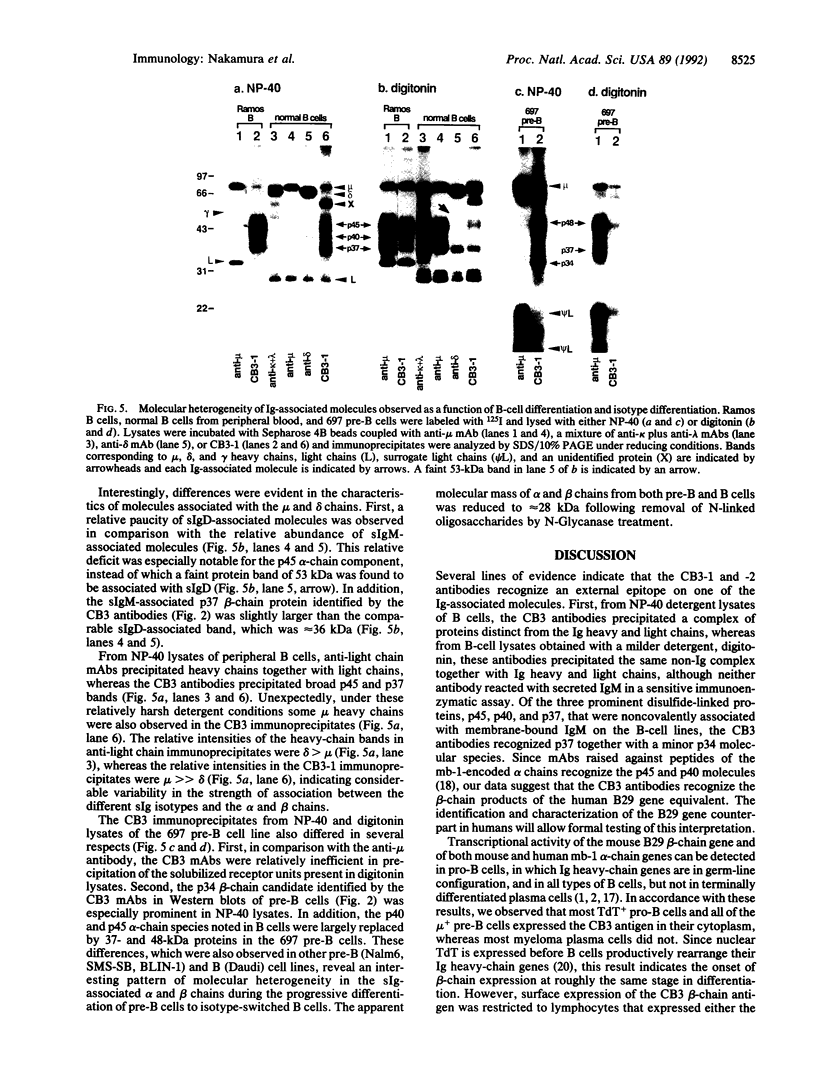
Abstract
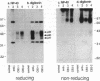
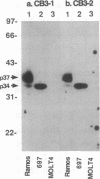
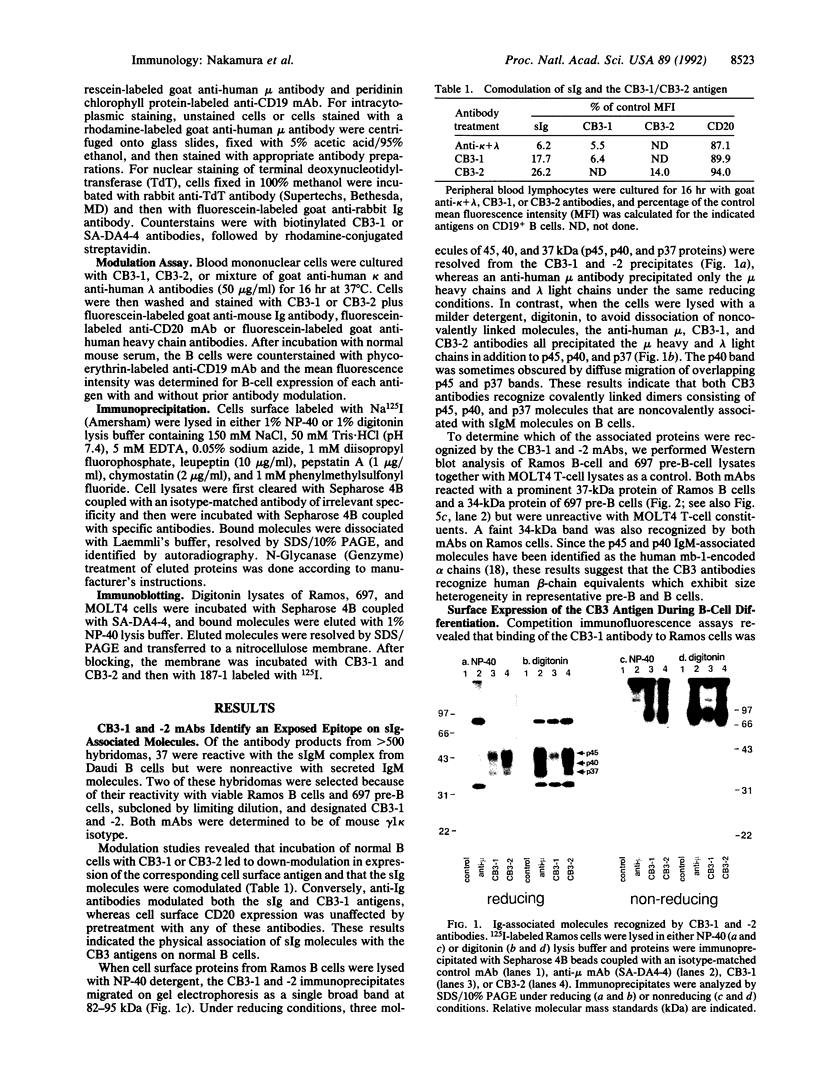
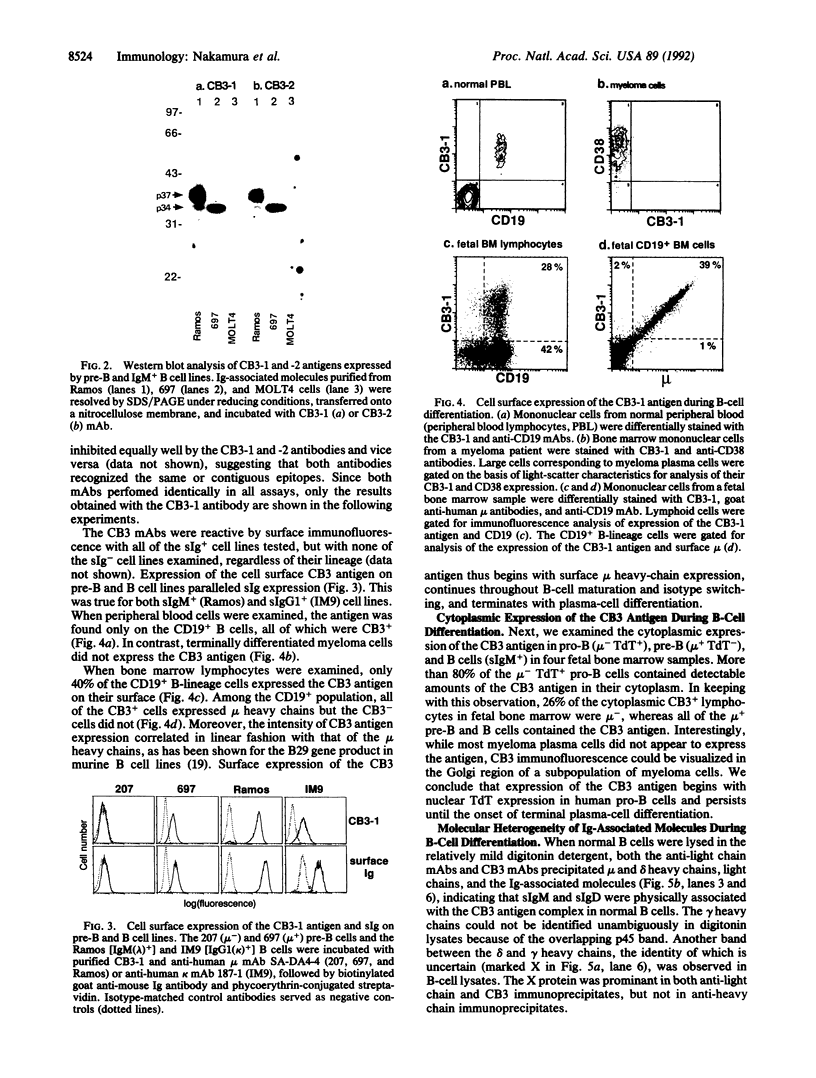
Two covalently linked transmembrane molecules, encoded in mice by the mb-1 and B29 genes, have been defined as integral components of the antibody receptor units expressed on B cells. We have produced monoclonal antibodies against an exposed extracellular epitope on the putative human equivalent of the mouse B29 product. These antibodies, CB3-1 and -2, were used to show that cytoplasmic expression of this molecule begins in human pro-B cells (terminal deoxynucleotidyltransferase-positive, mu chain-negative), whereas surface expression coincides strictly with surface immunoglobulin expression of all isotypes. Immunochemical analysis of the human immunoglobulin-associated molecules revealed greater molecular heterogeneity than has been noted for the murine analogues. This molecular heterogeneity of immunoglobulin-associated molecules varied as a function of differentiation stage and the immunoglobulin isotypes expressed by B-lineage cells. Our data support the hypothesis that biochemical heterogeneity of the surface immunoglobulin-associated molecules may contribute to the variability in biological effects of antigen receptor crosslinkage on B cells of different maturational stages. Because the CB3 antibodies are capable of down-modulating the antigen receptors on all B cells, they may prove therapeutically useful as universal B-cell suppressants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anasetti C., Tan P., Hansen J. A., Martin P. J. Induction of specific nonresponsiveness in unprimed human T cells by anti-CD3 antibody and alloantigen. J Exp Med. 1990 Dec 1;172(6):1691–1700. doi: 10.1084/jem.172.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith M., Urba W. J., Ferris D. K., Freter C. E., Kuhns D. B., Moratz C. M., Longo D. L. Anti-IgM-mediated growth inhibition of a human B lymphoma cell line is independent of phosphatidylinositol turnover and protein kinase C activation and involves tyrosine phosphorylation. J Immunol. 1991 Oct 1;147(7):2411–2418. [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Cambier J. C. Alpha-chains of IgM and IgD antigen receptor complexes are differentially N-glycosylated MB-1-related molecules. J Immunol. 1991 Sep 1;147(5):1575–1580. [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Z., Stall A. M., Herzenberg L. A., Herzenberg L. A. Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J. 1990 Jul;9(7):2117–2124. doi: 10.1002/j.1460-2075.1990.tb07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Kearney J. F., Gathings W. E., Lawton A. R. Effects of anti-Ig antibodies on the development and differentiation of B cells. Immunol Rev. 1980;52:29–53. doi: 10.1111/j.1600-065x.1980.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Matsuuchi L., Kelly R. B., DeFranco A. L. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H. J., Kubagawa H., Burrows P. D. Molecular cloning and expression pattern of a human gene homologous to the murine mb-1 gene. J Immunol. 1992 Mar 1;148(5):1526–1531. [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K., Wood W. J., Jr, Damore M., Hermanson G. G., Wall R., Kincade P. W. B29 gene products complex with immunoglobulins on B lymphocytes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):633–637. doi: 10.1073/pnas.89.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. M., Ishigami T., Hata D., Higaki Y., Morita M., Yamaoka K., Mayumi M., Mikawa H. Anti-IgM but not anti-IgD antibodies inhibit cell division of normal human mature B cells. J Immunol. 1992 Jan 1;148(1):29–34. [PubMed] [Google Scholar]
- Kiyotaki M., Cooper M. D., Bertoli L. F., Kearney J. F., Kubagawa H. Monoclonal anti-Id antibodies react with varying proportions of human B lineage cells. J Immunol. 1987 Jun 15;138(12):4150–4158. [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Cordell J. L., Tse A. G., van Dongen J. J., van Noesel C. J., Micklem K., Pulford K. A., Valensi F., Comans-Bitter W. M., Borst J. The IgM-associated protein mb-1 as a marker of normal and neoplastic B cells. J Immunol. 1991 Dec 1;147(11):2474–2482. [PubMed] [Google Scholar]
- Matsuo T., Kimoto M., Sakaguchi N. Direct identification of the putative surface IgM receptor-associated molecule encoded by murine B cell-specific mb-1 gene. J Immunol. 1991 Mar 1;146(5):1584–1590. [PubMed] [Google Scholar]
- Miyawaki T., Kubagawa H., Butler J. L., Cooper M. D. Ig isotypes produced by EBV-transformed B cells as a function of age and tissue distribution. J Immunol. 1988 Jun 1;140(11):3887–3892. [PubMed] [Google Scholar]
- Nomura J., Matsuo T., Kubota E., Kimoto M., Sakaguchi N. Signal transmission through the B cell-specific MB-1 molecule at the pre-B cell stage. Int Immunol. 1991 Feb;3(2):117–126. doi: 10.1093/intimm/3.2.117. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M. Three B-cell surface molecules associating with membrane immunoglobulin. Immunology. 1990 Feb;69(2):298–302. [PMC free article] [PubMed] [Google Scholar]
- Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- Wienands J., Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992 Mar 19;356(6366):246–248. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- Wienands J., Reth M. The B cell antigen receptor of class IgD can be expressed on the cell surface in two different forms. Eur J Immunol. 1991 Oct;21(10):2373–2378. doi: 10.1002/eji.1830211012. [DOI] [PubMed] [Google Scholar]
- Yellen A. J., Glenn W., Sukhatme V. P., Cao X. M., Monroe J. G. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes. Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J Immunol. 1991 Mar 1;146(5):1446–1454. [PubMed] [Google Scholar]