Abstract
Heat shock proteins (HSPs) present as a double edged sword. While they play an important role in maintaining protein homeostasis in a normal cell, cancer cells have evolved to co-opt HSP function to promote their own survival. As a result, HSPs such as HSP90 have attracted a great deal of interest as a potential anticancer target. These efforts have resulted in over 20 distinct compounds entering clinical evaluation for the treatment of cancer. However, despite the potent anticancer activity demonstrated in preclinical models, to date no HSP90 inhibitor has obtained regulatory approval. In this review we discuss the unique challenges faced in targeting HSPs that have likely contributed to their lack of progress in the clinic and suggest ways to overcome these so that the enormous potential of these compounds to benefit patients can finally be realized. We also provide a guideline for the future development of HSP-targeted agents based on the many lessons learned during the last two decades in developing HSP90 inhibitors.
Keywords: HSP90, chaperone, inhibitor, cancer
1. INTRODUCTION
Heat shock proteins (HSPs) are molecular chaperones which function to maintain protein homeostasis through the proper folding and activation of client proteins in the cell and are characterized by their ability to become overexpressed under conditions of stress [1-3]. There are a number of HSPs known, and these are classified according to their approximate molecular weight and include the small HSPs (i.e. HSP27), HSP40, HSP60, HSP70, HSP90 and HSP110. The study of the role of HSPs in the cause and progression of diseases such as cancer [4-5], neurodegeneration [6-7] and infection [8] continues to be an active area of research. The realization that the fundamental cause of a number of diseases can be attributed to the disruption of protein homeostasis has resulted in tremendous efforts aimed at identifying therapies directed towards HSPs. HSP90 and HSP70 have received the most interest and are the ones best understood in terms of drug discovery. The development of HSP90 inhibitors is by far most advanced, and there have been approximately 20 inhibitors that have undergone clinical evaluation [9-10]. Though none have yet been approved by the FDA, the hard lessons learned can serve as a blueprint for the future development of other HSP inhibitors. In this review we hope to convey the difficulties and challenges in developing these drugs, some of which are unique to chaperones and to offer some insight towards overcoming these challenges.
2. HSP90 AS A TARGET IN DISEASE
HSP90 is an ATP-dependent molecular chaperone that harnesses the energy derived from the hydrolysis of ATP towards the folding of its client proteins [11-12]. The mechanism whereby it accomplishes this is not fully understood, however, it is known to be a complex and highly regulated process involving numerous co-chaperones such as HSP70, HSP70-HSP90 organizing protein (HOP), HSP70-interacting protein (HIP), cell division cycle 37 (Cdc37), activator of HSP90 ATPase (Aha1) and p23 [13-15]. HSP90 is ubiquitously expressed and is one of the most abundant intracellular proteins in mammalian cells and folds a wide range of proteins involved in signal transduction, assembly and trafficking [16]. With more than 200 known client proteins, it has been recognized as a potential target for a number of diseases associated with aberrant protein signaling including cancer, neurodegeneration and infectious diseases [17-20]. The high abundance of HSP90, its presence in normal cells and its recognized housekeeping function would seem to suggest that it is a poor target and that efforts to develop drugs against it would be futile. However, the tremendous efforts that have been undertaken during the past 20 years to develop HSP90 inhibitors would seem to directly contradict this [9-10].
3. HSP90 AS AN ANTICANCER TARGET
Interest in HSP90 as an anticancer target derives from the properties observed from the ansamycin antibiotic geldanamycin (GM; Fig. 1). It was discovered as a hit in a screen of compounds for the ability to reverse the phenotype of v-src transformed cells [21]. Though its specific target was unknown at the time, the selective and potent anticancer activity of GM was intriguing. Subsequently, it was discovered to act directly as an HSP90 inhibitor by targeting the N-terminal nucleotide binding pocket [22-24]. However, an important question remained to be answered; how can an agent which targets a protein that is ubiquitously expressed exhibit selective toxicity towards cancer cells?
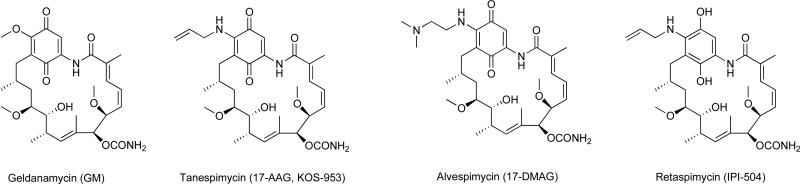
Fig. 1. Geldanamycin derivatives as HSP90 inhibitors.
Represented here are the chemical structures of inhibitors that are based on the benzoquinone ansamycin antibiotic geldanamycin. These inhibitors bind to the nucleotide-binding pocket situated at the N-terminal domain of HSP90.
Some initial insight into this was provided by the Neckers group in 1996 who found that stable expression of mutant p53, but not wild type p53, required tight association with HSP90 [25]. GM was able to selectively disturb the association of mutant p53 with HSP90 resulting in its degradation while not affecting wild type p53, thus demonstrating that client proteins have varying degrees of dependence upon HSP90 and that inhibition with a small molecule inhibitor does not indiscriminately result in the degradation of its client proteins. Rather, inhibition results in a graded response whereby certain client proteins can be degraded while others remain unaffected. A major breakthrough towards an explanation for the selectivity of GM came from Kamal et al. who showed that tumor cell-derived HSP90 exhibits a 100-fold higher binding affinity for 17-AAG (Fig. 1), a derivative of GM, than HSP90 from normal cells [26]. They showed that in tumor cells, HSP90 is present in a high-affinity conformation where it is present entirely in multi-chaperone complexes with activating co-chaperones p23 and HOP. They also showed that tumor cell-derived HSP90 complexes exhibited increased ATPase activity and possessed higher affinity for HSP90 inhibitors. In contrast to this, HSP90 in normal cells exists as an uncomplexed species with low AT-Pase activity and low affinity for HSP90 inhibitors. The fact that HSP90 exists in an altered high affinity conformation provided a suitable explanation for the selectivity observed with GM and other HSP90 inhibitors and for the first time provided a therapeutic rational for such agents as anticancer drugs.
These views were further refined by Moulick et al. who provided a clearer picture into the state of affairs of HSP90 in transformed cells [27]. Their findings suggest that not all HSP90 in cancer cells is present in high-affinity complexes. Rather, cancer cells harbor two distinct HSP90 populations; a major set being housekeeping chaperone HSP90 similar to the ones present in normal cell while the other fraction, constituting about 20-30% of total HSP90, consists of stressed HSP90 chaperone in complex with oncogenic partners (i.e. co-chaperones) that are essential for malignant transformations. Furthermore, this study showed that the HSP90 inhibitor PU-H71 (Fig. 3) selectively binds to stressed cancerspecific HSP90-oncoprotein networks; Bcr-Abl-HSP90 complex in K562 chronic myeloid leukemia cells, mutant B-RAF-HSP90 in SkMel28 melanoma cells and Her3-HSP90 and Raf1-HSP90 complex in MDA-MB-468 breast cancer cells. This characteristic, however, was not observed for all HSP90 inhibitors, and only certain compounds selectively targeted the stress chaperones, suggesting that inhibitors have varying ability to discriminate for the oncogenic HSP90 fraction.
Fig. 3. Purine derivatives as Hsp90 inhibitors.
Represented here are the chemical structures of HSP90 inhibitors that are based on the purine scaffold. These inhibitors target the nucleotide-binding pocket situated at the N-terminal domain of HSP90.
This oncogenic HSP90 fraction represents a cell stressspecific form of chaperone complex, that is expanded and constitutively maintained in the tumor cell context, and that may execute functions necessary to maintain the malignant phenotype. Such roles are not only to regulate the folding of overexpressed (i.e. HER2), mutated (i.e. mB-Raf, mEGFR) or chimeric proteins (i.e. Bcr-Abl), but also to facilitate scaffolding and complex formation of molecules involved in aberrantly activated signaling complexes (i.e. STAT5, BCL6) [27]. This species is a highly co-chaperone dependent HSP90 that certain cancer cells use to maintain the altered proteins and protein networks that are needed to drive the malignant phenotype. For instance, the PU-H71-bound Bcr-Abl-HSP90 complexes also bound HSP70, HSP40, HOP and HIP [27].
Further understanding has begun to reveal the dramatic effects of post-translational modifications (PTMs) on the function of HSP90 and in defining its oncogenic nature [28]. HSP90 can be modified by a number of PTMs including phosphorylation, acetylation, ubiquitination, S-nitrosylation, SUMOylation and oxidation. Studies have shown that these PTMs influence HSP90 chaperone activity in numerous ways including affecting the binding and hydrolysis of ATP as well as the binding of co-chaperones and client proteins [28]. Additionally, PTMs can affect inhibitor binding to HSP90 [29-31]. A recent study showed that two chemically distinct HSP90 inhibitors, GM and PU-H71, both of which bind to the N-terminal nucleotide binding pocket, could access overlapping but distinct HSP90 populations in the cell [30]. The binding of GM and PU-H71 is differentially affected by HSP90 phosphorylation, which suggests that PTMs may impact selectivity and recognition of various HSP90 conformational populations. The extent and type of PTMs in normal vs. cancer cells are also different and can impart drug selectivity towards tumor cells. For instance, SUMOylation of HSP90 has recently been found to enhance sensitivity of cancer cells to inhibitors, an effect which directly correlated to increased SUMOylation observed in cancer cells relative to non-transformed cells [31]. PTMs are also likely to play a significant role in determining sensitivity between various cancer cell types.
Under stressed conditions such as the tumor microenvironment, there is a greater need for HSP chaperone function, which in turn requires activated HSPs. Cancer cells are able to selectively modulate HSP90 activity through favorable complexes as well as through PTMs that together fine tune the cellular pool of HSP90 to satisfy the cells requirement to survive. Cancer cells possess a distinct milieu from normal cells that provide an elevated protein folding environment that is required for rapidly proliferating cells. An interesting interplay between complex formation and PTMs that cancer cells use to promote their survival has recently been shown for HOP and carboxy terminus HSP70 interacting protein (CHIP) [32-33]. HOP and CHIP are two co-chaperones of HSP90 that compete for binding through their tetratricopeptide repeat domains and have diametrically opposed functions that are both necessary for protein homeostasis. Whereas HOP promotes proper folding and activation of client proteins, CHIP promotes their degradation through its ubiquitin ligase function. The dynamic equilibrium of HOP/CHIP binding can serve to shift the equilibrium to either folding or degradative pathways. It has recently been shown that cancer cells are able to promote folding by increasing levels of HOP and by increasing affinity of HSP90 for HOP through phosphorylation [32-33]. Phosphorylation of the C-terminal region of HSP90 enhances affinity for HOP and diminishes affinity for CHIP, whereas phosphorylation of the C-terminal region of HSP70 does not appear to impact binding to HOP but diminishes binding to CHIP. Overall, phosphorylation favors formation of HSP90/70-HOP complexes in cancer cells and profolding pathways essential for survival [32].
4. TARGETING HSP90 WITH SMALL MOLECULE INHIBITORS
HSP90 consists of three functional domains; the N-terminal domain, a middle domain and the C-terminal domain; and has specific sites for its binding partners. ATP/ADP binds in the nucleotide-binding pocket located in the N-terminal domain. The binding and subsequent hydrolysis of ATP delivers the energy required for protein folding and trafficking. The C-terminal domain is the site of dimerization, a key aspect for HSP90 function, as well as a putative ATP binding pocket, which may also serve to allosterically regulate HSP90 function. The middle domain is essential for client protein and co-chaperone interactions.
The HSP90 chaperone cycle is a dynamic process mediated by interactions with co-chaperones and other binding partners to form a multi-chaperone complex machinery. HSP90 exists in diverse conformational states and cochaperones may function by favoring distinct conformations that help to drive the cycle [1]. The initial phase of the chaperone cycle involves binding of HSP40 to a client protein followed by the recruitment of HSP70 [14]. HOP binds to this complex and facilitates the formation of HSP70-HOP-HSP90 complex by interacting with the C-terminal tetratricopeptide repeat domains of HSP70 and HSP90. This interaction promotes client transfer from HSP70 to HSP90 forming an intermediate complex. ATP binding then results in N-terminal dimerization of HSP90 and forms a molecular clamp around the client protein, which is stabilized by p23. Subsequent recruitment of Aha1 to the middle domain of HSP90 stimulates ATP hydrolysis, client folding and release of mature client protein.
The dynamic nature of the HSP90 chaperone cycle lends itself to modulation by small molecule ligands through a number of distinct mechanisms that include targeting; 1. the N-terminal domain 2. the C-terminal domain 3. cochaperone binding and 4. client protein binding. The majority of known ligands function by competitively inhibiting the binding of ATP to the nucleotide binding pocket in the N-terminal domain. Some ligands are known to interact with the C-terminal domain, while a smaller subset affect cochaperone or client protein binding.
4.1. Targeting the N-Terminal Nucleotide Binding Domain
HSP90 has a unique nucleotide-binding pocket situated in the N-terminal domain that binds ATP in a bent conformation [34]. The distinct nature of this pocket, which is only shared by the GHKL (G=DNA gyrase subunit B; H=HSP90; K=histidine kinases; L=MutL) family of proteins, provides an opportunity to selectively target the HSP90 ATPase domain. Inhibitors targeting the ATP pocket of the N-terminal domain have been extensively studied with a number of them having advanced to clinical trials. These ligands function by inhibiting ATP binding and hydrolysis and induce proteasomal degradation of HSP90 clients. The first HSP90 inhibitor to be identified, GM (Fig. 1), could not advance to the clinic due to several limitations including poor solubility, limited metabolic stability and hepatotoxicity in animals [35]. In order to overcome these limitations, derivatives of GM, including 17-AAG, 17-DMAG and IPI-504 with improved properties were developed (Fig. 1).
17-AAG was the first HSP90 inhibitor to enter clinical trials, however, due to poor solubility, limited bioavailability, dose, schedule and formulation related toxicities as well as poor patient selection, the single agent clinical studies of 17-AAG had limited success [9-10]. Despite limited efficacy as a single agent, 17-AAG exhibited promising results in combination with trastuzumab in Phase II for advanced HER2-positive breast cancer in patients previously treated with trastuzumab and also in combination with bortezomib in Phase I/II studies for multiple myeloma. Bristol-Myers Squibb later discontinued the development of 17-AAG. 17-DMAG was also pursued in Phase I studies [9-10], however, Kosan Biosciences discontinued the clinical development of 17-DMAG in 2008 as a result of unfavorable off-site toxicities. IPI-504, a water-soluble hydroquinone hydrochloride salt derivative of 17-AAG, exhibited a better potency and improved toxicity profile compared to its parent compound in preclinical studies [36]. As a result, IPI-504 was evaluated in clinical studies for non-small cell lung cancer, HER2-positive breast cancer (in combination with trastuzumab) and gastrointestinal stromal tumor (GIST) [37-39]. The trials for GIST and HER2-positive breast cancer were eventually terminated due to drug-related fatalities and lack of adequate clinical response respectively. Overall, it seems that the ansamycins are not able to overcome the liabilities initially observed for GM, and it appears they are no longer being pursued in the clinic.
The resorcinol core of another naturally occurring HSP90 inhibitor, radicicol (Fig. 2), which is required for interaction with the ATP binding pocket, has served as a template for the development of numerous clinical candidates including NVP-AUY922, STA-9090, KW-2478 and AT-13387 (Fig. 2). NVP-AUY922 (Luminespib) was developed by researchers at Vernalis through optimization of a potent hit from a high-throughput screen (HTS) involving inhibition of yeast HSP90 ATPase activity. Novartis had then pursued the clinical development of the compound in multiple Phase I and II clinical trials [9-10]. However, in December 2014 Novartis discontinued the clinical development of Luminespib and relinquished rights to the compound back to Vernalis [40]. STA-9090 (Ganetespib), a resorcinol triazole inhibitor developed by Synta Pharmaceuticals, is being pursued in numerous clinical studies for hematologic and solid tumors as a single agent as well as in combination with agents such as docetaxel or trastuzumab [9-10]. AT-13387 (Onalespib) was developed through optimization of a resorcinol based hit identified through fragment based drug discovery. This compound has entered Phase I studies for solid tumors and has also advanced to Phase II studies for GIST. Various combination Phase I/II trials for AT-13387 are also underway. KW-2478 arose through various drug development efforts including lead optimization, microbial screening, X-ray crystallography, cell-based assay and in vivo studies [41]. Phase I clinical evaluation for KW-2478 in multiple myeloma, chronic lymphocytic leukemia and B-cell non-Hodgkin's lymphoma showed that KW-2478 is well tolerated with no dose limiting toxicity observed at doses up to 99 mg/m2 [42]. Phase I/II study of KW-2478 in combination with bortezomib in relapsed/refractory multiple myeloma has also been recently completed in which the combination was well tolerated with an overall response rate of 39% [43].
Fig. 2. Resorcinol derivatives as Hsp90 inhibitors.
Represented here are the chemical structures of compounds consisting of a common resorcinol core. This series of compounds bind to the nucleotide-binding pocket situated at the N-terminal domain of HSP90.
Rational drug design efforts based on the X-ray crystal structure of ADP, ATP and GM bound to the N-terminal nucleotide-binding domain of HSP90 have led to the discovery of various synthetic inhibitors. Chiosis et al. at Memorial Sloan Kettering Cancer Center (MSKCC) reported the first synthetic HSP90 inhibitor, PU3, whose purine core has been utilized as a common pharmacophore by various research groups to design clinical candidates such as BIIB021/CNF2024, PU-H71, MPC-3100 and CUDC-305 (Fig. 3) [44]. BIIB021/CNF2024 became the first synthetic HSP90 inhibitor to start clinical trials and was evaluated in Phase I trials for chronic lymphocytic leukemia and GIST [9]. Biogen Idec eventually discontinued BIIB021/CNF2024 as a result of a strategic change in focus away from oncology [45]. Another purine derivative, PU-H71, was discovered by the Chiosis lab at MSKCC and is currently undergoing Phase I clinical evaluation in patients with previously treated solid tumors, lymphoma and myeloproliferative diseases. Preliminary results have recently been reported at the 2015 ASCO annual meeting [46]. 40 patients have received doses ranging from 10-400 mg/m2 on days 1, 4, 8 and 11 on a 21 day cycle. Although the primary objective of the study was determination of the maximum tolerated dose, encouragingly a number of tumor regressions have been observed. These include a tumor regression of 20.6% in a marginal zone lymphoma patient (50 mg/m2), 22.6% in a cervical squamous cell carcinoma patient (140 mg/m2), 8.3% tumor regression in a triple negative breast cancer patient (300 mg/m2), 20.8% tumor regression in a penile squamous cell carcinoma patient (350 mg/m2) and 25.6% tumor regression in an estrogen receptor positive breast cancer patient (400 mg/m2).
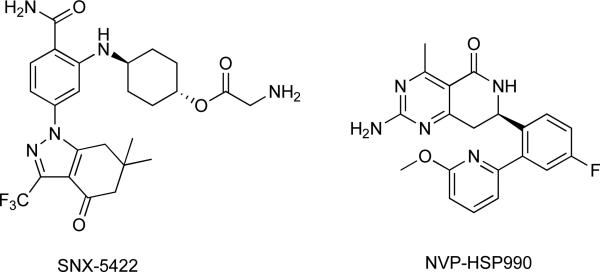
Other inhibitors based on the purine scaffold include MPC-3100 and CUDC-305 which have both advanced to clinical studies. In a Phase I study in recurrent or refractory tumor, MPC-3100 was found to be safe at doses below 600 mg per day with adverse effects that were manageable and reversible upon drug discontinuation. Other HSP90 inhibitors include SNX-5422 and NVP-HSP990, which were both developed through lead optimization of hits from HTS (Fig. 4) [9-10]. SNX-5422, which was discovered by Serenex and later by Pfizer, was initially discontinued as a clinical candidate due to concerns regarding ocular toxicity. This compound has now been acquired by Esanex and is being advanced to Phase I/II clinical trials. In contrast, clinical development of NVP-HSP990 was discontinued due to lack of adequate clinical response.
Fig. 4. Hsp90 inhibitors.
Both compounds represented here were discovered via lead optimization of hits obtained from HTS screening.
4.2. Targeting the C-Terminal Binding Domain
The C-terminal domain consists of a second putative ATP-binding site, which may be involved in allosteric regulation of HSP90 and offers an alternative strategy to modulate chaperone function [47]. Novobiocin (Fig. 5), a coumarin-type antibiotic was initially discovered as an inhibitor of DNA gyrase B. As both HSP90 and DNA gyrase B belong to the GHKL family of proteins consisting of structurally similar ATP-binding sites in the N-terminal domain, it was rational to conceive of it binding to this site. However, rather than binding to the N-terminal domain, novobiocin was identified as the first ligand binding to the C-terminal domain of HSP90 [48]. Interaction of novobiocin with HSP90, though with poor affinity (IC50 =700 μM in SkBr3), leads to the destabilization of various HSP90 client proteins such as Raf-1, v-src, mutant p53 and HER2 [48]. Subsequent development of novobiocin derivatives through structural modifications has resulted in compounds with improved HSP90 inhibition profile [49-50]. Beside novobiocin derivatives, several compounds such as cisplatin, epigallocatechin-3-gallate, taxol and withaferin A have also been reported to interact with the C-terminal domain of HSP90 (Fig. 5).
Fig. 5. Hsp90 inhibitors that exhibit alternative modes of action.
These inhibitors are reported to interact with the C-terminal binding domain of HSP90 and/or disrupt HSP90-cochaperone interaction.
A possible advantage of inhibiting the C-terminal domainof HSP90 is the reported absence of a heat shock response, which is a characteristic of the inhibitors that target the N-terminal nucleotide binding domain. This makes targeting the HSP90 C-terminus therapeutically attractive. However, clinical development has thus far been limited by few studies describing their in vivo activity.
4.3. Targeting HSP90-co-Chaperone Interaction
Cdc37 is a co-chaperone of HSP90 that promotes the recruitment of client proteins that are primarily kinases. The N-terminal domain of Cdc37 is known to interact with the catalytic domain of the kinase clients and enables interaction with HSP90 via its C-terminal domain [51]. Disruption of Cdc37-HSP90 association provides an alternative approach to target kinase driven cancers. siRNA mediated silencing of Cdc37 in human colon cancer cells resulted in the depletion of various kinases such as HER2, CDK4, CDK6, CRAF and AKT [52]. In addition, Cdc37 silencing sensitized cancer cells to HSP90 inhibitors and triggered cell cycle arrest and apoptosis. A gene expression based screening (GE-HTS) approach led to the identification of structurally similar triterpenoids, celastrol and gedunin (Fig. 5), as modulators of HSP90 pathway with androgen signaling signatures similar to HSP90 inhibitors 17-AAG, 17-DMAG and GM [53]. Both celastrol and gedunin did not compete with Cy3B-GM for binding to purified HSP90 in a fluorescence polarization assay, suggesting that these compounds act via a mechanism distinct from N-terminal ATPase inhibitors. Subsequent NMR and MS studies revealed that celastrol disrupts the HSP90-Cdc37 complex through covalent interaction with an active cysteine residue of Cdc37, which result in large conformational changes in the kinase binding domain and HSP90 binding domain of Cdc37 [54]. Celastrol and gedunin have also been reported to inactivate p23, a co-chaperone of HSP90 which facilitates client protein folding by stabilizing mature complexes of HSP90 [55-56]. Therefore, celastrol and gedunin are able to affect HSP90 function by multiple mechanisms.
Another HSP90 co-chaperone that is currently being targeted is HOP, a protein which facilitates the interaction of HSP90 with the HSP40-HSP70-client complex. The tetratricopeptide repeat domains of HOP, TPR1 and TPR2A, associate with HSP70 and HSP90 respectively through the EEVD motif located at the C-terminal tails of both proteins. Current endeavors are being made to target these proteinprotein interactions in order to disrupt the HSP70-HOP-HSP90 complex. A tetratricopeptide repeat domain mimic, CTPR390+ has been reported to specifically bind the HSP90 C-terminal tail with higher affinity (Kd = 1 μM) than the TPR2A motif of HOP. Disruption of HOP-HSP90 interaction by CTPR390+ results in HER2 degradation and growth inhibition in BT474 breast cancer cells [57]. An Alphascreen® technology based HTS assay has also been used to identify small molecules that inhibit the interaction between HSP90 and HOP TPR2A domain. The three active hits from this screen had in common a 7-azapteridine core (Fig. 5), and each exhibited antiproliferative effects and HER2 degradation in HER2 positive breast cancer cells [58]. A follow-up study on commercially available compounds containing a common 7-azpteridine core has been reported to kill TNBC cells through caspase-3/7 activation and induce depletion of several HSP90 clients including Cdk4, Raf-1, JNK1/2 and p38 [59].
Targeting interaction of Aha1 and HSP90 has also been studied as an alternative method to modulate HSP90 activity. Aha1 stimulates ATPase activity by binding to the N-terminal and middle domains of HSP90. siRNA silencing of Aha1 suggests that it might be required for activation of clients rather than their stabilization as it led to decreased Raf-1 kinase activity and reduced MEK1/2 and ERK1/2 phosphorylation in colon cancer cells but did not decrease the expression of client proteins such as HER2, CDK4 and Raf-1 [60]. Some of the C-terminal inhibitors (i.e. novobiocin, KU- 135, KU-174) have been shown to disrupt interaction of HSP90α and Aha1 in PC3-MM2 prostate cancer cells, resulting in dissociation of HSP90α/Aha1 complex and inhibition of cell migration, one of the key phases in tumor metastasis [61].
4.4. Targeting HSP90-Client Protein Interactions
Targeting interactions between HSP90 and its client proteins is another strategy that is currently being explored to inhibit chaperone activity. Targeting the HSP90-androgen modulate AR signaling in prostate cancer since AR is a key driver in prostate cancer progression, and AR-driven cancers are heavily dependent on HSP90 for survival [62]. It has been established that HSP90 is required for stabilization and activation of AR, and that the disruption of HSP90-AR complex is associated with cytosolic aggregation, inhibition of nuclear translocation, and eventual degradation of AR [63]. A study by Liu et al. has shown that disruption of the HSP90-AR complex by DNA topoisomerase I inhibitor camptothecin leads to the inhibition of AR transcriptional activity and decreased viability of androgen-positive prostate cancer cells [64]. These observations underscore the potential of therapeutic strategies directed towards the inhibition of HSP90-AR complex.
Recent studies have shown that dissociation of the HSP90-survivin complex initiates proteasomal degradation of survivin, triggers mitochondrial apoptosis and inhibits cell proliferation [65]. These effects make inhibition of HSP90-survivin interaction a potential target for anticancer therapy. Plescia et al. have reported a cell-permeable peptidomimetic compound, shepherdin, that disrupts the interaction of HSP90 and survivin [66]. Molecular dynamics docking simulations and mutagenesis studies suggest that shepherdin makes key contacts with several residues in the ATP binding pocket of HSP90. Domain-specific binding studies using recombinant HSP90 also support the association of shepherdin specifically with the N-terminal domain. Upon association with the N-terminal domain nucleotide-binding pocket, shepherdin induces cell death in various cancer cells and depletes HSP90 clients including survivin, Akt, CDK4 and CDK6 in PC3 prostate carcinoma cells. Furthermore, in MCF-7 xenograft models, shepherdin treatment led to decreased tumor growth and complete loss of Akt.
5. THERAPEUTIC TARGETING OF HSPs: EXPLOITING THE FUNCTIONAL STATE OF HSP90 IN CANCER AS MEANS FOR SELECTIVITY
The usefulness of any therapeutic can be determined by weighing the potential benefit against its potential toxicity. These two properties comprise the therapeutic index of a drug and determine whether it can be delivered in doses that are both efficacious as well as safe. The development of anticancer agents is invariably driven by attempts to balance these two interlinked properties, and it is most desirable that the therapeutic index be high as this would allow for the safe administration of drug with the best chance of eliciting the desirable beneficial effects.
A cancer cell contains a complex mixture of HSP chaperone complexes. While most of these complexes carry out housekeeping functions akin to those in normal cells, cancer cells also harbor a finely-tuned, active HSP fraction that buffers the proteome that is altered in the process of malignant transformation [27, 67]. The efficacy of an agent targeting a chaperone in cancer cells is therefore determined by its ability to sample and engage such “oncogenic complexes”. Small molecules that can interact specifically with the active HSP complexes selectively affect these complexes and will not act on the housekeeping HSPs unless higher or saturating concentrations of the compound are employed. Selective targeting of the active versus the housekeeping HSP complexes in the development of HSP drugs is advantageous for two reasons. First, lower toxicity through interference with HSP functions in normal cells would be expected. Second, more drug can be administered to the patient and thus a better engagement of the target at the site of disease is possible.
The therapeutic targeting of HSPs represents an interesting challenge from a drug discovery perspective because they are in fact atypical targets. Rather than being a clearly defined protein, the cellular targets are a pool of HSP90 complexes bound to various binding partners that can also be modified by PTMs. The impact of these two basic regulatory mechanisms must be considered if it is to be fully appreciated as an anticancer drug target. Agents that are directed towards HSP90 in cancer are in fact being directed towards HSP90-complexes which are also subject to PTMs. The diverse nature of HSP90 in cancer cells is likely the reason for the general property of inhibitors being retained in tumor for prolonged periods of time while being rapidly cleared from normal tissues and plasma [68-72]. This results in long residence time of drug selectively in tumor and suggests that the off rate (koff) of drug bound to HSP90 in tumor tissues and normal tissues is very different and that it is much lower in tumor HSP90 (tumor koff << nontumor koff).
Residence time of the drug on its target, rather than affinity, is more likely to be an important factor which determines in vivo efficacy [73-74]. In other words, the longer a drug is bound to its target, the more enduring will be its effect. This is especially relevant in vivo since the drug is actively being cleared away (i.e. metabolism, protein binding etc.) and will have an effect on dosing regimens which invariably extend also towards potential toxicity. High residence time of drug in tumor and rapid clearance from normal tissues suggest that on-target toxicities associated with HSP90 inhibition in normal tissues could be minimized by low exposure. Coupled with proper dosing strategies that consider the unique character of tumor HSP90, toxicity should be manageable.
6. A NOVEL PARADIGM IN DRUG DEVELOPMENT OF HSP-TARGETED AGENTS
How can oncogenic HSP90 be targeted selectively in the clinic so as to maximize the therapeutic benefit while minimizing potential toxicity? The simple answer is to ensure that residence time of drug in tumor is high and in normal tissues is low. In other words, AUC for tumor (AUCtumor) should be high while at the same time the AUC in normal tissues (AUCtissue) should be low. It should be stressed that it is not only the ratio of AUCtumor/AUCtissue that must be high, rather it is the absolute value of AUCtumor that should be high and that AUCtissue must be low. In this way, exposure to normal tissues is minimized, and thus, exposure to non-oncogenic HSP90 is low. As described earlier, HSP90 inhibitors in general exhibit high AUCtumor, however, they are likely distinguished by their relative exposures to other tissues. In fact, the ocular toxicity observed for some inhibitors but not others can be linked to this difference [75]. In this study, NVP-AUY922 and 17-DMAG, two inhibitors that had been previously linked to ocular toxicity in clinical trials, showed slower clearance and longer retention in the retina as compared to 17-AAG and STA-9090, two inhibitors which did not display ocular toxicity. These results reveal that retina/plasma exposure ratio and the rate of elimination of the drug plays a vital role in ocular toxicity and directly correlates prolonged drug exposure in retinal tissue to increased photoreceptor cell death. They also show that it is not possible to predict such profiles based on inhibitor structure alone. 17-DMAG and 17-AAG are two closely related analogs with divergent ocular toxicity profile. Similarly, NVP-AUY922 and STA-9090 are both resorcinol class of inhibitors and only NVP-AUY922 displays ocular toxicity.
The normal paradigms of drug discovery and development do not necessarily apply towards the development of HSP90-targeted agents. Traditionally, drugs have been developed by modifying pharmaceutical properties (i.e. lipophilicity, permeability, metabolic stability, plasma protein binding, etc.) that sought to optimize in vivo plasma pharmacokinetics (PK). Plasma PK has typically served as a surrogate for tissue concentrations since it is more practical to obtain plasma samples in the clinic. However, we know that for HSP90 inhibitors, plasma PK does not correlate with tumor PK [68-72]. This optimization paradigm results in compounds with relatively high plasma half-life, meaning compounds remain in circulation for a long time and as such are exposed to all tissues. Unfortunately, this is exactly contrary to what is necessary for a useful HSP90 targeted agent. In contrast, compounds that are rapidly cleared from plasma (low plasma half-life) and from normal tissues while concentrating into the tumor for prolonged period of time (high tumor half-life) are desired. In this way, the beneficial effects of targeting oncogenic HSP90 while minimizing potential toxicities as a result of inhibition of HSP90 in normal cells can be realized. This can be accomplished in the preclinical phase by performing PK studies measuring distribution to all relevant tissues (tumor, brain, liver, lung, heart, retina, etc.) that would allow for the identification of compounds that are rapidly cleared from normal tissues while being retained in tumor for prolonged periods of time. Such studies would help to identify those molecules with high AUCtumor and low AUCtissue and are crucial in preventing the further advancement of molecules that display poor properties for a HSP-targeted agent.
7. HSP90 INHIBITION, RELEVANT BIOMARKERS AND COMPANION DIAGNOSTICS: NEED FOR NON-INVASIVE METHODS IN THE CLINIC
In the development of targeted agents, it is becoming increasingly important to be able to select patients whose tumors depend on these molecules prior to treatment and then be able to assess modulation of the target during treatment. The exclusion of patients in clinical trials unlikely to benefit from therapy reduces the possibility that an active agent would be falsely rejected and gives the greatest chance for successful outcome. Hence, there is a pressing need to identify relevant biomarkers and to develop diagnostic tools capable of identifying appropriate patients prior to treatment and for determining target modulation during treatment.
There are many HSP90 inhibitors known and approximately 20 have entered into clinical evaluation. The progress of this class has been slow and a major reason for this has been a lack of a good understanding of the context in which these drugs should be used. Good predictive biomarkers for HSP90 inhibitor therapy have thus far remained elusive. Unlike the case of certain tyrosine kinase inhibitors whereby overexpression or mutation of the target protein may confer a degree of sensitivity to the agent [76], this has not proven to be true for HSP90 inhibitors. It is not necessarily levels of HSP90 that determine sensitivity to inhibitor but rather it is the functional state as determined by complex formation as well as PTMs [26-27, 67]. In other words, it is the presence of “oncogenic” HSP90 that make tumors more susceptible to HSP90 inhibitors and therefore may potentially serve as a useful biomarker. Determining the impact of complex formation and PTMs towards inhibitor sensitivity and identifying these in patients will be critical for the successful outcomes of these agents in the clinic.
While in the preclinical phase, it is possible to obtain tissue samples and determine PK/pharmacodynamic (PD) relationships following administration of drug, this is generally not feasible during the clinical phase of development for tissues other than plasma. As such, determination of drug concentrations as well as PD endpoints for measuring target engagement have typically been performed using plasma samples. Increased HSP70 induction is commonly used as a measure for HSP90 inhibition in the clinic [77], but whether it is a good marker is highly doubtful. This is because peripheral-blood mononuclear cells (PBMCs), which are essentially noncancerous blood cells, are often used as tissue samples to evaluate HSP70 induction and other biomarkers [78-80]. Since HSP90 inhibitors have a higher affinity for “oncogenic” HSP90 that is found only in cancer cells, HSP70 induction in noncancerous cells such as PBMCs most likely will not provide an accurate measure for HSP90 inhibition. And as stated earlier, plasma drug concentrations are not indicative of tumor drug concentrations. This emphasizes the need for evaluating PD markers in cancerous tissues.
Pre- and post-treatment tumor biopsies from patients treated with 17-AAG have been assayed in the past to determine target modulation including cRAF1 inhibition, HSP70 induction and CDK4 depletion [80]. However, the major limitations of this process include requirement of invasive procedures to obtain tumor biopsies, limited sample size, lack of tumor homogeneity and lack of sensitive immunohistochemical procedures that detect biomarkers [77]. Therefore, non-invasive ways to accurately measure target engagement and indirectly determine drug concentrations in tumors and normal tissues are required in order to determine AUCtumor and AUCtissue.
One potential technology that can be used for this purpose is positron emission tomography (PET). PET is a widely used non-invasive diagnostic that is ideally suited to quantitate uptake, concentration, clearance, and distribution of an appropriately labeled agent in a spatiotemporal manner. An appropriately labeled PET-tracer, that is one that can account for the long tumor half-life of HSP90 inhibitors, can potentially address a number of fundamental questions related to the drug in the clinic. First is whether the drug has actually reached the intended site of action and is in fact engaging its target. Though it might seem obvious, failure to do so can result in difficulties in explaining potential lack of clinical efficacy [81]. PET can also be used to determine the acceptable exposure to normal tissue (AUCtissue) to minimize risk of toxicity, as well as the minimal exposure required to the tumor (AUCtumor) to elicit a beneficial antitumor effect. Importantly, it can also be used to potentially address two major hurdles that have hindered the development of HSP90 inhibitors. These are determining which patients would be expected to benefit from HSP90 inhibitor therapy and the design of optimal dosing strategies.
A number of PET tracers are available to monitor the effects of HSP90 inhibition and three different imaging-based approaches have been used in clinical trials. These are based on (1) measuring changes in tumor metabolism, (2) measuring changes in key oncoproteins and (3) measuring distribution, uptake and retention with a radiolabeled isotopologue of a suitable HSP90 inhibitor.
Imaging changes in tumor metabolism by FDG-PET for assessing tumor glucose metabolism is used to monitor early drug response to anticancer agents [82-86]. The changes in the uptake of FDG in tumor are quantified to assess the PD effect of diminished tumor metabolism as a consequence of HSP90 inhibitor administration. This assay has been applied to monitor tumor response in various clinical trials of HSP90 inhibitors including 17-AAG, BIIB021 and NVP-AUY922 [87-89]. However, recent studies indicate that changes in tumor glycolysis do not necessarily correlate with early tumor response to HSP90 inhibition. Degradation of HER2 did not correlate with the rate of glycolysis suggesting that in this particular study, HER2-PET was able to better predict the early response of 17-AAG treatment than FDG-PET [88].
Target based imaging of HSP90 inhibition by visualizing the depletion of oncoproteins that are clients of HSP90 as a consequence of inhibitor administration have proven to be earlier predictors of tumor growth inhibition by an HSP90 targeted therapy. PET as a non-invasive molecular imaging technique can provide information about the molecular alterations as an early response to HSP90 inhibition. Because many oncoproteins are clients of HSP90 there are several molecular imaging target opportunities for non-invasive monitoring of HSP90 inhibition [90-91]. PET imaging of EGFR degradation with 64Cu-cetixumab has been used for PD monitoring of the HSP90 inhibitor 17-AAG in PC-3 human prostate cancer xenografts [92]. HER2 imaging has also been used as a likely better readout than EGFR imaging since HER2 is the client protein more sensitive to HSP90 inhibition [93]. Therefore, for HER2 positive cancers, molecular imaging of HER2 expression during HSP90 inhibiting therapies has potential for PD monitoring. 89Zr-trastuzumab has been successfully utilized as a means to non-invasively monitor HER2 downregulation in xenograft mouse models [94-95]. 89Zr-trastuzumab and 89Zr-bevacizumab PET imaging of NVP-AUY922 effects on HER2 and VEGF downregulation has also been validated in patients with metastatic breast cancer where the PET results at 3 weeks was consistent with the size of individual lesions obtained through CT [82].
In contrast to the two previous approaches, the use of a directly radiolabeled HSP90 inhibitor can provide a spatially and temporally resolved quantitative measurement of the distribution and concentration of the drug and can potentially allow for the identification of tumors in clinic which express “oncogenic” HSP90, which, in turn enables for the selection of patient groups most likely to benefit from HSP90 targeted therapy. Furthermore, this can be used to determine the extent of target engagement and inform on optimal dosing schedules. 124I-PU-H71, a radiolabeled PU-H71 where the iodine atom (127I) is replaced with the radioisotope 124I, is being developed as a PET imaging agent to monitor drug pharmacokinetics, metabolism and radiation densitometry. In fact, 124I-PU-H71 is currently being used in a microdose Phase 0 clinical trial with the intent of studying the PK of 124I-PU-H71 in patients with solid malignancy, myeloproliferative neoplasm, myeloma, and/or lymphoma [96]. It is also being co-administered with PU-H71 in a Phase I clinical trial as a non-invasive way to determine tumor PK and intratumoral concentration [97]. The preliminary results suggest that this technique can successfully determine intratumoral drug concentration with a good correlation between inhuman PET imaging and LC-MS/MS determined drug concentrations of patient biopsy specimens [46, 98].
CONCLUSION
HSPs have become interesting targets for the treatment of various diseases, and in this review we discuss several concepts related to the specific targeting of chaperones in cancer using HSP90 as an example. We now know it is especially important to consider that the actual target of such agents are complexes that function to regulate chaperone activity in a highly dynamic manner in conjunction with PTMs that in turn are affected by the cellular milieu. Therefore, rather than targeting distinct proteins, such agents need to be fine-tuned to engage cancer specific complexes. Primarily, it is the selective targeting of cancer-specific complexes in cells by small molecule inhibitors that can effectively engage them, and in doing so affect their ability to survive. Presumably, this is true for other HSP targeted agents, and the ability of such agents to specifically bind to components of the “stress chaperome” containing activating co-chaperones and cellspecific oncoproteins will directly affect the usefulness of the drug. Furthermore, we also discuss the limitations of traditional plasma PK to predict tumoral concentrations of these agents and of the need to ultimately determine tumor drug concentration and target engagement in a non-invasive manner for clinical utility.
The use of HSP agents is by no means limited to cancer. A broad variety of neurodegenerative disorders are characterized by specific patterns of neuronal cell death caused by the accumulation of abnormally processed or mutant proteins, which subsequently misfold and aggregate. Among these disorders are Alzheimer's disease (AD), Parkinson's disease (PD), and the polyglutamine expansion disorders, such as Huntington's disease (HD) and spinal and bulbar muscular atrophy (SBMA). Critical proteins that form these pathological deposits, such as tau (in AD), α-synuclein (in PD), huntingtin (in HD), and the polyglutamine androgen receptor (in SBMA), are client proteins of HSP90 [99]. Therefore, HSP90 inhibitors may have application in neurodegenerative disorders. However, this has been hampered by the inability to develop compounds that both favorably permeate the blood brain barrier and moreover, achieve therapeutic brain concentrations at non-toxic doses [100-102].
Much has been learned during the last two decades in developing HSP90 inhibitors for clinical application. Although this work has not yet resulted in an approved compound, many hard lessons have been learned that can be applied towards the future development of other HSP agents. Despite all that we have learned during this time, it is still not clear how to best use these agents. This remains a major hurdle that needs to be overcome in order to ensure that these agents play a role in the treatment of cancer in the future.
ACKNOWLEDGEMENTS
The authors acknowledge funding from the David Rubenstein Center for Pancreatic Cancer Research and the National Institutes of Health grant 1P01 CA177575-01A1 (T.T.), MSKCC Brain Tumor Center (A.B.) and Susan G Komen for the Cure (H.J.P.).
Biography
 Tony Taldone
Tony Taldone
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 2.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Cancer. 2013;13:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 4.Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013;19:347–365. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br. J. Cancer. 2009;100:1523–1529. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul S, Mahanta S. Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door? Mol. Cell Biochem. 2014;386:45–61. doi: 10.1007/s11010-013-1844-y. [DOI] [PubMed] [Google Scholar]
- 7.Adachi H, Katsuno M, Waza M, Minamiyama M, Tanaka F, Sobue G. Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int. J. Hyperthermia. 2009;25:647–654. doi: 10.3109/02656730903315823. [DOI] [PubMed] [Google Scholar]
- 8.Rochani AK, Singh M, Tatu U. Heat shock protein 90 inhibitors as broad spectrum anti-infectives. Curr. Pharm. Des. 2013;19:377–386. doi: 10.2174/138161213804143608. [DOI] [PubMed] [Google Scholar]
- 9.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jhaveri K, Ochiana SO, Dunphy MP, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J, 3rd, Modi S, Chiosis G. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert. Opin. Investig. Drugs. 2014;23:611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taipale M, Krykbaeva I, Whitesell L, Santagata S, Zhang J, Liu Q, Gray NS, Lindquist S. Chaperones as thermodynamic sensors of drug-target interactions reveal kinase inhibitor specificities in living cells. Nat. Biotechnol. 2013;31:630–637. doi: 10.1038/nbt.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Buchner J. Structure, function and regulation of the hsp90 machinery. Biomedical J. 2013;36:106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Soroka J, Buchner J. The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta. 2012;1823:624–635. doi: 10.1016/j.bbamcr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Prodromou C. The ‘active life’ of Hsp90 complexes. Biochim. Biophys. Acta. 2012;1823:614–623. doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clare DK, Saibil HR. ATP-driven molecular chaperone machines. Biopolymers. 2013;99:846–859. doi: 10.1002/bip.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul S, Mahanta S. Association of heat-shock proteins in various neurodegenerative disorders: is it a master key to open the therapeutic door? Mol. Cell Biochem. 2014;386:45–61. doi: 10.1007/s11010-013-1844-y. [DOI] [PubMed] [Google Scholar]
- 18.Hong DS, Banerji U, Tavana B, George GC, Aaron J, Kurzrock R. Targeting the molecular chaperone heat shock protein 90 (HSP90): lessons learned and future directions. Cancer Treat. Rev. 2013;39:375–387. doi: 10.1016/j.ctrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Veri A, Cowen LE. Progress and prospects for targeting Hsp90 to treat fungal infections. Parasitology. 2014;141:1127–1137. doi: 10.1017/S0031182013002072. [DOI] [PubMed] [Google Scholar]
- 20.Gillan V, Devaney E. Nematode Hsp90: highly conserved but functionally diverse. Parasitology. 2014;141:1203–1215. doi: 10.1017/S0031182014000304. [DOI] [PubMed] [Google Scholar]
- 21.Uehara Y, Hori M, Takeuchi T, Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol. Cell Biol. 1986;6:2198–2206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 24.Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 25.Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 27.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, Hatzi K, Vu LP, Zhao X, Zatorska D, Taldone T, Smith-Jones P, Alpaugh M, Gross SS, Pillarsetty N, Ku T, Lewis JS, Larson SM, Levine R, Erdjument-Bromage H, Guzman ML, Nimer SD, Melnick A, Neckers L, Chiosis G. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat. Chem. Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta. 2012;1823:648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton-Diaz A, Khan S, Bourboulia D, Trepel JB, Neckers L, Mollapour M. Contributions of co-chaperones and posttranslational modifications towards Hsp90 drug sensitivity. Future Med. Chem. 2013;5:1059–1071. doi: 10.4155/fmc.13.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beebe K, Mollapour M, Scroggins B, Prodromou C, Xu W, Tokita M, Taldone T, Pullen L, Zierer BK, Lee MJ, Trepel J, Buchner J, Bolon D, Chiosis G, Neckers L. Posttranslational modification and conformational state of Heat Shock Protein 90 differentially affect binding of chemically diverse small molecule inhibitors. Oncotarget. 2013;4:1065–1074. doi: 10.18632/oncotarget.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mollapour M, Bourboulia D, Beebe K, Woodford MR, Polier S, Hoang A, Chelluri R, Li Y, Guo A, Lee MJ, Fotooh-Abadi E, Khan S, Prince T, Miyajima N, Yoshida S, Tsutsumi S, Xu W, Panaretou B, Stetler-Stevenson WG, Bratslavsky G, Trepel JB, Prodromou C, Neckers L. Asymmetric Hsp90 N domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Mol. Cell. 2014;53:317–329. doi: 10.1016/j.molcel.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 33.Ruckova E, Muller P, Nenutil R, Vojtesek B. Alterations of the Hsp70/Hsp90 chaperone and the HOP/CHIP co-chaperone system in cancer. Cell. Mol. Biol. Lett. 2012;17:446–458. doi: 10.2478/s11658-012-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chène P. ATPases as drug targets: learning from their structure. Nat. Rev. Drug Discov. 2002;1:665–673. doi: 10.1038/nrd894. [DOI] [PubMed] [Google Scholar]
- 35.Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332:275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14:e358–369. doi: 10.1016/S1470-2045(13)70169-4. [DOI] [PubMed] [Google Scholar]
- 37.Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, Gray JE, Iafrate AJ, Katayama R, Hafeez N, Sweeney J, Walker JR, Fritz C, Ross RW, Grayzel D, Engelman JA, Borger DR, Paez G, Natale R. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J. Clin. Oncol. 2010;28:4953–4960. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner AJ, Chugh R, Rosen LS, Morgan JA, George S, Gordon M, Dunbar J, Normant E, Grayzel D, Demetri GD. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft tissue sarcomas. Clin. Cancer Res. 2013;19:6020–6029. doi: 10.1158/1078-0432.CCR-13-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modi S, Saura C, Henderson C, Lin NU, Mahtani R, Goddard J, Rodenas E, Hudis C, O'Shaughnessy J, Baselga J. A multicenter trial evaluating retaspimycin HCL (IPI-504) plus trastuzumab in patients with advanced or metastatic HER2-positive breast cancer. Breast Cancer Res. Treat. 2013;139:107–113. doi: 10.1007/s10549-013-2510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. [9/21/2015]; http://www.vernalis.com/media-centre/latest-releases/2014-releases/695-update-on-auy922-development-programme.
- 41.Nakashima T, Ishii T, Tagaya H, Seike T, Nakagawa H, Kanda Y, Akinaga S, Soga S, Shiotsu Y. New molecular and biological mechanism of antitumor activities of KW-2478, a novel nonansamycin heat shock protein 90 inhibitor, in multiple myeloma cells. Clin. Cancer Res. 2010;16:2792–2802. doi: 10.1158/1078-0432.CCR-09-3112. [DOI] [PubMed] [Google Scholar]
- 42.Cavenagh JD, Yong K, Byrne J, Cavet J, Johnson P, Morgan G, Williams CD, Akinaga S, Francis G, Kilborn J. The safety, pharmacokinetics and pharmacodynamics of KW-2478, a novel Hsp90 antagonist, in patients with B-cell malignancies: A first-in-man, phase I, multicentre, open-label, dose escalation study. 50th ASH annual meeting and exposition. 2008;112:958–959. [Google Scholar]
- 43.Jamie C, Baylon HG, Caguioa PB, Davies FE, Gharibo M, Akinaga S, Kurman MR, Novak BA, Yong KL. A phase 1/2 study of KW-2478, an Hsp90 inhibitor, in combination with bortezomib (BTZ) in patients (Pts) with relapsed/refractory (R/R) multiple myeloma (MM). Blood. 2013;122:1967. [Google Scholar]
- 44.Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem. Biol. 2001;8:289–299. doi: 10.1016/s1074-5521(01)00015-1. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell P. Biogen Idec restructures, sharpens neurology focus. Nat. Biotechnol. 2011;29:7–8. doi: 10.1038/nbt0111-7. [DOI] [PubMed] [Google Scholar]
- 46.Gerecitano JF, Modi S, Rampal R, Drilon AE, Fury MG, Gounder MM, Harding JJ, Hyman DM, Varghese AM, Voss MH, France FO, Taldone T, DaGama EG, Uddin M, Chiosis G, Lewis JS, Lyashchenko SK, Larson SM, Pressl C, Dunphy M. Phase I trial of the HSP-90 inhibitor PUH71. J. Clin. Oncol. (Meeting Abstracts) 2015:33. no. 15_suppl 253. [Google Scholar]
- 47.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Curr. Med. Chem. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J. Biol. Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 49.Audisio D, Methy-Gonnot D, Radanyi C, Renoir JM, Denis S, Sauvage F, Vergnaud-Gauduchon J, Brion JD, Messaoudi S, Alami M. Synthesis and antiproliferative activity of novobiocin analogues as potential hsp90 inhibitors. Eur. J. Med. Chem. 2014;83:498–507. doi: 10.1016/j.ejmech.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 50.Zhao H, Garg G, Zhao J, Moroni E, Girgis A, Franco LS, Singh S, Colombo G, Blagg BS. Design, synthesis and biological evaluation of biphenylamide derivatives as Hsp90 C-terminal inhibitors. Eur. J. Med. Chem. 2015;89:442–466. doi: 10.1016/j.ejmech.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle. 2009;8:362–372. doi: 10.4161/cc.8.3.7531. [DOI] [PubMed] [Google Scholar]
- 52.Smith JR, Clarke PA, de Billy E, Workman P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 2009;28:157–169. doi: 10.1038/onc.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, Maloney KN, Clardy J, Hahn WC, Chiosis G, Golub TR. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Sreeramulu S, Gande SL, Göbel M, Schwalbe H. Molecular mechanism of inhibition of the human protein complex Hsp90-Cdc37, a kinome chaperone-cochaperone, by triterpene celastrol. Angew. Chem. Int. Ed. Engl. 2009;48:5853–5855. doi: 10.1002/anie.200900929. [DOI] [PubMed] [Google Scholar]
- 55.Chadli A, Felts SJ, Wang Q, Sullivan WP, Botuyan MV, Fauq A, Ramirez-Alvarado M, Mer G. Celastrol Inhibits Hsp90 Chaperoning of Steroid Receptors by Inducing Fibrillization of the Co-chaperone p23. J. Biol. Chem. 2010;285:4224–4231. doi: 10.1074/jbc.M109.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BSJ, Chadli A. Gedunin Inactivates the Co-chaperone p23 Protein Causing Cancer Cell Death by Apoptosis. J. Biol. Chem. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortajarena AL, Yi F, Regan L. Designed TPR modules as novel anticancer agents. ACS Chem. Biol. 2008;3:161–166. doi: 10.1021/cb700260z. [DOI] [PubMed] [Google Scholar]
- 58.Yi F, Regan L. A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pimienta G, Herbert KM, Regan L. A compound that inhibits the HOP-Hsp90 complex formation and has unique killing effects in breast cancer cell lines. Mol. Pharm. 2011;8:2252–2261. doi: 10.1021/mp200346y. [DOI] [PubMed] [Google Scholar]
- 60.Holmes JL, Sharp SY, Hobbs S, Workman P. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2008;68:1188–1197. doi: 10.1158/0008-5472.CAN-07-3268. [DOI] [PubMed] [Google Scholar]
- 61.Ghosh S, Shinogle HE, Garg G, Vielhauer GA, Holzbeierlein JM, Dobrowsky RT, Blagg BS. Hsp90 C-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90α/Aha1 complex in PC3-MM2 cells. ACS Chem. Biol. 2015;10:577–590. doi: 10.1021/cb5008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centenera MM, Fitzpatrick AK, Tilley WD, Butler LM. Hsp90: still a viable target in prostate cancer. Biochim. Biophys. Acta. 2013;1835:211–218. doi: 10.1016/j.bbcan.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Georget V, Térouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Yuan Y, Okumura Y, Shinkai N, Yamauchi H. Camptothecin disrupts androgen receptor signaling and suppresses prostate cancer cell growth. Biochem. Biophys. Res. Commun. 2010;394:297–302. doi: 10.1016/j.bbrc.2010.02.164. [DOI] [PubMed] [Google Scholar]
- 65.Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, Sessa WC, Altieri DC. Regulation of survivin function by Hsp90. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plescia J, Salz W, Xia F, Pennati M, Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, Gabrilovich DI, Colombo G, Altieri DC. Rational design of shepherdin, a novel anticancer agent. Cancer Cell. 2005;7:457–468. doi: 10.1016/j.ccr.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 67.Taldone T, Ochiana SO, Patel PD, Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 2014;35:592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A, Guo Y, Wu N, de Stanchina E, White J, Gross SS, Ma Y, Varticovski L, Melnick A, Chiosis G. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shimamura T, Perera SA, Foley KP, Sang J, Rodig SJ, Inoue T, Chen L, Li D, Carretero J, Li YC, Sinha P, Carey CD, Borgman CL, Jimenez JP, Meyerson M, Ying W, Barsoum J, Wong KK, Shapiro GI. Ganetespib (STA-9090), a nongeldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin. Cancer Res. 2012;18:4973–4985. doi: 10.1158/1078-0432.CCR-11-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bao R, Lai CJ, Qu H, Wang D, Yin L, Zifcak B, Atoyan R, Wang J, Samson M, Forrester J, DellaRocca S, Xu GX, Tao X, Zhai HX, Cai X, Qian C. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin. Cancer Res. 2009;15:4046–4057. doi: 10.1158/1078-0432.CCR-09-0152. [DOI] [PubMed] [Google Scholar]
- 71.Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P. Pharmacokinetic-pharmacodynamics relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin. Cancer Res. 2005;11:7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 72.Eiseman JL, Lan J, Lagattuta TF, Hamburger DR, Joseph E, Covey JM, Egorin MJ. Pharmacokinetics and pharmacodynamics of 17-demethoxy 17-[[(2-dimethylamino)ethyl]amino] geldanamycin (17DMAG, NSC 707545) in C.B-17 SCID mice bearing MDA-MB-231 human breast cancer xenografts. Cancer Chemother. Pharmacol. 2005;55:21–32. doi: 10.1007/s00280-004-0865-3. [DOI] [PubMed] [Google Scholar]
- 73.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 2006;5:730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 74.Lu H, Tonge PJ. Drug-target residence time: critical information for lead optimization. Curr. Opin. Chem. Biol. 2010;14:467–474. doi: 10.1016/j.cbpa.2010.06.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou D, Liu Y, Ye J, Ying WW, Ogawa LS, Inoue T, Tatsuta N, Wada Y, Koya K, Huang Q, Bates RC, Sonderfan AJ. A rat retinal damage model predicts for potential clinical visual disturbances induced by Hsp90 inhibitors. Toxicol. Appl. Pharm. 2013;273:401–409. doi: 10.1016/j.taap.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 76.Gandhi J, Zhang J, Xie Y, Soh J, Shigematsu H, Zhang W, Yamamoto H, Peyton M, Girard L, Lockwood WW, Lam WL, Varella-Garcia M, Minna JD, Gazdar AF. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4(2):e4576. doi: 10.1371/journal.pone.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jhaveri K, Ochiana SO, Dunphy MPS, Gerecitano JF, Corben AD, Peter RI, Janjigian YY, Gomes-DaGama EM, Koren J, Modi S, Chiosis G. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin. Inv. Drug. 2014;23:611–628. doi: 10.1517/13543784.2014.902442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA, Horneffer YR, Juwara L, Melillo G, Ivy SP, Merino M, Neckers L, Steeg PS, Conley BA, Giaccone G, Doroshow JH, Murgo AJ. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur. J. Cancer. 2010;46:340–347. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, Lan J, Potter DM, Ivy SP, Ramalingam S, Brufsky AM, Wong MK, Tutchko S, Egorin MJ. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin. Cancer Res. 2005;11:3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- 80.Banerji U, O'Donnell A, Scurr M, Pacey S, Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M, Walton M, Lakhani S, Kaye S, Workman P, Judson I. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J. Clin. Oncol. 2005;23:4152–4161. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 81.Simon GM, Niphakis MJ, Cravatt BF. Determining target engagement in living systems. Nat. Chem. Biol. 2013;9:200–205. doi: 10.1038/nchembio.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaykema SB, Schröder CP, Vitfell-Rasmussen J, Chua S, Munnink THO, Brouwers AH, Bongaerts AH, Akimov M, Fernandez-Ibarra C, Lub-de Hooge MN, de Vries EG, Swanton C, Banerji U. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin. Cancer Res. 2014;20:3945–3954. doi: 10.1158/1078-0432.CCR-14-0491. [DOI] [PubMed] [Google Scholar]
- 83.Friedberg JW, Chengazi V. PET scans in the staging of lymphoma: current status. Oncologist. 2003;8:438–447. doi: 10.1634/theoncologist.8-5-438. [DOI] [PubMed] [Google Scholar]
- 84.Torizuka T, Nakamura F, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, Kobayashi M, Ouchi Y. Early therapy monitoring with FDG-PET in aggressive non-Hodgkin's lymphoma and Hodgkin's lymphoma. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:22–28. doi: 10.1007/s00259-003-1333-8. [DOI] [PubMed] [Google Scholar]
- 85.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J. Nucl. Med. 2005;46:983–995. [PubMed] [Google Scholar]
- 86.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin. Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 87.Dickson MA, Okuno SH, Keohan ML, Maki RG, D'Adamo DR, Akhurst TJ, Antonescu CR, Schwartz GK. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann. Oncol. 2013;24:252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J. Nucl. Med. 2006;47:793–796. [PMC free article] [PubMed] [Google Scholar]
- 89.Samuel TA, Sessa C, Britten C, Milligan KS, Mita MM, Banerji U, Pluard TJ, Stiegler P, Quadt C, Shapiro G. AUY922, a novel HSP90 inhibitor: Final results of a first-in-human study in patients with advanced solid malignancies. J. Clin. Oncol. 2010;2815s(suppl) abstr 2528. [Google Scholar]
- 90.Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann. Oncol. 2003;14:1169–1176. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- 91.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Niu G, Cai W, Chen K, Chen X. Non-invasive PET imaging of EGFR degradation induced by a heat shock protein 90 inhibitor. Mol. Imaging Biol. 2008;10:99–106. doi: 10.1007/s11307-007-0123-2. [DOI] [PubMed] [Google Scholar]
- 93.Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, Neckers L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- 94.Munnink THO, de Korte MA, Nagengast WB, Timmer-Bosscha H, Schroder CP, de Jong JR, van Dongen GAMS, Jensen MR, Quadt C, Lub-de Hooge MN, de Vries EGE. Zr-89-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur. J. Cancer. 2010;46:678–684. doi: 10.1016/j.ejca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Holland JP, Caldas-Lopes E, Divilov V, Longo VA, Taldone T, Zatorska D, Chiosis G, Lewis JS. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using Zr-DFO-trastuzumab. PLoS One. 2010;5:e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunphy M. PET Imaging of Cancer Patients Using 124I-PUH71: A pilot study. available from: https://clinicaltrials.gov/ct2/show/NCT01269593, verified May 2015.
- 97.Gerecitano J. The first-in-human phase I trial of PU-H71 in patients with advanced malignancies. available from: https://clinicaltrials.gov/ct2/show/NCT01393509, verified April 2015.
- 98.Gerecitano JF, Modi S, Gajria D, Taldone T, Alpaugh M, DaGama EG, Uddin M, Chiosis G, Lewis JS, Larson SM, Pillarsetty NVK, Jhaveri KL, Krichevsky B, Chen MH, Dixit P, Dunphy M. Using I-124-PU-H71 PET imaging to predict intratumoral concentration in patients on a phase I trial of PU-H71. J. Clin. Oncol. 2013;31(suppl) abstr 11076. [Google Scholar]
- 99.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29:15–32. doi: 10.1016/s0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 100.Waza M, Adachi H, Katsuno M, Minamiyama M, Tanaka F, Sobue G. Alleviating neurodegeneration by an anticancer agent: an Hsp90 inhibitor (17-AAG). Ann. N.Y. Acad. Sci. 2006;1086:21–34. doi: 10.1196/annals.1377.012. [DOI] [PubMed] [Google Scholar]
- 101.Tokui K, Adachi H, Waza M, Katsuno M, Minamiyama M, Doi H, Tanaka K, Hamazaki J, Murata S, Tanaka F, Sobue G. 17-DMAG ameliorates polyglutamine-mediated motor neuron degeneration through well-preserved proteasome function in an SBMA model mouse. Hum. Mol. Genet. 2009;18:898–910. doi: 10.1093/hmg/ddn419. [DOI] [PubMed] [Google Scholar]
- 102.Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J. Biol. Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]