphenidate self-administration or dopamine markers, nor were there any differences in the current study between treated and untreated animals.
In conclusion, we have shown that exposure to cues associated with cocaine reinforcement, when compared to exposure to stimuli never associated with cocaine availability, produced a pattern of increases in glucose utilization in a network of brain regions that included the anterior cingulate cortex, medial prefrontal cortex, dorsal striatum and precuneus region of the parietal cortex in a nonhuman primate model of cocaine self-administration. These findings are highly consistent with reports in human addicts, suggesting the validity of this approach as a platform for investigations of potential cocaine treatment medications and of key factors contributing to the severity of craving without the confounds inherent in human studies.
Acknowledgments
The authors would like to acknowledge the technical assistance of Tonya Calhoun. Support: NIH/NIDA Grants P50 DA06634 and R01 DA20648.
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SJ, Sayette MA. Neuroimaging craving: urge intensity matters. Addiction. 2015;110:195–203. doi: 10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yalachkov Y, Kaiser J, Naumer MJ. Functional neuroimaging studies in addiction: multisensory drug stimuli and neural cue reactivity. Neurosci Biobehav Rev. 2012;36:825–835. doi: 10.1016/j.neubiorev.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 7.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 9.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 11.Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and frontostriatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill KE, Pierre PJ, Daunais J, Bennett AJ, Martelle S, Gage HD, et al. Chronic treatment with extended release methylphenidate does not alter dopamine systems or increase vulnerability for cocaine self-administration: a study in nonhuman primates. Neuropsychopharmacology. 2012;37:2555–2565. doi: 10.1038/npp.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martelle SE, Porrino LJ, Nader MA. Effects of chronic methylphenidate in adolescence on later methylphenidate self-administration in rhesus monkeys. Behav Pharmacol. 2013;24:478–481. doi: 10.1097/FBP.0b013e328364bfee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 17.Maldjian JA, Daunais JB, Friedman DP, Whitlow CT. Vervet MRI atlas and label map for fully automated morphometric analyses. Neuroinformatics. 2014;12:543–550. doi: 10.1007/s12021-014-9231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010;4:212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2:181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porrino LJ, Hampson RE, Opris I, Deadwyler SA. Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacology (Berl) 2013;225:105–114. doi: 10.1007/s00213-012-2798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, et al. The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction. 2014;109:2062–2070. doi: 10.1111/add.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict Biol. 2014;19:240–249. doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 25.Leeman RF, Robinson CD, Waters AJ, Sofuoglu M. A critical review of the literature on attentional bias in cocaine use disorder and suggestions for future research. Exp Clin Psychopharmacol. 2014;22:469–483. doi: 10.1037/a0037806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, et al. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology. 2014;39:1135–1147. doi: 10.1038/npp.2013.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson AM, Larson GA, Zahniser NR. Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. J Pharmacol Exp Ther. 2009;331:985–997. doi: 10.1124/jpet.109.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto DJ, Nelson AM, Mandt BH, Larson GA, Rorabaugh JM, Ng CM, et al. Rats classified as low or high cocaine locomotor responders: a unique model involving striatal dopamine transporters that predicts cocaine addiction-like behaviors. Neurosci Biobehav Rev. 2013;37:1738–1753. doi: 10.1016/j.neubiorev.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, et al. Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. J Neurosci. 2013;33:10027–10036. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and ancestry modulate brain’s DAT availability in healthy humans. PLoS One. 2011;6:e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- 32.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 34.Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, et al. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 36.Miller GM, Madras BK. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry. 2002;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- 37.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Jong JW, Roelofs TJ, Mol FM, Hillen AE, Meijboom KE, Luijendijk MC, et al. Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 40.Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 42.Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- 44.Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 45.Czoty PW, Gage HD, Nader SH, Reboussin BA, Bounds M, Nader MA. PET imaging of dopamine D2 receptor and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J Addict Med. 2007;1:33–39. doi: 10.1097/ADM.0b013e318045c038. [DOI] [PubMed] [Google Scholar]