Abstract
Background
The long-term outcomes of aortic valve sparing (AVS) root replacement in Marfan syndrome (MFS) patients remain uncertain. We sought to determine the utilization and outcomes of AVS root replacement in MFS patients enrolled in the Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC).
Methods
At the time of this analysis, 788 patients with MFS were enrolled in the GenTAC Registry, of whom 288 have undergone aortic root replacement. Patients who have undergone AVS procedures were compared to those who have undergone aortic valve replacing (AVR) procedures.
Results
AVS root replacement was performed in 43.5% of MFS patients and the frequency of AVS increased over the past 5 years. AVS patients were younger at the time of surgery (31.0 vs. 36.3 years, p=0.006) and more likely to have had elective rather than emergency surgery compared to AVR patients. AVR patients were more likely to have had aortic valve dysfunction and aortic dissection as a primary indication for surgery. After mean follow-up of 6.2 (SD=3.6) years, none of the 87 AVS patients have required reoperation; in contrast, after mean follow up of 10.5 (SD=7.6) years, 11.5% of AVR patients have required aortic root reoperation. Aortic valve function has been durable with 95.8% of AVS patients with aortic insufficiency graded as mild or less.
Conclusions
AVS root replacement is performed commonly in the MFS population. The durability of the aortic repair and aortic valve function have been excellent to date. These results justify the continued use of the procedure in the elective setting. The GenTAC Registry will be a useful resource to assess the long-term durability of AVS root replacement in the future.
Keywords: aortic root, aortic valve, aneurysm
Marfan syndrome (MFS) is an autosomal dominant inherited disorder caused by mutations in the gene that encodes fibrillin-1 (1,2). This leads to systemic connective tissue manifestations most prominently involving the ocular, skeletal, and cardiovascular systems. The primary cardiovascular lesion is aortic root aneurysm. Aortic dissection and rupture are the most common causes of death in MFS patients with a peak incidence in the third and fourth decades of life (3,4). The life expectancy of patients with MFS has improved dramatically since aortic root replacement with a composite prosthetic valve conduit was first reported in 1967 (5,6). Aortic root replacement procedures are now routinely performed under elective conditions with low morbidity and mortality (7-12).
Techniques for aortic valve sparing (AVS) root replacement have been developed over the past several decades since their initial descriptions by Yacoub in 1979 (remodeling) and David in 1988 (reimplantation) (7,13). Concern about the durability of the native aortic valve following AVS root replacement has led to controversy regarding the suitability of AVS procedures in MFS patients (11,14,15). This concern has led to the development of a prospective study that is ongoing to address this issue directly (16). Despite the controversy, AVS root replacement procedures have been rapidly adopted for MFS patients in many centers. The National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) has established a biospecimen respository and bioinformatics infrastructure to enable research to determine the best practices for management of genetically triggered thoracic aortic aneurysms (17,18). In this study, we have investigated the current utilization and mid-term outcomes of AVS root replacement in MFS patients enrolled in the GenTAC Registry.
Patients and Methods
GenTAC Registry
The GenTAC Registry contains longitudinal observational data on patients with conditions related to genetically induced thoracic aortic aneurysms (17). It also has a biospecimen repository and bioinformatics infrastructure created to support research to determine the optimal clinical management of genetically triggered thoracic aortic aneurysms and related complications. The National Heart Lung and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases are co-sponsors of GenTAC.
Patients included in this study were recruited from 6 regional clinical centers that treat patients from a wide geographic catchment area within the United States: Baylor College of Medicine, Johns Hopkins University, Oregon Health & Science University, University of Pennsylvania, University of Texas Health Science Center at Houston, and Weill Cornell Medical College. Research Triangle Institute International serves as the data coordinating center responsible for data management, coordination of training, logistics, and statistical design and analysis.
The targeted enrollment of patients includes adults and children who fall into 1 or more of 12 diagnosis categories, including MFS, Loeys-Dietz syndrome, vascular Ehlers-Danlos syndrome, Turner syndrome, bicuspid aortic valve with ascending aortic aneurysm, and familial thoracic aortic aneurysm and dissection. Available clinical data, imaging results, and blood and tissue samples from each patient are processed and stored by GenTAC to provide a resource that combines clinical and biological data from a large and diverse population of patients with inherited thoracic aortic aneurysm disorders.
Patients
The diagnosis of MFS was made at the regional clinical centers and was based on the revised Ghent criteria (19). From the 788 patients with MFS who were enrolled in GenTAC as of October 2012, we selected those who had undergone aortic root replacement procedures. Our analyses focused on procedure information, imaging findings, and quality of life, which was measured with the Karnofsky Performance Status Score (20).
Analysis
We used SAS software (SAS Institute, Inc.; Cary, NC) to extract data from a secure enterprise network database to create reports and summary tables and to perform ad hoc statistical analyses. We used t-tests to examine between-group differences with continuous outcomes. To examine between-group differences with categorical outcomes, we used Chi-squared tests unless the sample sizes were small, in which case Fisher exact tests were performed. For data security purposes, all analyses were performed and all data were stored in a password-protected remote workspace.
Institutional Review Boards and Consent
Institutional Review Board approval was obtained for this study at each of the 6 participating GenTAC regional clinical centers. Individual informed consent was obtained from each GenTAC Registry patient.
Results
At the time of the analysis, 788 patients with MFS were enrolled in GenTAC. Of these, 288 (37%) had undergone aortic root replacement. These patients were divided into two groups: those who had undergone aortic valve replacing (AVR) vs. aortic valve sparing (AVS) root replacement procedures. Two hundred patients (AVR 113, AVS 87) had detailed clinical data available for analysis. As shown in Table 1, patients who had undergone AVS root replacement were younger at the age of enrollment and were also more likely to have been treated with Losartan. Other demographic variables were similar between the AVR and AVS groups.
Table 1.
Patient demographics.
| Variable | Statistic | Total Marfan Patients (n=788) | Patients with Valve Replacing (n=113) | Patients with Valve Sparing (n=87) | p-value* (VR vs. VS) | |||
|---|---|---|---|---|---|---|---|---|
| Age at enrollment | mean(SD) | 31.9 | (17.7) | 44.6 | (15.2) | 33.7 | (12.8) | < 0.0001 |
| < 5 years | n (%) | 34 | (4.3) | 0 | 1 | (1.2) | < 0.0001 | |
| 5-17 years | n (%) | 177 | (22.5) | 2 | (1.8) | 6 | (6.9) | |
| 18-39 years | n (%) | 307 | (39.0) | 42 | (37.2) | 53 | (60.9) | |
| 40-69 years | n (%) | 252 | (32.0) | 61 | (54.0) | 26 | (29.9) | |
| > 69 years | n (%) | 18 | (2.3) | 8 | (7.1) | 1 | (1.2) | |
| Age at diagnosis | ||||||||
| Gender (Male) | n (%) | 421 | (53.4) | 74 | (65.5) | 61 | (70.1) | 0.54 |
| White race | n (%) | 703 | (89.2) | 105 | (92.9) | 84 | (96.6) | 0.36 |
| Hispanic | n (%) | 73 | (9.3) | 8 | (7.1) | 10 | (11.5) | 0.32 |
| Height (cm) | mean(SD) | 176.1 | (22.3) | 185.2 | (11.2) | 185.9 | (14.8) | 0.72 |
| Weight (kg) | mean(SD) | 74.2 | (28.7) | 87.0 | (22.0) | 84.0 | (26.5) | 0.41 |
| Medications (ever) | ||||||||
| Beta-blocker | n(%) | 624 | (79.2) | 105 | (92.9) | 84 | (96.6) | 0.35 |
| Losartan | n(%) | 318 | (40.4) | 52 | (46.0) | 55 | (63.2) | 0.02 |
| Other ARB | n(%) | 76 | (9.6) | 17 | (15.0) | 13 | (14.9) | 0.99 |
| ACE-inhibitor | n(%) | 124 | (15.7) | 45 | (39.8) | 13 | (14.9) | 0.0001 |
| Statins | 92 | (11.7) | 29 | (25.7) | 13 | (14.9) | 0.07 | |
| Verapamil/diltiazem | n(%) | 65 | (8.2) | 18 | (15.9) | 12 | (13.8) | 0.7 |
| Other calcium channel blocker | n(%) | 72 | (9.1) | 23 | (20.4) | 11 | (12.6) | 0.15 |
| Other BP lowering drug | n(%) | 83 | (10.5) | 34 | (30.1) | 8 | (9.2) | 0.0003 |
| Karnofsky performance | mean(SD) | 90.6 | (12.9) | 85.4 | (17.0) | 87.9 | (14.1) | 0.28 |
Chi-squared/Fisher exact test for categorical, and T-test for continuous variables.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; VR, valve replacing; VS, valve sparing.
Although AVR surgery was more common than AVS surgery overall, in recent years AVS surgery has become more common. Figure 1 depicts the number of AVR and AVS procedures performed by year. The first AVR surgery in the Registry was performed in 1981. The first AVS procedure was performed in 1997. For patients operated on up to 2007, AVS surgery comprised only 33.3% of procedures. In the period 2008-2012, AVS surgery was more common than AVR surgery (54.4% vs. 46.6%). This change over time (p-value = 0.002) likely reflects centers’ increasing experience and confidence in the early and medium-term outcomes of the AVS procedure in the MFS patient population.
Figure 1.
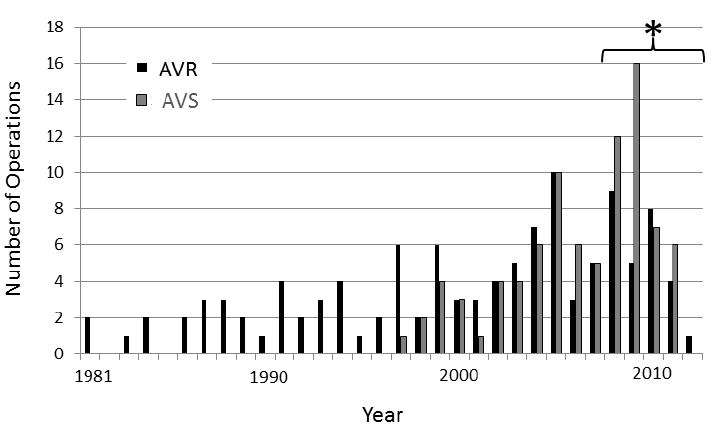
Number of Aortic root replacement procedures by year. * AVS surgery comprised only 33.3% of procedures prior to 2008. In the period 2008-2012, AVS surgery comprised 54.4% of procedures (p-value = 0.002). AVR, aortic valve replacing; AVS, aortic valve sparing.
Table 2 depicts details of the initial aortic root replacement procedure for the 200 patients in the primary analysis. AVS patients were significantly younger at the time of their root operations and more likely to have their surgery in elective circumstances than AVR patients. Emergency AVS surgery was rare. Aneurysm was more common as an indication for surgery in the AVS group than in the AVR group. The average aortic root diameter of AVS patients prior to surgery was 4.81 cm, which is smaller than previously published guidelines for elective repair in MFS patients (21). Although aortic regurgitation was present in 48.0% of AVS patients, aortic valve dysfunction was more common in the AVR group. Dissection as an indication for surgery was more common in the AVR group than in the AVS group (31.9 vs. 3.5%), reflecting the higher frequency of emergency surgery in this group.
Table 2.
Aortic root replacement procedures.
| Variable | Patients with Valve Replacing (n=113) | Patients with Valve Sparing (n=87) | p-value* |
|---|---|---|---|
| Age at surgery (mean years) | 36.3 | 31.0 | 0.006 |
| Elective surgery (%) | 68.0 | 95.4 | <0.0001 |
| Emergency surgery (%) | 32.0 | 4.6 | |
| Aortic root diameter at time of surgery (cm) | 5.25 (n=21) | 4.81 (n=37) | 0.23 |
| Indication for surgery | |||
| Aneurysm (%) | 85.8 | 98.9 | 0.001 |
| Aortic regurgitation (%) | 68.0 | 48.0 | 0.005 |
| Dissection (%) | 31.9 | 3.5 | <0.0001 |
| Associated procedures | |||
| CABG (%) | 8.0 | 2.3 | 0.12 |
| Mitral valve repair (%) | 7.1 | 10.3 | 0.41 |
| Mitral valve replacement (%) | 8.0 | 1.1 | 0.04 |
| Patients requiring reoperation on aortic root (%) | 11.5 | 0 | 0.0011 |
| Mean time (years) between operation and follow up | 10.5 | 6.2 | <0.0001 |
Chi-squared/Fisher exact test for categorical, and T-test for continuous variables. CABG, coronary artery bypass graft.
Associated procedures were common. Mitral valve procedures were performed in 15.1% of AVR patients and 11.4% of AVS patients. Mitral valve replacement was much more common in AVR patients than in AVS patients, suggesting that inability to perform mitral valve repair in patients with double valve disease influenced the choice of aortic root replacement procedure.
Importantly, the durability of AVS procedures in the GenTAC population has been excellent. At mean follow up of 6.2 (SD = 3.6) years, no AVS patient has required a reoperation on the aortic root. In comparison, at mean follow up of 10.5 (SD=7.6) years, 11.5% of AVR patients have required reoperation. This relatively high reoperation frequency for AVR patients is likely related to the longer duration of follow up for the AVR group, the high incidence of dissection, and the high frequency of stentless tissue and homograft aortic root replacements that were performed in this group. Of the 13 AVR patients requiring reoperation, 8 had significant valve dysfunction of a stentless tissue or homograft aortic root at the time of their second surgery. Figure 2 depicts a Kaplan-Meier curve showing freedom from reoperation following initial AVR or AVS root replacement surgery.
Figure 2.
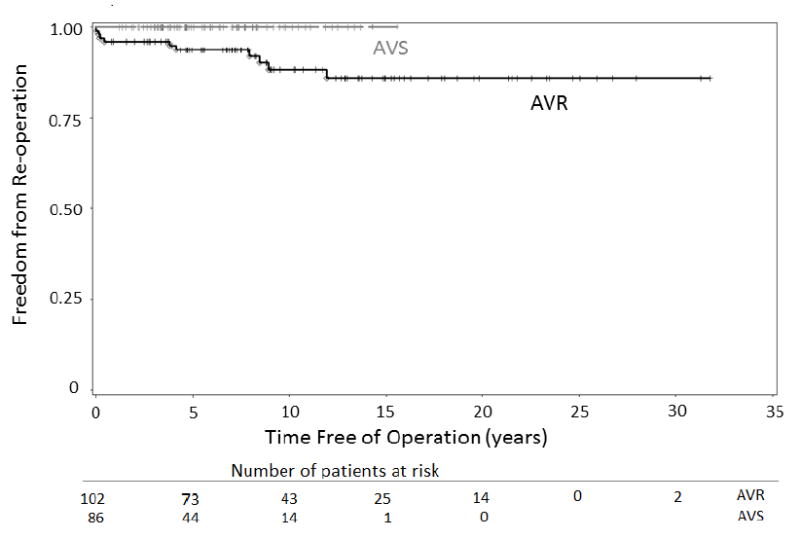
Kaplan-Meier curve showing freedom from reoperation following initial AVR or AVS root replacement. AVR, aortic valve replacing; AVS, aortic valve sparing.
Limited postoperative imaging data was available through the GenTAC registry (Table 3). A total of 89 echocardiograms were analyzed (42 AVR, 47 AVS). The mean duration of imaging follow-up was substantially longer for AVR patients than for AVS patients (9.7 (SD=7.4) vs. 4.2 (SD=3.7) years). The majority of patients in both groups had mild, trivial, or no aortic regurgitation. More AVR patients than AVS patients had trivial or no regurgitation, while mild regurgitation was substantially more common in AVS patients than in AVR patients. The frequency of moderate or severe aortic insufficiency was similarly low in both groups (7.2% AVR, 4.2% AVS). Figure 3 depicts a Kaplan-Meier curve showing freedom from moderate or severe aortic regurgitation following AVR or AVS root replacement surgery.
Table 3.
Postoperative aortic valve regurgitation
| Variable | Patients with Valve Replacing (n=42) | Patients with Valve Sparing (n=47) | p-value* |
|---|---|---|---|
| None (%) | 2.4 | 12.7 | 0.0002 |
| Trivial (%) | 83.3 | 44.7 | |
| Mild (%) | 7.1 | 38.3 | |
| Moderate (%) | 4.8 | 2.1 | |
| Severe (%) | 2.4 | 2.1 | |
| Mean time between operation and echo follow up (years) | 9.7 | 4.2 | <0.0001 |
Chi-squared/Fisher exact test for categorical, and T-test for continuous variables.
Figure 3.
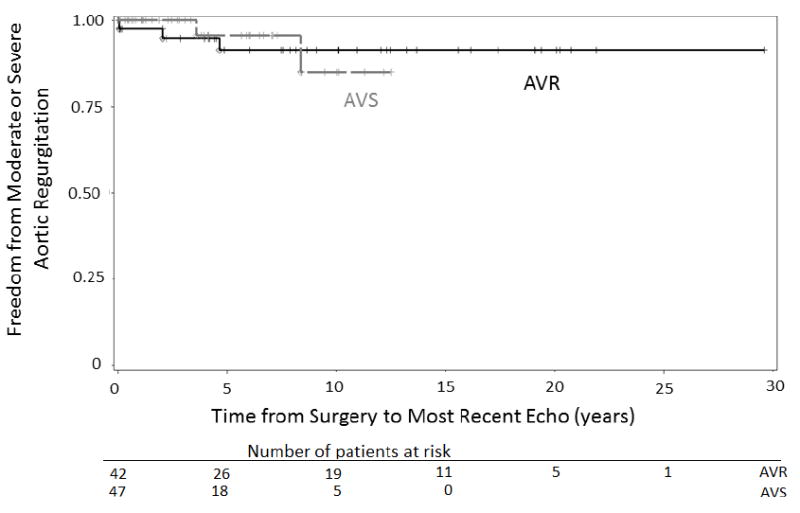
Kaplan-Meier curve showing freedom from moderate or severe aortic regurgitation following AVR or AVS root replacement. AVR, aortic valve replacing; AVS, aortic valve sparing; Echo, echocardiogram.
Comments
This study was undertaken to assess the current utilization and outcomes of AVS root replacement procedures among patients with MFS enrolled in the GenTAC Registry. The durability of AVS surgery in MFS patients has been called into question by the spectrum of outcomes that have been reported by centers performing the procedure. David reported no reoperations for aortic regurgitation among 77 MFS patients who had undergone AVS surgery with the reimplantation technique (22). Other experienced centers, however, have found that MFS is an independent predictor of late failure after AVS surgery and have reported reoperation rates as high as 18.5% at a mean time of 1.7 years (9,12).
Despite this uncertainty, we found that AVS surgery has become increasingly common in the GenTAC MFS population and over the past 5 years has become more prevalent than AVR surgery. Patients having elective surgery with smaller aneurysm size were found to be more likely to have AVS surgery. The presence of aortic regurgitation at the time of surgery does not preclude AVS surgery in this population. The outcomes of AVS surgery at mid-term follow up are encouraging. No aortic root reoperations have been reported in the GenTAC Registry following AVS surgery. The limited imaging data available shows that at mid-term follow up, the great majority of AVS patients in the Registry have mild or less aortic regurgitation.
This study has limitations related to the GenTAC Registry design. Historical clinical data abstracted from medical records may be less accurate than prospectively collected data. Patients were enrolled through 1 of 6 regional clinical centers participating in GenTAC; the treatments and outcomes described in this report may have been biased by the clinical practices and guidelines used at the enrolling centers. All GenTAC registrants are alive at the time of enrollment, and therefore a survivor bias is inherent in both the AVS and AVR groups analyzed here. The GenTAC sites are high volume surgery centers and outcomes from this study may not be generalizable to all medical centers. The imaging follow up was incomplete. We do not have follow up data related to several important forms of valve related morbidity, such as bleeding and thromboembolic complications. The second phase of GenTAC has been initiated which will address the weaknesses in the clinical and imaging follow up of patients in this study in the future (23).
Despite these limitations, the results of the study demonstrate that the real world experience of AVS root replacement among GenTAC clinical centers has been excellent to date. The results of this study are encouraging and justify the current approach of performing AVS root replacement surgery in select MFS patients, particularly in elective settings for patients with moderate-sized aneurysms. This approach offers the possibility that MFS patients, who are typically young adults at the time of surgery, may be able to benefit from prophylactic aortic root replacement without being exposed to the potential complications related to mechanical prosthetic valve replacement, including permanent warfarin anticoagulation. Phase two of the GenTAC Registry will continue to evaluate the outcomes of AVS surgery in MFS patients at longer durations of follow up.
Acknowledgments
The GenTAC Registry has been supported in full with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract Numbers HHSN268200648199C and HHSN268201000048C. Additional support was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Oregon Clinical and Translational Research Institute (Portland, OR), grant UL1 RR024140 from the National Center for Research Resources (Bethesda, MD), and by the Weill Cornell Medical College Clinical Translational Science Center (New York, NY), grant UL1RR024996.
Footnotes
Stephen N. Palmer, PhD, ELS, contributed to the editing of this manuscript.
This manuscript was presented at the 49th Annual Meeting of the Society of Thoracic Surgeons in Los Angeles, CA on January 28, 2013.
References
- 1.Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–9. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 2.Maslen CL, Corson GM, Maddox BK, Glanville RW, Sakai LY. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991;352:334–7. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- 3.Keane MG, Pyeritz RE. Medical management of Marfan syndrome. Circulation. 2008;117:2802–13. doi: 10.1161/CIRCULATIONAHA.107.693523. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez F, Dietz HC. Marfan syndrome: from molecular pathogenesis to clinical treatment. Current Opinion in Genetics & Development. 2007;17:252–8. doi: 10.1016/j.gde.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Saunders KB, Bentall HH. Aneurysm of the aortic root with gross aortic incompetence: successful surgical correction. Proceedings of the Royal Society of Medicine. 1967;60:726–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. American Journal of Cardiology. 1995;75:157–60. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 7.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. Journal of Thoracic & Cardiovascular Surgery. 1992;103:617–21. discussion 622. [PubMed] [Google Scholar]
- 8.Gott VL, Cameron DE, Alejo DE, et al. Aortic root replacement in 271 Marfan patients: a 24-year experience. Annals of Thoracic Surgery. 2002;73:438–43. doi: 10.1016/s0003-4975(01)03336-7. [DOI] [PubMed] [Google Scholar]
- 9.Karck M, Kallenbach K, Hagl C, Rhein C, Leyh R, Haverich A. Aortic root surgery in Marfan syndrome: Comparison of aortic valve-sparing reimplantation versus composite grafting. Journal of Thoracic & Cardiovascular Surgery. 2004;127:391–8. doi: 10.1016/j.jtcvs.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 10.LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Annals of Thoracic Surgery. 2006;81:2063–78. doi: 10.1016/j.athoracsur.2006.01.070. discussion 2078. [DOI] [PubMed] [Google Scholar]
- 11.Miller DC. Valve-sparing aortic root replacement in patients with the Marfan syndrome. Journal of Thoracic & Cardiovascular Surgery. 2003;125:773–8. doi: 10.1067/mtc.2003.162. [DOI] [PubMed] [Google Scholar]
- 12.Zehr KJ, Orszulak TA, Mullany CJ, et al. Surgery for aneurysms of the aortic root: a 30-year experience. Circulation. 2004;110:1364–71. doi: 10.1161/01.CIR.0000141593.05085.87. [DOI] [PubMed] [Google Scholar]
- 13.Fagan A, Pillai R, Radley Smith R, Yacoub MH. Results of new valve conserving operation for treatment of aneurysms or acute dissection of the aortic root. British Heart Journal. 1983;49:302. [Google Scholar]
- 14.Cameron DE, Vricella LA. Valve-sparing aortic root replacement in Marfan syndrome. Seminars in Thoracic & Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 2005:103–11. doi: 10.1053/j.pcsu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Miller DC. Valve-sparing aortic root replacement: current state of the art and where are we headed? Annals of Thoracic Surgery. 2007;83:S736–9. doi: 10.1016/j.athoracsur.2006.10.101. discussion S785-90. [DOI] [PubMed] [Google Scholar]
- 16.Volguina IV, Miller DC, LeMaire SA, et al. Valve-sparing and valve-replacing techniques for aortic root replacement in patients with Marfan syndrome: Analysis of early outcome. Journal of Thoracic & Cardiovascular Surgery. 2009;137:1124–32. doi: 10.1016/j.jtcvs.2009.03.023. [Republished from J Thorac Cardiovasc Surg. 2009 Mar;137(3):641-9] [DOI] [PubMed] [Google Scholar]
- 17.Eagle KA, Gen TACC. Rationale and design of the National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) American Heart Journal. 2009;157:319–26. doi: 10.1016/j.ahj.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song HK, Bavaria JE, Kindem MW, et al. Surgical treatment of patients enrolled in the national registry of genetically triggered thoracic aortic conditions. Annals of Thoracic Surgery. 2009;88:781–7. doi: 10.1016/j.athoracsur.2009.04.034. discussion 787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. Journal of Medical Genetics. 2010;47:476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 20.Karnofsky DA, Borchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: CM M, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 21.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Annals of Thoracic Surgery. 2002;74:S1877–80. doi: 10.1016/s0003-4975(02)04147-4. discussion S1892-8. [DOI] [PubMed] [Google Scholar]
- 22.David TE, Armstrong S, Maganti M, Colman J, Bradley TJ. Long-term results of aortic valve-sparing operations in patients with Marfan syndrome. Journal of Thoracic & Cardiovascular Surgery. 2009;138:859–64. doi: 10.1016/j.jtcvs.2009.06.014. discussion 863-4. [DOI] [PubMed] [Google Scholar]
- 23.Kroner BL, Tolunay HE, Basson CT, et al. The National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC): results from phase I and scientific opportunities in phase II. American Heart Journal. 2011;162:627–632.e1. doi: 10.1016/j.ahj.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


