Abstract
Statins (HMG-CoA reductase inhibitors) lower low-density lipoprotein cholesterol (LDL-C) and prevent cardiovascular disease. However, there is wide individual variation in LDL-C response. Drugs targeting proprotein convertase subtilin/kexin type 9 (PCSK9) lower LDL-C and will be used with statins. PCSK9 mediates the degradation of LDL receptors (LDLRs). Therefore, a greater LDL-C response to statins would be expected in individuals with PCSK9 loss-of-function (LOF) variants because LDLR degradation is reduced. To examine this hypothesis, the effect of 11 PCSK9 functional variants on statin response was determined in 669 African-Americans. One LOF variant, rs11591147 (p.R46L) was significantly associated with LDL-C response to statin (p=0.002). In the 3 carriers, there was a 55.6% greater LDL-C reduction compared to non-carriers. Another functional variant, rs298362261 (p.N425S), was marginally associated with statin response (p=0.0064).The effect of rs11591147 was present in individuals of European ancestry (N=2388, P=0.054). The therapeutic effect of statins may be modified by genetic variation in PCSK9.
Cardiovascular disease (CVD) affects over 84 million Americans and is the leading cause of death in all major ethnic groups; it accounts for 1 of every 3 deaths in the U.S. (1) Elevated low-density lipoprotein cholesterol (LDL-C) is a key reversible risk factor for CVD (2).
Statins (HMG-CoA reductase inhibitors) lower LDL-C concentrations and decrease CVD by approximately 20–30% (3,4), and in some populations by as much as 50% (5). Despite the overall benefits of statins in populations, there is wide variation in LDL-C response among individuals (6–8). Thus, there is interest in defining the genetic determinants of statin response.
Statins inhibit HMG-CoA reductase activity, lower circulating and cellular cholesterol concentrations and increase synthesis of LDL receptors (LDLRs) (9–11). LDLRs play a key role in the uptake and subsequent lysosomal hydrolysis of LDL-C (9,10); however, they are subject to degradation mediated by an enzyme, proprotein convertase subtilin/kexin type 9 (PCSK9) (9,10). Thus, PCSK9 reduces the number of LDLRs whereas statins have the opposite effect. However, statins also up-regulate the PCSK9 gene and this effect limits their ability to lower LDL-C (12). Therefore, individuals with PCSK9 variants of reduced function would be expected to have an increased response to statins because LDLR degradation would be reduced.
The PCSK9 gene is highly polymorphic and several gain-of-function (GOF) and loss-of-function (LOF) variants are reported to increase and decrease LDL-C concentrations, respectively (13–23). However, there is little information about the relationship between PCSK9 variants and the LDL-C response to statins. In one study of 25 subjects who responded particularly well to statin therapy, 3 had PCSK9 LOF variants (24), but in another study candidate PCSK9 variants were not associated with response to statin therapy (25). In a genome-wide association study of statin response in 6989 subjects of European ancestry, a PCSK9 LOF variant (rs11591147, R46L) associated to a greater response to rosuvastatin with locus-wide significance (26). The relationship between PCSK9 variants and statin response remains unclear but is important because many individuals who may receive PCSK9-inhibitor drugs are also likely to be receiving a statin.
Historically, the importance of PCSK9 LOF variants was defined in populations of African ancestry in whom uncommon variants were discovered (15). Thus, to define the relationship between PCSK9 variants and statin response, we studied African-Americans (AAs) who had been treated with a statin in clinical practice and had genotyping performed using the MetaboChip (27,28). We identified 11 previously reported functional variants at the PCSK9 locus (2 GOF and 9 LOF variants) and examined the hypothesis that these functional variants affected the magnitude of the LDL-C response to statin therapy.
Methods
Study Population
The study was approved by the Institutional Review Board of Vanderbilt University. The study cohort consisted of self-identified African-American (AA) and European American (EA) patients who had both a statin response phenotype (described later) and genotyping information available in the Vanderbilt DNA biobank (BioVU). BioVU accrues DNA samples from blood drawn for routine clinical testing after these samples have been retained for 3 days and are scheduled to be discarded. BioVU and sample handling have been described (29). Samples and existing genotypes in BioVU are linked to a de-identified version of each individual’s electronic health record (EHR).
Phenotype and covariates
We used the definition of statin response adopted by the Genomic Investigation of Statin Therapy (GIST) consortium (30) - the difference between the natural log-transformed LDL-C level on and off statin treatment for each patient. The beta of the corresponding regression thus reflects the fraction of differential LDL lowering in carriers versus non-carriers of the SNP. Statin exposure and LDL-C measures were extracted from each individual’s EHR by applying natural language processing algorithms that we have developed and validated (8,31,32). Each study participant was required to have at least one off-treatment LDL-C measurement and at least one on-treatment LDL-C measurement. Off-treatment LDL-C was defined as the median LDL-C level in the 20 months before the first mention of a statin in the EHR. The on-treatment LDL was defined as lowest LDL-C level within 20 months after the first mention of a statin in the EHR. Even though patients may achieve LDL-C lowering rapidly after statin exposure, LDL-C levels are seldom measured more frequently than at 6–12 month intervals in usual clinical practice. We have selected the 20-month window based on local clinical practice patterns. Given that many patients had <2 LDL-C measurements within 18 months after the first statin prescription we used that time frame but allowed an additional window of 2 months for appointment variations; e.g., an appointment for 6 months could actually occur in 7 or 8 months. Thus, we selected the 20-month window to define on-treatment LDL-C levels. A further analysis showed no association between LDL-C change and time of statin exposure (p=0.715, r2=−0.0002). On average, each patient had 9.8 ± 8.9 LDL-C measurements. The equation for calculating LDL-C response to statin treatment is shown below.
LDL response to statin treatment = ln(on-treatment LDL) − ln(off-treatment LDL)
Covariates including age, sex, and statin type and dose were extracted from the EHR. The statin type and dose closest to the lowest on-treatment LDL-C measurement was identified using methods we developed (8). A statin dose-equivalent was calculated for each patient as defined in Supplementary Table 1 (30,33). Population stratification was assessed by applying principal component analyses implemented in EIGENSOFT (34,35).
Manual chart review was performed for (1) Individuals with low (<50 mg/dl) and high (>250mg/dl) off-treatment LDL-C levels; (2) Individuals with very large LDL-C responses to statin treatment (LDL-C change >250mg/dl); (3) individuals who increased LDL-C levels after taking a statin; (4) individuals with multiple statin types or ambiguous doses.
Genotype
Genotyping was performed using the Illumina MetaboChip (27,28) a custom BeadChip targeting 196,725 genetic variants in AA individuals, the Illumina Infinium ExomeChip, targeting ~250,000 variants across the protein coding region of the genome, in individuals of European ancestry (EA). Genotyping data were curated for quality control using PLINK (36). We removed SNPs with a call rate less than 95%. No SNPs in this study deviated significantly from Hardy–Weinberg equilibrium (HWE) (<0.01). We removed samples (1) with per-individual call rate <95%; (2) with mismatch between genetic and EHR sex; (3) with a cryptic relationship closer than a third-degree relative. In the AA cohort, a total of 29 individuals were removed from the analysis. Genotypes of 105 SNPs within the PCSK9 locus were extracted from MetaboChip. We selected 12 LOF or GOF variants based on both association studies and functional studies. One variant, rs67608943 (Y142X), was monomorphic, thus, 11 functional variants were tested. Specifically, we selected variants based on the following criteria: (1) the variant had been associated with altered LDL-C levels in large-scale association studies or GWAS (rs11206510, rs2479409, rs11591147, rs11583680, rs11800243, rs72646508, rs28362261, rs28362263, rs562556, rs505151 and rs28362286) (13,16–19,21–23,37–39); or (2) the variant had been associated with altered circulating PCSK9 levels (rs2479409, rs11591147) (14). Two variants, rs11591147 and rs28362286 had also been reported as strong predictor for CHD risk (15,40).
To replicate the findings (described later) with rs11591147 in the AA cohort, we extracted statin response phenotypes for a cohort of 2388 Caucasian statin users in BioVU who had rs11591147 genotypes available from Illumina Infinium ExomeChip genotyping.
Statistical analysis
We used the R statistical package (41) to analyze the correlations between LDL-C response to statin therapy and clinical covariates, which included age, sex, body mass index (BMI), statin dose equivalent, co-morbidity (hypertension (HTN) and type 2 diabetes (T2DM)), off-treatment LDL-C levels and time between 1st statin exposure and LDL-C measurement. For correlation analysis, Kendall’s tau correlation coefficients were determined. One-way ANOVA analysis was used to compare LDL-C responses in individuals receiving different statin dose-equivalents. A p-value of less than 0.05 was considered statistically significant.
Genetic association analysis was performed using PLINK (36). No SNPs in this study deviated significantly from HWE (<0.01). We tested the associations between PCSK9 variants and (1) off-treatment LDL-C levels; (2) the difference between natural log-transformed on- and off-treatment LDL-C levels. An additive inheritance model was assumed and tested using a linear regression model. The analysis was adjusted for age (at the time of the on-treatment LDL-C measurement), sex, and two primary principal components (PCs) for ancestry. In order to control for possible associations with off-treatment LDL-C level, the analysis of LDL-C response to statin was additionally adjusted for off-treatment LDL-C (natural log transformed) and the statin dose-equivalent. A p-value < 0.0045 (0.05/11) was considered statistically significant. For rs11591147, we also performed a Wilcoxon rank sum test as a non-parametric test to limit the dependence of our test on the normal distribution. To account for the importance of covariates in our analysis, we performed a linear regression analysis testing the impact of only the covariates on LDL-C response and included the residuals in a Wilcoxon rank sum test with the genetics (42).
We tested the significant association with rs11591147 in statin users of European ancestry in BioVU (N=2388). Genetic analysis was performed using PLINK as described above. A p-value of less than 0.05 was considered statistically significant.
We further tested the collective effect of 11 PCSK9 variants in the AA group using a burden test. We collapsed the functional variants into single risk score. Specifically, we defined the risk score as number of GOF minus number of LOF variants for each individual. The burden test was adjusted for age, sex, natural log-transformed off-treatment LDL-C, statin dose-equivalent and two primary PCs for ancestry. A p-value of less than 0.05 was considered statistically significant.
Results
There were 669 AA statin users who had both statin response phenotype and genotype available (Table 1). The mean age was 55.4 ± 13.9 years. More than half of the cohort took simvastatin (352, 52.6%), and 155 patients (23.2%) took atorvastatin. Statin treatment lowered LDL-C from 139.1 ± 44.9 mg/dl to 93.0 ± 36.2 mg/dl.
Table 1.
Demographic Characteristics of AA and CA cohorts
| AA(N=669) | CA(N=2388) | ||
|---|---|---|---|
| Gender | male | 261 (39%) | 1186(49.7%) |
| female | 408 (61%) | 1202 (50.3%) | |
| Age (years) | 55.4 ± 13.9 | 61.9 ± 14.3 | |
| Hypertension | 631 (94.3%) | 2072(86.8%) | |
| Type 2 Diabetes | 270 (40.4%) | 613(25.7%) | |
| Statin Type | simvastatin | 352 | 1168 |
| atorvastatin | 155 | 685 | |
| pravastatin | 86 | 237 | |
| lovastatin | 45 | 158 | |
| fluvastatin | 20 | 55 | |
| rosuvastatin | 13 | 71 | |
| Off-statin LDL-C (mg/dl) | 139.1 ± 44.9 | 131.7 ± 37.5 | |
| On-statin LDL-C (mg/dl) | 93.0 ± 36.2 | 82.4 ± 29.3 | |
Data are presented as Mean ± SD or Number (percent)
Effect of covariates
There was no significant association between response to statin therapy and covariates such as age, sex, BMI and presence of HTN and T2DM (Supplementary Table 2). Of the 10 ancestry principal components (PCs) calculated using EIGENSOFT (34,35) two primary PCs accounted for majority of ancestry variation but were not associated with response to statin (p=0.075 and 0.94, respectively). Off-treatment LDL-C levels influenced LDL-C response to statin treatment strongly (p=3.14 × 10−15) as did statin dose (measured as statin dose-equivalents) (p=9.78 × 10−13).
Effect of PCSK9 variants
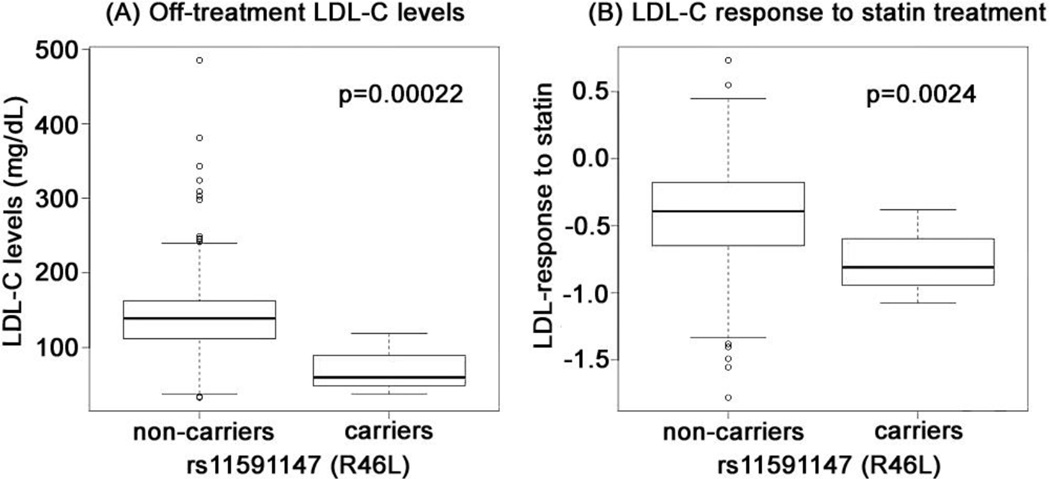
The LDL-C response to statin was significantly associated with rs11591147 (R46L), a LOF variant, (p=0.0024) (Table 2). The minor allele of the variant (T allele) was associated with a 55.6% greater LDL-C reduction (Figure). In the non-parametric test, rs11591147 remained significantly associated with LDL-C response to statin treatment (Wilcoxon rank sum test, unadjusted-p = 0.096, after adjustment-p = 0.026). This variant was also the most significant predictor for off-treatment (baseline) LDL-C levels (p=2.2×10−4) (Table 2, Figure). The 3 carriers of rs11591147 had an off-treatment LDL-C of 72.33 ± 41.9 mg/dL compared to an average off-treatment LDL-C of 139.4 ± 44.8 mg/dL among non-carriers (Figure). Another LOF variant, rs28362261 (N425S) was associated with statin response (p=0.0064) and off-treatment LDL-C levels (p=0.067) with borderline significance. The collective effect of all the functional was associated with LDL-C response to statin (burden test, p=0.035; Supplementary Table 3).
Table 2.
Association between PCSK9 variants and LDL-C response to statin treatment in AAs (N=669)
| rsNumber | MAF | Association with LDL-C response to statin | Association with off- treatment (baseline) LDL-C |
Reported Function 5 | |||
|---|---|---|---|---|---|---|---|
| Beta | % Extra reduction1 |
P-value2 | P-value3 | LOF / GOF | Amino Acid Change |
||
| rs11206510 | 0.1405 | −0.008671 | 0.8671 | 0.7236 | 0.702 | LOF | |
| rs2479409 | 0.2735 | 0.006977 | −0.6977 | 0.7192 | 0.2343 | GOF | |
| rs11591147 | 0.002242 | −0.5562 | 55.62 | 0.002366 | 0.0002164 | LOF | p.Arg46Leu |
| rs11583680 | 0.02167 | −0.01173 | 1.173 | 0.8451 | 0.9105 | LOF | p.Ala53Val |
| rs11800243 | 0.03587 | −0.009405 | 0.9405 | 0.8375 | 0.3367 | LOF | |
| rs726465084 | 0.0007474 | - | - | - | - | LOF | p.Leu253Phe |
| rs28362261 | 0.01424 | −0.1989 | 19.89 | 0.006384 | 0.06758 | LOF | p.Asn425Ser |
| rs28362263 | 0.09595 | −0.007844 | 0.7844 | 0.7848 | 0.2336 | LOF | p.Ala443Thr |
| rs562556 | 0.2138 | −0.00601 | 0.601 | 0.7714 | 0.9662 | LOF | p.Ile474Val |
| rs505151 | 0.2827 | 0.03808 | −3.808 | 0.05067 | 0.06105 | GOF | p.Glu670Gly |
| rs28362286 | 0.004491 | −0.1122 | 11.22 | 0.3832 | 0.5926 | LOF | p.Cys679Ter |
This percentage reflects the % additional LDL-C lowering in carriers versus non-carriers of the SNP.
P-value has been adjusted for age, sex, statin dose, PCs for ancestry, and ln(baseline LDL-C)
P-value has been adjusted for age, sex, and PCs for ancestry
There was only one carrier of rs72646508, so the variant was removed from single variant association analysis, but included in collective effect analysis
Figure. Effect of rs11591147 on baseline LDL-C concentrations and statin response.
Off-treatment LDL-C levels (A) and statin response (B) in carriers and non-carriers of rs11591147. The analysis was adjusted for age (at the time of the on-treatment LDL-C measurement), sex, and two primary principal components (PCs) for ancestry. In order to control for possible associations with off-treatment LDL-C level, the analysis of LDL-C response to statin was additionally adjusted for off-treatment LDL-C (natural log transformed) and the statin dose-equivalent. A p-value < 0.0045 (0.05/11) was considered statistically significant.
Effect in the European Americans
We validated the top hit in a EA cohort (Table 1) in whom the rs11591147 had a MAF of 3.4% and was strongly associated with off-treatment LDL-C levels (p=2.7×10−7); similar to a previous report (26) it was associated with an additional 7.0% reduction of LDL-C after statin treatment compared to non-carriers (p=0.054).
Discussion
The current study represents the first comprehensive analysis of the association between PCSK9 functional variants and statin response in an AA cohort. The major finding was that a PCSK9 variant rs11591147 was associated with a 55.6% increase in LDL-C reduction compared to non-carriers (p=0.0024) AAs; the association was also present in EAs, with a smaller effect size (7%, p=0.054). Thus, this study provides genetic evidence of synergy between HMG-CoA-reductase inhibition and PCSK9 inhibition in treatment of hyperlipidemia.
PCSK9 regulates circulating LDL-C levels primarily by binding to LDLRs and facilitating their degradation, and there is a positive correlation between circulating PCSK9 and LDL-C levels (43,44). Variants in the PCSK9 gene were initially associated with autosomal dominant hypercholesterolemia in patients carrying rare GOF variants (45). By screening the coding region of PCSK9 in 128 subjects, Cohen et al. identified two LOF PCSK9 variants (40) - carriers of those LOF variants not only had 40% lower LDL-C levels, but also were protected from CVD (p=0.008) (15,40).
Statins increase the number of LDLRs as well as PCSK9 expression (12,46). This increase in PCSK9, and the consequent increase in LDLR degradation, may be why the LDL-C response to statin treatment plateaus (12). Individuals who carry PCSK9 LOF variants have low expression or reduced activity of PCSK9 and would be expected to achieve greater LDL-C reduction with statins compared to non-carriers.
Rs11591147 is a well-characterized LOF variant in PCSK9. Carriers of rs11591147 were reported to have a reduced risk for ischemic heart disease and myocardial infarction (18,47–49). The nucleotide alteration results in an amino acid change at Arg46 to Leu46 (R46L) next to an Ser47 phosphorylation site and PCSK9 L46 was more susceptible to proteolytic degradation (50).
We found a relationship between the presence of rs11591147 and the LDL-C response to statin therapy in both AAs and EAs. This is concordant with a study performed in patients with familial hypercholesterolemia in whom carriers of rs11591147 had lower than expected LDL-C levels while receiving statin treatment (24). Nevertheless, previous observations from Caucasian populations for relationships between rs11591147 (R46L) and statin response are inconclusive. While rs11591147 has previously been associated with LDL-C response to both rosuvastatin (26) and atorvastatin (51), Polisecki et al. observed no association between the variant and pravastatin treatment in an elderly cohort (aged 70–82) (52). Clinical covariates (for example, statin type and age at statin exposure) may influence statin response and without such knowledge and appropriate adjustment, the ability to detect genetic predictors may be limited. This might explain why a recent meta-analyses of >20,000 Caucasians failed to identify rs11591147 as a predictor of statin response (30).
Although we observed an effect of rs11591147 on statin response in AAs and EAs, the effect size was larger in AAs. Population-specific allele frequency might contribute to the variation in effect size. It is also possible that the effect of rs11591147 is further modified by ethnic-specific genetic or environmental factors. Also, there were only 3 AA carriers of the allele and thus the point estimate of the effect size may not be well defined.
We also observed population-specific allele frequency in other PCSK9 genetic variants. Rs11583680 has MAF of 13% in European and only 1% in African populations (1000 Genomes Project). In our AA cohort, rs11583680 had a MAF of 2%. This variant was not associated with statin response in either Caucasians (recent meta-analyses of >20,000 individuals) (30) or AAs (our cohort). Rs28362261 is an AA-exclusive variant with MAF of 2% in Africans and 0% in Caucasians (1000 Genomes Project). In our AA statin cohort, rs28363361 associated with statin response (p= 0.006). The MAF in Caucasians is too low to replicate its effect in that population group. The variation in genetic architecture between AAs and EAs may contribute to ancestry-related variation of statin response. Further trans-ethnic studies in larger AA and EA populations as well as populations of other ethnicity are needed.
We identified 11 functional PCSK9 variants from both genetic association studies and functional studies (13–23). One might expect all the functional variants to be associated with alterations in off-treatment LDL-C (LDL-C lower with LOF and higher with GOF variants). This was not the case and several explanations are possible. The likely explanation is that the effect of some variants was small or was modified by variants in other genes or by non-genetic factors affecting LDL-C concentrations. Similarly, not all PCSK9 functional variants were associated with statin response. Most of the variants had either low frequency or small effect size, factors that would limit detection of an effect. However, it was interesting to note that even though the p-values did not reach significance, all variants had the expected direction of effect for statin response as predicted from their GOF or LOF characterization (Table 2) and the burden test of the collective effect was significantly associated with LDL-C response to statin therapy (P=0.03).
It is critical to understand the genetic architecture of the AA population and its relation to response to medications for several reasons. First, testing the generalizability of genetic findings from EA populations to AA populations will reduce the false positive findings caused by LD structure. Second, considering the specific genetic structures and richness of sequence variations, genetic studies in AA populations have the potential to reveal novel mechanisms not detected in by studying EAs. Last, understanding the variation of the magnitude of effect in different populations will contribute to translating genetic findings to clinical practice. However, genetic studies in AAs often have the limitation that large cohorts are not readily available. Although our study was one of the largest statin response studies in AAs, the sample size was a limitation.
We conclude that some LOF variants in PCSK9 are associated with enhanced statin response. This finding is of interest since the Food and Drug Administration recently approved the first PCSK9 inhibitors, alirocumab, as a new lipid lowering medication (53).
Supplementary Material
Acknowledgments
This study was supported by GM109145, UL1 RR024975, P50GM115305, U19HL065962, U01HL069757 and K23AR064768. The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center's resources, BioVU and the Synthetic Derivative, which are supported by institutional funding and by the Vanderbilt National Center for Advancing Translational Science grant 2UL1 TR000445-06 from NCATS/NIH. Existing genotypes in BioVU were funded by NIH grants RC2GM092618 from NIGMS/OD and U01HG004603 from NHGRI/NIGMS.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update A Report From the American Heart Association. Circulation. 2015 Jan 27;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010 Dec;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010 Nov 13;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012 Aug 11;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 6.Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (l-tap): A multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med. 2000 Feb 28;160(4):459–467. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 7.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, Shiboski S, et al. Phenotypic Predictors of Response to Simvastatin Therapy Among African-Americans and Caucasians: The Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol. 2006 Mar 15;97(6):843–850. doi: 10.1016/j.amjcard.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 8.Wei W-Q, Feng Q, Jiang L, Waitara MS, Iwuchukwu OF, Roden DM, et al. Characterization of Statin Dose-response within Electronic Medical Records. Clin Pharmacol Ther. 2013 Oct 4; doi: 10.1038/clpt.2013.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009 Apr;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein JL, Brown MS. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell. 2015 Mar 26;161(1):161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovanen PT, Bilheimer DW, Goldstein JL, Jaramillo JJ, Brown MS. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, et al. Statins Upregulate PCSK9, the Gene Encoding the Proprotein Convertase Neural Apoptosis-Regulated Convertase-1 Implicated in Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004 Aug 1;24(8):1454–1459. doi: 10.1161/01.ATV.0000134621.14315.43. [DOI] [PubMed] [Google Scholar]
- 13.Chen SN, Ballantyne CM, Gotto AM, Tan Y, Willerson JT, Marian AJ. A Common PCSK9 Haplotype, Encompassing the E670G Coding Single Nucleotide Polymorphism, Is a Novel Genetic Marker for Plasma Low-Density Lipoprotein Cholesterol Levels and Severity of Coronary Atherosclerosis. J Am Coll Cardiol. 2005 May 17;45(10):1611–1619. doi: 10.1016/j.jacc.2005.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chernogubova E, Strawbridge R, Mahdessian H, Mälarstig A, Krapivner S, Gigante B, et al. Common and low-frequency genetic variants in the PCSK9 locus influence circulating PCSK9 levels. Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1526–1534. doi: 10.1161/ATVBAHA.111.240549. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N Engl J Med. 2006 Mar 23;354(12):1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 16.Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013 Nov;45(11):1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathiresan S, Willer CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41(1):56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, et al. A Spectrum of PCSK9 Alleles Contributes to Plasma Levels of Low-Density Lipoprotein Cholesterol. Am J Hum Genet. 2006 Mar;78(3):410–422. doi: 10.1086/500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shioji K, Mannami T, Kokubo Y, Inamoto N, Takagi S, Goto Y, et al. Genetic variants in PCSK9 affect the cholesterol level in Japanese. J Hum Genet. 2004 Jan 15;49(2):109–114. doi: 10.1007/s10038-003-0114-3. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull C, Perdeaux ER, Pernet D, Naranjo A, Renwick A, Seal S, et al. A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat Genet. 2012 Jun;44(6):681–684. doi: 10.1038/ng.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterworth DM, Ricketts SL, Song K, Chen L, Zhao JH, Ripatti S, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2264–2276. doi: 10.1161/ATVBAHA.109.201020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008 Feb;40(2):161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Waite LL, Jackson AU, Sheu WH-H, Buyske S, Absher D, et al. Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained. PLoS Genet. 2013 Mar 21;9(3):e1003379. doi: 10.1371/journal.pgen.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berge KE, Ose L, Leren TP. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1094–1100. doi: 10.1161/01.ATV.0000204337.81286.1c. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JM, Cerda A, Hirata MH, Rodrigues AC, Dorea EL, Bernik MMS, et al. Influence of PCSK9 polymorphisms on plasma lipids and response to atorvastatin treatment in Brazilian subjects. J Clin Lipidol. 2014 May;8(3):256–264. doi: 10.1016/j.jacl.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet. 2012 Apr 1;5(2):257–264. doi: 10.1161/CIRCGENETICS.111.961144. [DOI] [PubMed] [Google Scholar]
- 27.Crawford DC, Goodloe R, Brown-Gentry K, Wilson S, Roberson J, Gillani NB, et al. Characterization of the Metabochip in diverse populations from the International HapMap Project in the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) project. Pac Symp Biocomput Pac Symp Biocomput. 2013:188–199. [PMC free article] [PubMed] [Google Scholar]
- 28.Voight BF, Kang HM, Ding J, Palmer CD, Sidore C, Chines PS, et al. The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits. PLoS Genet. 2012 Aug 2;8(8):e1002793. doi: 10.1371/journal.pgen.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postmus I, Trompet S, Deshmukh HA, Barnes MR, Li X, Warren HR, et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat Commun. 2014;5:5068. doi: 10.1038/ncomms6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peissig P, Sirohi E, Berg RL, Brown-Switzer C, Ghebranious N, McCarty CA, et al. Construction of atorvastatin dose-response relationships using data from a large population-based DNA biobank. Basic Clin Pharmacol Toxicol. 2007;100:286–288. doi: 10.1111/j.1742-7843.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilke RA, Berg RL, Linneman JG, Zhao C, McCarty CA, Krauss RM. Characterization of low-density lipoprotein cholesterol-lowering efficacy for atorvastatin in a population-based DNA biorepository. Basic Clin Pharmacol Toxicol. 2008;103(4):354–359. doi: 10.1111/j.1742-7843.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 33.Stein EA. Extending therapy options in treating lipid disorders: a clinical review of cerivastatin, a novel HMG-CoA reductase inhibitor. Drugs. 1998;56(Suppl 1):25–31. doi: 10.2165/00003495-199856001-00004. discussion 33. [DOI] [PubMed] [Google Scholar]
- 34.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006 Dec;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006 Aug;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 36.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guella I, Asselta R, Ardissino D, Merlini PA, Peyvandi F, Kathiresan S, et al. Effects of PCSK9 genetic variants on plasma LDL cholesterol levels and risk of premature myocardial infarction in the Italian population. J Lipid Res. 2010 Nov;51(11):3342–3349. doi: 10.1194/jlr.M010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005 Feb;37(2):161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 41.R Development Core Team. R: A language and environment for statistical computing. [Internet] R Foundation for Statistical Computing. 2010 Available from: http://www.R-project.org. [Google Scholar]
- 42.LaVange LM, Koch GG. Rank score tests. Circulation. 2006 Dec 5;114(23):2528–2533. doi: 10.1161/CIRCULATIONAHA.106.613638. [DOI] [PubMed] [Google Scholar]
- 43.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and Metabolic Determinants of Plasma PCSK9 Levels. J Clin Endocrinol Metab. 2009 Jul;94(7):2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert G, Ancellin N, Charlton F, Comas D, Pilot J, Keech A, et al. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin Chem. 2008 Jun;54(6):1038–1045. doi: 10.1373/clinchem.2007.099747. [DOI] [PubMed] [Google Scholar]
- 45.Abifadel M, Varret M, Rabès J-P, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003 Jun;34(2):154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 46.Mayne J, Dewpura T, Raymond A, Cousins M, Chaplin A, Lahey KA, et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 2008;7:22. doi: 10.1186/1476-511X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010 Jun 22;55(25):2833–2842. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 48.Humphries SE, Neely RDG, Whittall RA, Troutt JS, Konrad RJ, Scartezini M, et al. Healthy individuals carrying the PCSK9 p.R46L variant and familial hypercholesterolemia patients carrying PCSK9 p.D374Y exhibit lower plasma concentrations of PCSK9. Clin Chem. 2009 Dec;55(12):2153–2161. doi: 10.1373/clinchem.2009.129759. [DOI] [PubMed] [Google Scholar]
- 49.Kathiresan S Myocardial Infarction Genetics Consortium. A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med. 2008 May 22;358(21):2299–2300. doi: 10.1056/NEJMc0707445. [DOI] [PubMed] [Google Scholar]
- 50.Dewpura T, Raymond A, Hamelin J, Seidah NG, Mbikay M, Chrétien M, et al. PCSK9 is phosphorylated by a Golgi casein kinase-like kinase ex vivo and circulates as a phosphoprotein in humans. FEBS J. 2008 Jul;275(13):3480–3493. doi: 10.1111/j.1742-4658.2008.06495.x. [DOI] [PubMed] [Google Scholar]
- 51.Thompson JF, Hyde CL, Wood LS, Paciga SA, Hinds DA, Cox DR, et al. Comprehensive Whole-Genome and Candidate Gene Analysis for Response to Statin Therapy in the Treating to New Targets (TNT) Cohort. Circ Cardiovasc Genet. 2009 Apr 1;2(2):173–181. doi: 10.1161/CIRCGENETICS.108.818062. [DOI] [PubMed] [Google Scholar]
- 52.Polisecki E, Peter I, Simon JS, Hegele RA, Robertson M, Ford I, et al. Genetic variation at the NPC1L1 gene locus, plasma lipoproteins, and heart disease risk in the elderly. J Lipid Res. 2010 May 1;51(5):1201–1207. doi: 10.1194/jlr.P001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Press Announcements - FDA approves Praluent to treat certain patients with high cholesterol [Internet] [cited 2015 Jul 26]; Available from: h://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455883.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.