Abstract
Purpose
We sought to develop and characterize a model of human vitamin D nutritional insufficiency/deficiency in the adult mouse, which could have broad utility in examining health consequences of this common condition.
Methods
Adult mice were fed diets containing cholecalciferol contents of 0.05IU/g, 0.25IU/g, 0.5IU/g or 1.5IU/g for four months. We studied induction of steady-state vitamin D insufficiency, and its consequences on primary cholecalciferol metabolite levels, calcium homeostasis, parathyroid physiology, and bone morphology.
Results
All diets were well tolerated, without adverse effects on body weight. Diets containing 0.05IU/g and 0.25 IU/g cholecalciferol significantly lowered serum 25-hydroxyvitamin D levels (median 25OHD, 10.5 ng/ml, and 21.6 ng/ml, respectively), starting as early as 1 month following initiation of the diets, maintained through the 4-month experimental period. The 0.05IU/g diet significantly decreased 1,25-dihydroxyvitamin D (1,25OH2D) levels (median, 78 pg/ml). Despite these decreased 25OHD and 1,25OH2D levels, the diets did not alter parathyroid gland morphology or parathyroid cell proliferation. There were no statistical differences in the serum total calcium and serum PTH levels among the various dietary groups. Furthermore, the 0.05IU/g diet did not cause any alterations in the cortical and trabecular bone morphology, as determined by microCT.
Conclusions
The dietary manipulations yielded states of vitamin D insufficiency or modest deficiency in adult mice, with no overtly detectable impact on parathyroid and bone physiology, and calcium homeostasis. This model system may be of value to study health effects of vitamin D insufficiency/deficiency especially on extraskeletal phenotypes such as cancer susceptibility or immune function.
Introduction
The secosteroidal hormone vitamin D plays an important role in calcium homeostasis and is essential for skeletal health. In addition to its established skeletal effects there is growing evidence that vitamin D has broader effects on a variety of cell types. In particular, vitamin D's role in cancer, immunomodulation, and wound healing are under intense investigation (1-6). The term vitamin D broadly refers to its two forms vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), and its sources include diet as well as dermal synthesis by ultraviolet-radiation catalyzed conversion of 7-dehydrochoestrol. Vitamin D is hydroxylated in the liver to 25-hydroxyvitamin D (25OHD), which is the major circulating, albeit inactive, form of vitamin D. It is subsequently hydroxylated in the kidney by the enzyme CYP27B1 into its active metabolite, 1,25-dihydroxyvitamin D (1,25OH2D). Circulating 25OHD concentrations are used as markers of vitamin D status, but defining the level deemed to be adequate, insufficient and deficient has been controversial. The Institute of Medicine defines vitamin D deficiency when serum 25OHD is less than 12 ng/ml, with levels between 12-20 ng/ml considered insufficient (7). However, clinical practice guidelines from The Endocrine Society recommend that 25OHD levels below 20 ng/ml be considered deficient, with levels of 21-29 ng/ml categorized as insufficient (8). Thus, there is a need to critically examine and better understand vitamin D's pleotropic effects in these ranges, and thereby guide future recommendations regarding vitamin D status.
There is growing epidemiological evidence for vitamin D's role in a diverse group of human diseases, including cancer, diabetes, cardiovascular disease and Crohn's disease (9, 10). However, such epidemiological studies demonstrate associations between vitamin D status and disease and do not firmly establish a causal relationship for vitamin D nutrition in disease causation or prevention. Better information will be especially important in establishing nutritional guidelines for vitamin D intake, considering its roles beyond bone health. To this end, superimposition of dietary vitamin D deficiency on existing animal models of disease provide an attractive experimental approach to evaluate this hormone's role in modulating disease in the context of an intact animal (11-14). Typically, such studies have used diets that are completely depleted of cholecalciferol, thereby inducing severe vitamin D deficiency. However, it is of crucial importance to examine the effects of less severe vitamin D deficiency or vitamin D insufficiency, a large and growing clinical problem worldwide.
The nutritional requirements for cholecalciferol in laboratory mice are estimated at 1IU/g of diet (15). When modeling diet-induced vitamin D deficiency/insufficiency in mice, it is essential to characterize alterations in calcium homeostasis and parathyroid function, as these parameters could independently influence the endpoint under investigation. To this end, Fleet and colleagues determined vitamin D requirements in growing mice and rats, and described diets to establish vitamin D sufficiency and insufficiency in growing mice (16). However, the minimal amount of dietary cholecalciferol needed to maintain optimal vitamin D status in adult mice, or to achieve defined target levels of 25OHD, especially in the controversial insufficient range in adult mice, has not been directly determined. This is particularly important given that most of the diseases that are potentially impacted by non-calcemic actions of vitamin D, such as cancer, cardiovascular disease and diabetes, occur primarily in adulthood.
In general, most experimental rodent studies use standard maintenance diets. The cholecalciferol content of such standard diets varies considerably. For example, standard maintenance diets from one manufacturer (Lab Diets, St Louis, MO) have cholecalciferol contents that range from 2.2 IU/g to as high as 5 IU/g. Notably, unlike precision- and custom-formulated diets, the contents of key nutritional components in standard diets are not tightly controlled. Indeed, we found substantial batch-to-batch variation (up to 50% more than labeled) of cholecalciferol content in a standard, non-certified diet from a major manufacturer (Supplemental Table 1). Plausibly, such variations in vitamin D content could affect biological endpoints being examined.
Here we describe manipulation of dietary cholecalciferol content to induce steady-state vitamin D insufficiency in adult mice. We also characterize the effects of these dietary manipulations on cholecalciferol metabolites 25OHD and 1,25OH2D, biochemical parameters of calcium homeostasis and bone morphology.
Materials and Methods
Diets
We designed four custom fabricated diets containing varying amounts of cholecalciferol (Table 1). The dietary design was based on a review of several typical murine dietary formulations as well as on the recommended daily intake for mice. The cholecalciferol content in commercially available standard diets ranges from 1 IU/g to 5 IU/g. The recommended daily intake (RDI) for vitamin D in laboratory mice is estimated to be 1 IU/g of diet (15). To simulate a typical, standard mouse diet, we used a cholecalciferol concentration of 1.5 IU/g diet. In addition, we designed three diets that had reduced cholecalciferol concentrations relative to the murine RDI, as described in Table 1. The composition of the diets is listed in Supplemental Table 2. All diets were custom formulated to our precise requirements by Harlan Laboratories (Madison, WI). The four diets differ only in their cholecalciferol content. All diets contained 1.0% calcium and 0.3% phosphorous. All other ingredients, including fat, protein, carbohydrate and caloric content were identical (Supplemental Table 2). During manufacture, the vendor prepared the diets without cholecalciferol, and then added an appropriate amount of cholecalciferol stock (concentration determined by HPLC) to achieve the designed concentration. Following preparation, the diets were subjected to analytical testing (AOAC Official Method 982.29) by an independent commercial laboratory (Covance, Madison, WI) to ensure the levels of cholecalciferol were consistent with the designed concentrations. As expected, the cholecalciferol content in 0.05X and 0.25X diets were below the detection levels, and the 0.5X and 1.5X diets were consistent with the designed concentration. None of the diets had any detectable ergocalciferol.
Table 1.
Description and cholecalciferol content in the control and test diets.
| Diet | Description | Cholecalciferol (IU/g) |
|---|---|---|
| 1.5X | Typical cholecalciferol content in standard rodent diets | 1.5 |
| 0.5X | 50% of murine RDI* | 0.5 |
| 0.25X | 25% of murine RDI* | 0.25 |
| 0.05X | 5% of murine RDI* | 0.05 |
The murine RDI for cholecalciferol is 1 IU/g of diet.
Animals and housing
Four-month old adult FVB male and female mice were obtained from Jackson Laboratories. The animals were initially maintained on the standard house chow, containing approximately 2.4 IU/g of cholecalciferol and 1% calcium. After a 2-week acclimatization period, mice were switched to the custom test or control diets (n= 10 mice per dietary group). During the entire experimental period mice were housed with ad libitum access to water and food in a controlled-temperature room. Mice were housed in standard cages without shielding from UVB and maintained on a standard 12-h-light/-dark cycle. Mice were maintained on the custom diets for a period of four months. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Connecticut Health Center.
Biochemical measurements
At monthly intervals following initiation of the test and control diets, blood was collected by facial vein puncture. At termination of the experiment, blood was collected by cardiac puncture under isoflurane anesthesia. Following collection, blood was separated into plasma or serum and used for biochemical assays as described below.
To measure 25OHD3, we used a commercial ELISA kit (Immunodiagnostic Systems Inc., Fountain Hills, AZ, sensitivity 5ng/ml, inter-assay variation 6.4 to 8.7%). Total serum calcium was measured using the Cresolphthalein complexone method using a commercial kit (Stanbio Laboratory, Boerne, TX). PTH concentrations were measured using a commercially available ELISA kit (Immutopics International, San Clemente, CA). 1,25OH2D was measured using a commercially available RIA kit (DiaSorin Inc., Stillwater, MN).
PCNA immunohistochemistry
At the end of the 4-month experimental period, mice were euthanized as described above. The thyro-parathyroid tissue was dissected, fixed in 4% formaldehyde overnight and then saturated with 30% sucrose prior to embedding in freezing medium. The embedded tissue was sectioned in a cryotome and processed for histology and immunohistochemistry to detect PCNA, using a commercially available PCNA kit (Invitrogen, Carlsbad, CA) as described previously (17). This kit uses a biotinylated mouse monoclonal anti-PCNA antibody (Clone PC10).
Micro computed tomography analyses
Following euthanasia, femurs were harvested, the attached soft tissues carefully removed, and the bones were stored in 70% ethanol at 4°C. Femurs from mice in the 1.5X and 0.05X dietary groups (10/group) were analyzed for length, BMC, BMD, cortical area, and cortical thickness by micro–computed tomography (µCT; Skyscan 1172, Aartselaar, Belgium). The μCT images were acquired at 55 kVp and 72 μA at a resolution of 12 μm. Volumetric analysis was performed using the Skyscan software. For cortical analysis at the mid-diaphysis, the length of each femur bone was determined, and 40 mid-diaphyseal slices were used. For trabecular bone analysis, 200 slices per femur were measured, covering a total of 2.4 mm from the proximal growth plate to the shaft distally. The analysis of the secondary spongiosa begins at 0.048 mm below the most distal point of the primary spongiosa, which was defined as directly distal to the most distal portion of the growth plate. A hydroxyapatite phantom was used for BMD calibration. Nomenclature for the bone morphology parameters is as described (18).
Statistical analysis
All statistical analyses were performed using the Prism software program (GraphPad Software Inc, LaJolla, CA). Serum 25OHD, serum 1,25OH2D3, serum calcium, and serum PTH levels of mice from the 4 dietary groups were compared using the Kruskal-Wallis test, followed by Dunn's multiple comparison test. MicroCT bone parameters from mice on the 1.5X and 0.05X diets were compared using the Mann-Whitney test.
Results
Dietary modulation induced a steady state of vitamin insufficiency/deficiency
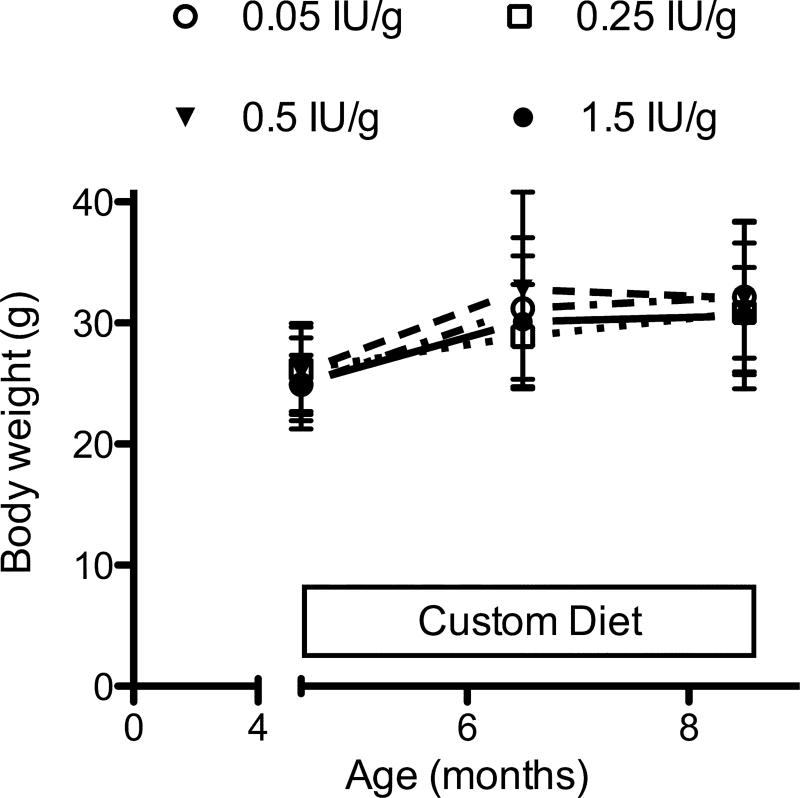
The animals tolerated all diets, and there were no deaths or evident adverse effects attributed to the dietary manipulations. Through the experimental period there was modest weight gain, with no significant difference between the four dietary groups (Fig. 1).
Figure 1.
Body weight changes during the experimental period. Mice were fed with the indicated diets starting at age 4.5 months. Symbols and error bars represent mean ± SD.
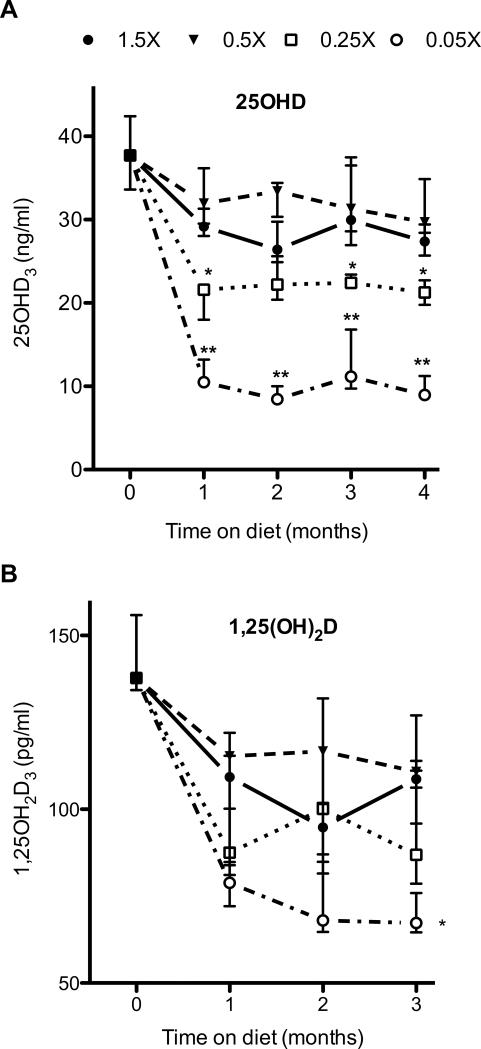
Prior to initiation of the control and test diets, all animals were maintained on a standard rodent chow, containing 2.4 IU/g of cholecalciferol. At the start of the dietary manipulation period, the median serum 25OHD concentration was 38 ng/ml (interquartile range: 34ng/ml to 42 ng/ml, Fig 2). One month after initiation of the experimental diets, the median serum 25OHD concentrations in mice fed with the 1.5X diet was 29 ng/ml (interquartile range: 28ng/ml to 31 ng/ml, Fig 1). These concentrations remained steady and did not significantly vary during the 4-month experimental diet period (Fig. 2). Over the 4-month period, the median serum 25OHD concentrations in individual mice fed with the 0.5X diet ranged from 30 to 33 ng/ml, and were not significantly different than those in mice fed with the 1.5X diet (median 27 ng/ml to 30 ng/ml). In contrast, the 0.25X and 0.05X diets markedly reduced serum 25OHD concentrations. Within one month after beginning the 0.25X and 0.05X diets, the median serum 25OHD concentrations decreased to 21.6 ng/ml (interquartile range: 18.0 to 22.1 ng/ml), and 10.5 ng/ml (interquartile range: 10 ng/ml to 13.2 ng/ml), respectively, and were significantly lower than those in mice fed with the vitamin D replete 1.5X diet (p≤0.05 and p≤0.001, respectively). Feeding mice with these cholecalciferol deficient diets for longer time periods did not yield further decreases in 25OHD concentrations, further emphasizing the point that a steady state had been achieved, and these 25OHD levels were maintained throughout the 4-month experimental period. Notably, there was a dose-dependent decrease in the 25OHD levels with decreasing cholecalciferol concentrations, most noticeable over the diet range of 0.05 IUg to 0.5IU/g.
Figure 2.
Effect of dietary cholecalciferol content on (A) serum 25OHD concentrations and (B) serum 1,25OH2D concentrations. Mice were fed with the indicated diets and blood was collected at monthly intervals. Symbols and error bars represent median ± interquartile range, *p ≤ 0.05, *p≤0.001, compared with 1.5X and 0.5X diets. For the 25OHD levels, n=27 for 0 months, and 8 to 10 mice per dietary group for months 1 to 4. For the 1,25OH2D levels, n=12 for 0 months, and 9 mice per dietary group for months 1 to 3.
We also examined the effects of these diets on the concentrations of vitamin D's active metabolite, 1,25OH2D in a subset of these mice. For these studies we analyzed 1,25OH2D concentrations over a 3-month period in all dietary groups. Within each dietary group, there was no variation in the 1,25OH2D concentrations with time. The 1,25OH2D concentrations in mice fed with the control (1.5X) diet was 101 ± 12 pg/ml. This was not significantly changed by the 0.5X diet (111 ± 16 pg/ml). The 0.25X yielded a slight decrease in these concentrations to 91± 5 pg/ml, although the difference was not statistically different. In contrast, 1,25OH2D concentrations were significantly lower in mice fed with the 0.05X diet (78 ± 3 pg/ml, p≤0.0001 compared with control diet).
Dietary modulation did not alter calcium homeostasis or parathyroid physiology
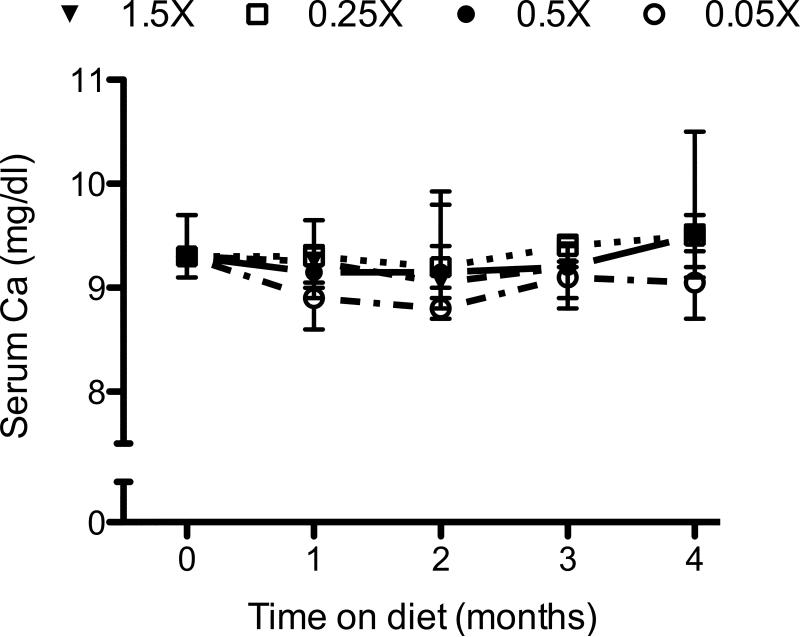
Vitamin D plays an important role in intestinal calcium absorption. To examine whether our dietary modulations disturbed calcium homeostasis, we examined the serum total calcium and PTH concentrations. The median serum calcium in mice at the start of the experiment was 9.3mg/dl (interquartile range: 9.1 mg/dl to 9.7 mg/dl). None of the diets significantly altered serum calcium concentrations. Throughout the experimental period there was no significant difference in the total serum calcium concentrations between the 4 dietary groups (Fig 3). At the end of the 4-month experimental period, the median serum calcium concentrations for the 1.5X, 0.5X, 0.25X and 0.05X dietary groups were 9.5 mg/dl, 9.5 mg/dl, 9.5 mg/dl, and 9.1 mg/dl, respectively.
Figure 3.
Effect of dietary cholecalciferol content on serum calcium concentrations. Mice were fed with the indicated diets for 4 months. Blood was collected at monthly intervals and analyzed for total serum calcium. Symbols represent medians ± interquartile range.
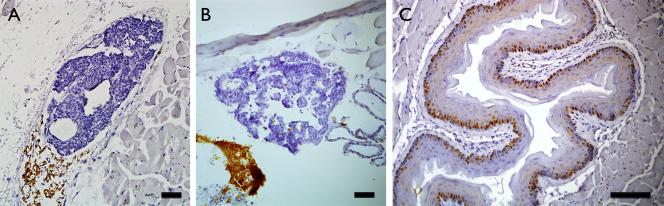
We also examined the effects of the dietary manipulations on the circulating PTH concentrations. For these analyses, we assayed the PTH concentrations in mice after 3 months of dietary manipulation. Given the rapid decrease in 25OHD3 levels as described above, we considered that the 3-month period would be sufficient to induce any secondary increase in PTH secretion. The median PTH concentrations for the 1.5X, 0.5X, 0.25X and 0.05X dietary groups were 252, 167, 362 and 265 pg/ml, respectively. Comparison between the four dietary groups did not reveal any statistical difference in the PTH concentrations. To further confirm absence of any effect on the parathyroid gland, we examined the glands from these mice by histology and immunohistochemistry. On gross histology there was no evidence of parathyroid hyperplasia or neoplasia. Furthermore, we also examined parathyroid cell proliferation in a subset of the mice. For these analyses, we compared parathyroid glands from mice maintained on the control diet or the 0.05X diet, the latter having yielded the lowest 25OHD concentrations. Parathyroid glands from mice treated with the replete diet showed minimal cell proliferation, consistent with previous reports. A similar low level of PCNA immunoreactivity was observed in the parathyroid glands from mice fed with the 0.05X diets (Fig 4).
Figure 4.
Representative PCNA immunoreactivity in the parathyroid glands of mice fed with the (A) 1.5X diet, or (B) 0.05X diet. Note sparse PCNA immunoreactivity with both diet types. (C) Positive PCNA immunoreactivity in esophagi on the same sections served as positive controls. Magnification bars = 250 μm
Dietary modulation did not alter bone morphology
Vitamin D plays an important role in skeletal health. Thus, we examined whether the decreased 25OHD levels caused by dietary manipulation resulted in alterations in the trabecular and cortical bone morphology. For these studies, we used microCT to analyze the vertebrae and femurs from mice fed with the control and 0.05X diets, the latter representing the group with the lowest 25OHD concentrations. No differences in cortical or trabecular bone parameters were observed between the 2 dietary groups (Tables 2 and 3).
Table 2.
Cortical bone parameters in femurs from mice fed with the 1.5X and 0.05X diets.
| 1.5X Diet | 0.05X Diet | |
|---|---|---|
| Tt.Ar. | 1.75 ± 0.05 | 1.77 ± 0.06 |
| Cs.Th | 0.190 ± 0.005 | 0.188 ± 0.002 |
| Cortical.Th. | 0.127 ± 0.003 | 0.126 ± 0.001 |
Table 3.
Trabecular bone parameters in femurs from mice fed with the 1.5X and 0.05X diets.
| 1.5X diet | 0.05X diet | |
|---|---|---|
| TV (mm3) | 3.80 ± 0.21 | 3.95 ± 0.20 |
| BV (mm3) | 0.27 ± 0.09 | 0.27 ± 0.02 |
| BV/TV (%) | 7.1 ± 0.03 | 6.8 ± 0.50 |
| Tb.Th. (mm) | 0.068 ± 0.002 | 0.066 ± 0.001 |
| Tb. N. (mm−1) | 1.05 ± 0.08 | 1.10 ± 0.07 |
| Tb.Sp. (mm) | 0.40 ± 0.02 | 0.35 ± 0.01 |
Discussion
Vitamin D plays an important role in calcium homeostasis. In addition to its established role in bone health, it is now recognized that vitamin D has roles in cell proliferation, cell differentiation and immunomodulation (19). There is increasing interest in these non-calcemic effects of vitamin D, especially its involvement in cancer, immune function and cardiovascular disease. Animal models provide an attractive approach to study the effects of vitamin D on these processes in a manner that simulates human pathophysiology— manipulation of the dietary vitamin D content will permit direct examination of the influence of vitamin D deficiency on specific endpoints. However, given vitamin D's calcemic effects, these dietary manipulations could result in altered calcium homeostasis or skeletal effects and these latter effects could potentially confound the endpoint under investigation. Mice fed with vitamin D-depleted diets, without any added cholecalciferol, manifest severe vitamin D deficiency (20). However, the precise vitamin D content in murine diets that can be reliably used to induce various states of vitamin D deficiency and insufficiency in adult mice has not been adequately described. Importantly, the minimum dietary requirement of vitamin D for adult mice with regards to maintaining a normal 25OHD3 concentration has not been scientifically validated.
We wish to emphasize the rigor in the fabrication and testing of the diets that we found to be necessary. This included independent laboratory assays to confirm the concentration of the cholecalciferol stock premixes to ensure that the prepared deficient and insufficient diets contained the appropriate cholecalciferol content. An important contribution of our study is the definition of dietary formulations that rapidly and reliably decrease the serum 25OHD3 concentrations into the targeted ranges. Within one month of initiating the experimental dietary formulations, the median 25OHD3 concentrations decreased to 21.6 ng/ml with the 0.25X diet, and to 10.5 ng/ml with the 0.05X diet. Importantly, these decreases are at the thresholds for the insufficiency/optimal, and the deficiency/insufficiency ranges, respectively. Notably, these decreased levels remained consistent for the entire 4-month experimental period suggesting that the dietary manipulations induce a steady-state decrease of 25OHD3 levels. It is noteworthy that the serum 25OHD3 levels yielded by the 0.05X diets parallel the 25OHD3 levels in human vitamin D moderate deficiency/insufficiency. Such 25OHD levels have also been observed by Fleet et al. following dietary cholecalciferol restriction in growing mice (16). Many rodent studies evaluating the effects of vitamin D have used diets devoid of any vitamin D, which result in severe vitamin D deficiency. Of note, during the entire experimental period, the mice were housed under standard conditions and we did not attempt to restrict exposure to UVB lighting. The rapid dietary-induced decrease in 25OHD suggests that dermal vitamin D synthesis does not contribute significantly to the 25OHD levels in mice. Thus, our data suggest that efforts to shield against UVB radiation are not essential, at least to achieve the clinically relevant degrees of vitamin D deficiency and insufficiency, as demonstrated in our study. Strikingly, we also observed a linear trend between 25OHD levels and dietary cholecalciferol contents, at least between the dietary cholecalciferol contents of 0.05 IU/g to 0.5 IU/g. The new information from our study provides guidance to fabricate diets that would simulate various degrees of vitamin D deficiency/insufficiency in adult mice over a range of levels that are similar to that often found in human populations. This would facilitate future studies to examine the role of such states of vitamin D inadequacy in a variety of disease conditions, including cancer and immune response, and provide guidance to the optimal vitamin D status required to maintain health of specific physiological systems.
Importantly, we show that although these diets reduced 25OHD3 to insufficient and marginally-deficient levels, the lowered 25OHD3 levels did not result in hypocalcemia or secondary hyperparathyroidism, as determined by biochemical analyses, and by histological and immunohostochemical examination of the parathyroid glands from these mice. Furthermore, microCT examination of the bones from mice with such lowered 25OHD3 levels did not demonstrate any abnormalities in the cortical and trabecular bone parameters. Thus, these dietary formulations will enable experimental investigation of vitamin D's effects without potential confounding effects from hypocalcemia and hyperparathyroidism.
Given vitamin D's role in intestinal calcium absorption, the lowered 25OHD3 concentrations could have been expected to induce hypocalcemia and subsequent increase in PTH secretion, in turn enhancing renal 1,25OH2D3 synthesis. Accordingly, the notable absence of hypocalcemia and hyperparathyroidism observed in our mice with lowered 25OHD3 is intriguing. A potential explanation lies in the calcium concentration of our experimental diets. Although a calcium concentration of 1% is used in several standard laboratory rodent diets, it is considerably higher than the estimated murine RDI for calcium (0.5%). It is likely that the abundant dietary supplementation of calcium in our study may have been sufficient to compensate for any decrease in calcium absorption resulting from the lowered vitamin D status. Similar findings have been observed in rat models, where a dietary calcium level of as low as 0.4% was sufficient to maintain normocalcemia without severe secondary HPT even when the mice were fed with a vitamin D deplete diet (21). Likewise, high dietary calcium rescues the skeletal phenotype of VDR-null mice, where vitamin D signaling is ablated (22). Our data are consistent with prior observations emphasizing the importance of dietary calcium, and its ability to compensate for vitamin D deficiency in maintenance of skeletal health in adult mice. This suggests that even a 0.4% calcium dietary content may be in excess of the minimum level required for the maintenance of calcium homeostasis. Indeed, in vitamin D replete rats normocalcemia can be maintained with a diet containing as low as 0.1% Ca (23).
Decrease in the circulating concentrations 25OHD3 levels may lead to enhanced renal 1,25(OH)2D3 synthesis. Thus, it is intriguing that we found a decrease in 1,25(OH)2D3 even in mice fed with deficient levels of vitamin D. Possibly, substrate limitation may in part contribute to this decreased 1,25(OH)2D3 level. Additionally, these effects may represent the higher calcium content in the diets used. For example in one study using a rat model, increasing dietary calcium from 0.4% to 1% reduced 1,25D levels even in rats fed a vitamin D deplete diet (23), and has been attributed to upregulation of renal CYP24A1. Overall, these findings emphasize the complex regulation of 1,25(OH)2D3.
Our finding of a lack of skeletal effects is especially significant given that bone health is often considered the sole parameter to evaluate the nutritional vitamin D requirement. Clearly our dietary interventions did not induce any skeletal effects, but did dramatically reduce 25OHD3 concentrations, maintaining the potential to investigate adverse extraskeletal consequences from vitamin D nutritional deficiency. Indeed, in ongoing studies in our laboratory, we have applied these dietary manipulations in transgenic mice to study the effects of vitamin D nutrition on parathyroid pathophysiology and oral cancer development. In these studies, we have maintained mice on these diets for almost 7 months, and continue to observe steady-state decreases in 25OHD3. Importantly, the general well being of the mice maintained on these various diets underscores that such dietary manipulations do not yield any overt health effects, and thus will allow investigators to study the contribution of vitamin D deficiency/insufficiency in mice, closely simulating the context of vitamin D deficiency/insufficiency in humans.
Currently, the RDI of cholecalciferol in mice is estimated to be 1IU/g. However, it has not been scientifically validated that this is the minimal cholecalciferol intake needed to maintain normal 25OHD3 levels or skeletal health in mice. Our study demonstrates that decreasing dietary cholecalciferol content to 0.5 IU/g did not adversely impact 25OHD and 1,25OH2D levels in mice. This lack of deterioration in primary cholecalciferol metabolites underscores that a dietary cholecalciferol content of 0.5IU/g can be sufficient to maintain normal vitamin D status for maintenance of calcium homeostasis in mice. Our data therefore, suggest the need to redefine the RDI for cholecalciferol in mice, at least for the FVB strain, and to investigate this issue for other commonly used strains.
In summary, we report on a comprehensive evaluation of the effect of dietary cholecalciferol restriction on the primary metabolites of cholecalciferol, and its impact on calcium/bone homeostasis in adult mice. These data provide important guidance to designing studies that aim to evaluate the effects of vitamin D nutrition in preclinical mouse models of human disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Thomas Carpenter (Yale University, New Haven, CT) for performing assays of serum 1,25OH2D.
SMM and AA designed research; SMM, KRC, EAS, FFY, HQT, KS, and EA conducted research; SMM, KRC, EAS, FFY, HQT, KS, EA, ST and AA analyzed data; and SMM and AA wrote the paper. SMM and AA had primary responsibility for final content. All authors read and approved the final manuscript
Funding
Research reported in this publication was supported by the National Institutes of Health under award numbers R01DK066411 (AA) and R03DE022598 (SM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was supported in part by the Murray-Heilig Fund in Molecular Medicine (AA), by the American Institute of Cancer Research grant 10A046 (SM), and by a seed grant from the UCLA School of Dentistry (SM).
Footnotes
Conflicts of interest: The authors do not have any conflicts of interest.
References
- 1.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21(6):375–84. doi: 10.1016/j.tem.2010.01.003. Epub 2010/02/13. doi: S1043-2760(10)00005-6 [pii] 10.1016/j.tem.2010.01.003. PubMed PMID: 20149679; PubMed Central PMCID: PMC2880203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441(1):61–76. doi: 10.1042/BJ20110744. Epub 2011/12/16. doi: 10.1042/BJ20110744. PubMed PMID: 22168439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. American journal of physiology. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. PubMed PMID: 15951480. [DOI] [PubMed] [Google Scholar]
- 5.Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Archives of biochemistry and biophysics. 2012;523(1):107–14. doi: 10.1016/j.abb.2011.10.019. doi: D - nlm: nihms337760 D - NLM: PMC3295909 EDAT- 2011/11/17 06:00 MHDA- 2012/08/07 06:00 CRDT- 2011/11/17 06:00 PHST- 2011/09/21 [received] PHST- 2011/10/31 [accepted] PHST- 2011/11/09 [aheadofprint] AID - S0003-9861(11)00356-0 [pii] AID - 10.1016/j.abb.2011.10.019 [doi] PST - ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, et al. The impact of vitamin D status on periodontal surgery outcomes. J Dent Res. 2011;90(8):1007–12. doi: 10.1177/0022034511407771. Epub 2011/05/11. doi: 10.1177/0022034511407771. PubMed PMID: 21555774; PubMed Central PMCID: PMC3170167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine . In: Dietary Reference Intakes for Calcium and Vitamin D. Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. National Academy of Sciences; Washington DC: 2011. [PubMed] [Google Scholar]
- 8.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. Epub 2011/06/08. doi: 10.1210/jc.2011-0385. PubMed PMID: 21646368. [DOI] [PubMed] [Google Scholar]
- 9.Moreno LA, Valtuena J, Perez-Lopez F, Gonzalez-Gross M. Health effects related to low vitamin D concentrations: beyond bone metabolism. Ann Nutr Metab. 2011;59(1):22–7. doi: 10.1159/000332070. Epub 2011/11/30. doi: 10.1159/000332070. PubMed PMID: 22123633. [DOI] [PubMed] [Google Scholar]
- 10.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn's disease. Gastroenterology. 2012;142(3):482–9. doi: 10.1053/j.gastro.2011.11.040. Epub 2011/12/14. doi: 10.1053/j.gastro.2011.11.040. PubMed PMID: 22155183; PubMed Central PMCID: PMC3367959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastie CC, Gaffney-Stomberg E, Lee TW, Dhima E, Pessin JE, Augenlicht LH. Dietary Cholecalciferol and Calcium Levels in a Western-Style Defined Rodent Diet Alter Energy Metabolism and Inflammatory Responses in Mice. The Journal of nutrition. 2012 doi: 10.3945/jn.111.149914. Epub 2012/03/23. doi: 10.3945/jn.111.149914. PubMed PMID: 22437564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang K, Lamprecht SA, Shinozaki H, Fan K, Yang W, Newmark HL, et al. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. The Journal of nutrition. 2008;138(9):1658–63. doi: 10.1093/jn/138.9.1658. PubMed PMID: 18716166. [DOI] [PubMed] [Google Scholar]
- 13.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151(6):2423–32. doi: 10.1210/en.2010-0089. Epub 2010/04/16. doi: 10.1210/en.2010-0089. PubMed PMID: 20392825; PubMed Central PMCID: PMC2875827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovalenko PL, Zhang Z, Yu JG, Li Y, Clinton SK, Fleet JC. Dietary vitamin D and vitamin D receptor level modulate epithelial cell proliferation and apoptosis in the prostate. Cancer Prev Res (Phila) 2011;4(10):1617–25. doi: 10.1158/1940-6207.CAPR-11-0035. Epub 2011/08/13. doi: 10.1158/1940-6207.CAPR-11-0035. PubMed PMID: 21836023; PubMed Central PMCID: PMC3188351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nutrition. NRCUSSoLA. Nutrient requirements of laboratory animals. National Research Council (U.S.), Subcommittee on Laboratory Animal Nutrition, National Academy of Sciences; Washington, D.C.: 1995. p. 0309051266. [Google Scholar]
- 16.Fleet JC, Gliniak C, Zhang Z, Xue Y, Smith KB, McCreedy R, et al. Serum metabolite profiles and target tissue gene expression define the effect of cholecalciferol intake on calcium metabolism in rats and mice. The Journal of nutrition. 2008;138(6):1114–20. doi: 10.1093/jn/138.6.1114. Epub 2008/05/22. doi: 138/6/1114 [pii]. PubMed PMID: 18492843; PubMed Central PMCID: PMC2542586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallya SM, Gallagher JJ, Wild YK, Kifor O, Costa-Guda J, Saucier K, et al. Abnormal parathyroid cell proliferation precedes biochemical abnormalities in a mouse model of primary hyperparathyroidism. Mol Endocrinol. 2005;19(10):2603–9. doi: 10.1210/me.2005-0116. Epub 2005/06/02. doi: me.2005-0116 [pii] 10.1210/me.2005-0116. PubMed PMID: 15928311. [DOI] [PubMed] [Google Scholar]
- 18.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. Journal of bone and mineral research. 2010;25(7):1468–86. doi: 10.1002/jbmr.141. Epub 2010/06/10. doi: 10.1002/jbmr.141. PubMed PMID: 20533309. [DOI] [PubMed] [Google Scholar]
- 19.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–8. doi: 10.1210/jc.2009-1773. Epub 2010/02/06. doi: 95/2/471 [pii] 10.1210/jc.2009-1773. PubMed PMID: 20133466; PubMed Central PMCID: PMC2840860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70(5):1835–44. doi: 10.1158/0008-5472.CAN-09-3194. Epub 2010/02/18. doi: 10.1158/0008-5472.CAN-09-3194. PubMed PMID: 20160035. [DOI] [PubMed] [Google Scholar]
- 21.Anderson PH, Sawyer RK, May BK, O'Loughlin PD, Morris HA. 25-Hydroxyvitamin D requirement for maintaining skeletal health utilising a Sprague-Dawley rat model. J Steroid Biochem Mol Biol. 2007;103(3-5):592–5. doi: 10.1016/j.jsbmb.2006.12.086. Epub 2007/02/03. doi: 10.1016/j.jsbmb.2006.12.086. PubMed PMID: 17267207. [DOI] [PubMed] [Google Scholar]
- 22.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139(10):4391–6. doi: 10.1210/endo.139.10.6262. PubMed PMID: 9751523. [DOI] [PubMed] [Google Scholar]
- 23.O'Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511(1):313–22. doi: 10.1111/j.1469-7793.1998.313bi.x. doi: 10.1111/j.1469-7793.1998.313bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.