Abstract
Neurofibromatosis type 1 (NF1) is a hereditary tumor syndrome caused by mutations of the NF1 gene and resulting dysregulation of the Ras-pathway. In addition to peripheral nerve tumors, affected tissues include the musculoskeletal and cardiovascular system. The immune system has recently been suggested as a possible modulator NF1-related phenotypes. Therefore, we determined the immune phenotype in NF1 patients and investigated its relationship with the phenotypic severity of NF1-related tumor manifestations. We quantified global leukocytes and lymphocyte subpopulations of peripheral blood from 37 NF1 patients and 21 healthy controls by flow cytometry. To associate immune phenotype with tumor phenotype, all NF1 patients underwent whole-body magnetic resonance imaging and total internal tumor volume was calculated. The immunophenotypes were compared among four NF1 groups with different total internal tumor burdens and between NF1 patients and non-NF1 subjects. We found that NF1 patients show a generalized lymphopenia. Closer analysis revealed that the CD8+/CD27− and CD8+/CD57+ effector T cell fractions strongly increase in NF1 patients with low tumor load and decrease to levels below control in patients with high tumor load. Moreover, increased production of IL2, IFN-γ and TNF-α was found in T cells of NF1 patients upon phorbol-12-myristate acetate (PMA) stimulation compared to healthy controls. The data indicate that decreasing CD8+/CD57+ and CD27− T cell fractions correspond to increasing tumor load in NF1 patients, potentially making these populations useful marker for internal tumor burden.
Keywords: Neurofibromatosis type 1, Immune phenotype, T cell, Plexiform neurofibroma, Whole-body MRI
Introduction
Neurofibromatosis type 1 (NF1) is a rare autosomal dominant tumor syndrome, with an estimated incidence at birth of 1 in 3000 [1] and complete penetrance. NF1 is caused by heterozygous mutations in the NF1 gene, which codes for the tumor suppressor protein neurofibromin acting as a Ras-negative regulator via its Ras-guanosine triphosphate (GTP) activating domain (GAP)-domain. Mutations of NF1 lead to increased Ras-activity in affected cells [2, 3]. NF1 patients are prone to develop multiple phenotypic manifestations involving the skin, peripheral and central nervous system, musculoskeletal system and cardiovascular system. Multiple neurofibromas are the hallmark of the disease and affect almost all patients. Although benign, neurofibromas can cause severe secondary symptoms such as disfigurement and pain. Musculoskeletal phenotypes of NF1 include scoliosis, long-bone pseudarthrosis and generalized osteoporosis; cardiovascular symptoms may result from involvement of the heart itself or of cerebral, coronary, renal or other arteries. In addition, cognitive impairment and other neuropsychological problems are common [4]. Expressivity of the NF1 phenotype is highly variable and risk assessment problematic due to lack of predictive markers.
There is increasing evidence from animal models and clinical observations, supporting an association of immune phenotypes with several NF1 phenotypes. Abundant mast cells were recognized as a frequent histopathological feature of benign neurofibromas more than 35 years ago [5], an observation that has been repeatedly confirmed [6–8]. Although suggested that these inflammatory cells may be involved in the pathogenesis of neurofibromas in patients with NF1 [9], it was the observation that myeloid malignancies, which occur much more often than expected in children with NF1, frequently exhibit loss of activity of both NF1 alleles in people with NF1 [10, 11] that delivered evidence for the functional importance of neurofibromin in myeloid cells. The subsequent demonstration that development of neurofibromas in mouse models required Nf1 haploinsufficiency in myeloid cells of the tumor microenvironment [12–15] established the central role of these bone marrow-derived cells in the pathogenesis of NF1.
Likewise, the hematopoietic microenvironment has been shown to be involved in development of both NF1 vascular disease and NF1 bone disease. For example, NF1-associated vascular disease was associated with a striking increase in vascular inflammation and vascular monocytes in humans [16]. Experiments in a murine model have demonstrated that vascular neointima formation is enhanced by myeloid cells lacking Nf1 [17]. Similarly, deficient fracture healing and other skeletal manifestations of NF1 have been shown to require the presence of Nf1 haploinsufficiency in bone marrow mononuclear cells in a mouse model [18]. In addition, an increase in brain microglia in NF1 patients has been reported [19]. The available data substantiate increased circulating monocyte numbers and higher migratory and proliferative capacities of macrophages in NF1 patients and in NF1-deficient mice [8, 12–17]. It was furthermore demonstrated that NF1-deficient mice display aberrations in T cell development and function, with reduced proliferative responsiveness to interleukin2 and modulated numbers of naïve T cells [20, 21]. Of note, HLA-DR was found upregulated in NF1 −/− Schwann cells, while it is almost undetectable in normal Schwann cells [22]. Moreover, aberrant plasma cytokine levels in NF1 patients were found in several studies, indicating generalized immunological imbalances with a tendency to develop a systemic inflammatory environment [23–26].
In summary, there is growing evidence for an altered hematopoietic phenotype due to NF1 haploinsufficiency, which may play a role in the modulation of one or several of the multiple NF1 phenotypes such as vascular disease, idiopathic hypertension, fracture healing and other skeletal manifestations as well as tumorigenesis. However, association of immunological and clinical phenotypes has not yet been systematically assessed with quantifiable clinical data in a larger number of NF1 patients. In particular, we were interested in assessing the lymphocyte fraction of the adaptive hematopoietic cell repertoire and its association with the highly variable tumor phenotype in NF1 patients. Therefore, we profiled the cellular immune phenotype of NF1 patients and correlated it with the quantified internal tumor volume of plexiform neurofibromas (PNF) obtained by magnetic resonance imaging (MRI) of the whole body.
Materials and methods
Patients and samples
Adults and children with NF1 were recruited after informed consent at the University Hospital Hamburg-Eppendorf. Blood samples from adult healthy subjects were obtained at Charité University Medicine Berlin after informed consent. Altogether, 37 adult NF1 patients, 9 NF1 patients under 18 years and 21 healthy subjects were included. All NF1 patients were clinically diagnosed according to published guidelines and criteria [27]. Patients with microdeletions involving the NF1 gene, astrocytoma, optical pathway glioma, spinal tumors and schwannoma were excluded from the study. The study was approved by the internal review board (Ethics Committee of the Ärztekammer Hamburg number OB-089/04).
For flow cytometry, the following groups were used: healthy control subjects (n = 21, age range 18–60 years, mean 36 years, 11 male, 10 female) and NF1 patients (n = 37, only subjects over 20 years of age were included, range 20–66 years, mean 36 years, 18 male, 19 female). Correlation with tumor load was according to the following groups: no internal PNF tumors (n = 6), low tumor load (tumor volume <99 cm3; n = 11), medium tumor load (tumor volume 100–500 cm3; n = 9), high tumor load (tumor volume >500 cm3; n = 11).
Blood differential test
May–Grunwald–Giemsa-stained-whole-blood smears were microscopically examined independently by two persons using standard routine protocols in a double-blinded fashion. Standard reference ranges of leukocyte populations were used for comparison [28].
Flow cytometry
FACS of PBMC was used to analyze the ratio of different lymphocyte T cell populations. PBMC were prepared from 15 ml to 20 ml venous blood samples collected in sodium citrate as anticoagulant by density gradient in Leucosep™ tubes and Ficoll-Paque Plus solution (Amersham Biosciences) according to manufacturer’s instructions. PBMC were washed with sterile PBS (Biochrom), re-suspended, counted and cryopreserved with 10 % DMSO in FBS (5x10E6 cells/vial) and stored in liquid nitrogen. Flow cytometric analyses were conducted with FITC-, PE-, PE-cyanin 7 (PE-Cy7)-, PerCP-, electron coupled dye (ECD)-, Alexa Fluor® 647-, Alexa Fluor 700-, Horizon™ V450-, Horizon™ V500-, allophycocyanin (APC)-, APC cyanin 7 (APC-Cy7)-, Qdot® 655 nanocrystal- and allophycocyanin-conjugated antibodies. The following antibodies were used: Anti-human CD8 (Life Technologies), CD336 (Becton–Dickinson), CD314 (Beckman Coulter), CD3 (Life Technologies), CD335 (R&D Systems), CD62L (Beckman Coulter), CD56 (Becton–Dickinson), CD4 (Becton–Dickinson), CD16 (Becton–Dickinson), CD25 (Becton–Dickinson), CD127(eBioscience), HLA-DR (Becton–Dickinson), CD57 (Miltenyi Biotec), CD45RO (Becton–Dickinson), CD27 (Life Technologies). Live/dead-discrimination staining dye (Life Technologies) was added to exclude dead cells. After thawing, cell number and viability was microscopically assessed with trypan blue (Sigma) before surface staining. Cells were incubated for 30 min at 2–8 °C and washed afterward. Cells were measured with a LSR-II flow cytometer (Becton–Dickinson), and FACS data were analyzed using FlowJo software (Tree Star Inc.). The following panels were used: T cells (TH/Tcyt) CD3+ CD4/CD8; naïve T cells CD4+/CD45RO−; effector/central memory T cells CD45RO/CD62L; naturally occurring regulatory T cells CD25+/CD127lo; late activation marker memory T cells CD4/CD8/CD57+; late differentiation T cells CD27−; early activation marker expressing T cells HLA-DR; natural killer (NK) cells CD3/CD56; cytotoxic NK cells CD16+/CD56dim; regulatory NK cells CD16+CD56bright.
Tumor quantification
Whole-body MRI was performed in NF1 patients to quantify total internal tumor volume. MRI and calculation of total tumor volume were performed in analogy to previous studies [29, 30]. All patients underwent an identical scan protocol with the same parameters for slice thickness, gap, orientation, field of view, imaging matrix, image resolution, echo time, repetition time and inversion time (Siemens Avanto 1.5T). The subjects were imaged in a supine position from head to knee in four steps (head, thorax, abdomen and legs) in accordance with the maximum range of table movement. Slice thickness is 5–10 mm without skips between slices. MRI analysis was performed by an experienced radiologist and a physician trained in image analysis of NF1 associated tumors. The analysis was carried out in a blinded manner. Tumor segmentation and volumetry were performed semiautomatically with the heuristics-based software MEDx (V 3.44) using fat-suppressed T2-short-Ti inversion-recovery (STIR) sequences. Differences in signal intensity of neurofibroma tissue and surrounding tissue were used to define tumor margins on axial slices. The method used for this automated volumetric analysis is sensitive (it detects volume changes as small as 10 %), reproducible (coefficient of variation 0.6–5.6 %) and produces results similar to manual tumor tracings (R = 0.999).
Whole-body cutaneous and subcutaneous neurofibroma burden were independently examined by two observers and averages used. Tumor number was estimated within set ranges of (0, 1–10, 11–50, 51–100, 101–500, 500+, 1000+).
Intracellular cytokine staining
Cytokine production was measured by CD4 and CD8 cells from age- and gender-matched NF1 patients and healthy controls after stimulation. PBMC were stimulated for 4 h with PMA/ionomycin (Sigma), and cytokine secretion was blocked with brefeldin A (BD Biosciences). Cell fixation and permeabilization were carried out with the BD Fixation/Permeabilization Solution kit. Antibodies directed against IL-2, TNF-α and IFN-γ (BD Biosciences) were used to label intracellular cytokines for FACS analysis with a LSR-II flow cytometer (Becton–Dickinson), and data were analyzed using FlowJo software (Tree Star Inc.).
Statistical analysis
To verify all data for normal distribution, the Kolmogorov–Smirnov test was performed. Stratified patient groups were compared using the Mann–Whitney U test for continuous nonparametric variables. For significance testing, the nonparametric Mann–Whitney U test followed by Bonferroni correction was performed. Two-site tests were used for all analyses. p values <0.05 were considered significant. Statistical analysis was performed using SPSS version 18 software (SPSS, Inc., IL, USA) and GraphPad Prism software 5.0 (GraphPad Software Inc., CA, USA).
Results and discussion
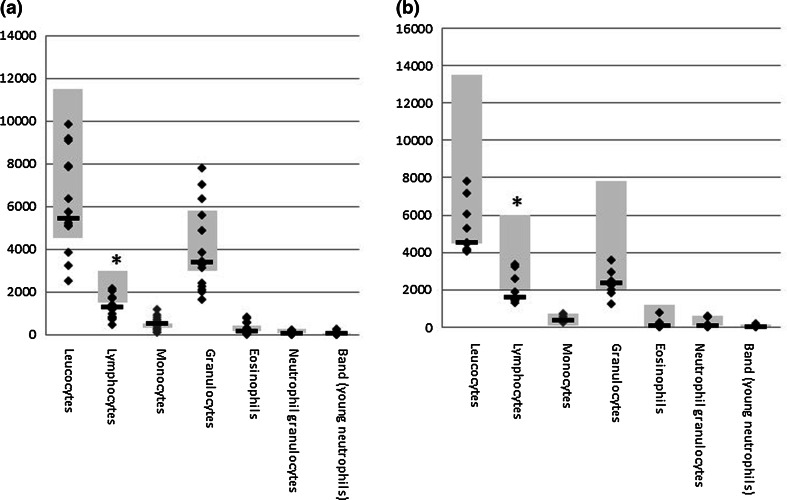
Global blood cell analysis showed a generalized low count of leukocytes in NF1 patients, independent of age. Granulocytes and monocytes are at the lower end of the normal range, while lymphocytes are below the standard reference range (Fig. 1). Our further analysis focused therefore on analyzing T cell subpopulations by flow cytometry of PBMC from NF1 patients over 18 years (n = 37) and healthy age-matched controls (n = 21) (Table 1). As we aimed at identifying correlations between the immune phenotype and the cancer phenotype, patients were analyzed by whole-body MRI to quantify their total internal tumor load. Children and NF1 patients below 20 years of age were excluded since the tumor phenotype is often not fully established at younger ages. No significant differences were observed between the overall cohort of NF1 patients and the non-NF1 controls for any of the T cell subpopulations (Fig. 2). However, we identified a trend for higher levels of regulatory T cells (Fig. 2a), fewer CD8+/HLA-DR+ T cells (Fig. 2b) as well as fewer CD4+CD27− effector T cells (Fig. 2e) in the general NF1 population. Although these trends were not significant (p = 0.06), the analysis clearly revealed groups of patients with very few memory and effector T cells (Fig. 2d, e).
Fig. 1.
Blood differential test of NF1 patients. Reference ranges of leukocyte populations in samples of healthy subjects are indicated by gray boxes. a Adults with NF1 (n = 15, age range 18–68, median 39 years), b NF1 patients under 18 years (n = 9, age range 4–17, median 13 years). The lymphocyte levels are below the normal range for both groups (p < 0.05)
Table 1.
Phenotype of NF1 patients with quantified dermal and internal tumors
| Number of dermal neurofibroma | Internal tumor (PNF) (volume in cm3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient no | Sex | Age | Subcutaneous | Cutaneous | None* | Low 1–99 cm3 | Medium 100–500 cm3 | High >500 cm3 | MPNST |
| 1 | M | 22 | 1 | 11–50 | x | ||||
| 2 | F | 40 | 3 | 1–10 | x | ||||
| 3 | M | 25 | 9 | 0 | x | ||||
| 4 | F | 38 | 0 | 101–500 | x | ||||
| 5 | F | 48 | 11–50 | 1000+ | x | ||||
| 6 | F | 26 | 0 | 11–50 | x | ||||
| 7 | F | 45 | 51–100 | 101–500 | 81 | ||||
| 8 | F | 66 | 15 | 11–50 | 22 | ||||
| 9 | M | 48 | 0 | 11–50 | 39 | ||||
| 10 | M | 44 | 0 | 1000+ | 28 | ||||
| 11 | F | 55 | 11–50 | 101–500 | 44 | ||||
| 12 | M | 41 | 0 | 11–50 | 16 | ||||
| 13 | M | 42 | 0 | 11–50 | 22 | ||||
| 14 | M | 45 | 0 | 51–100 | 74 | ||||
| 15 | M | 21 | 0 | 11–50 | 38 | ||||
| 16 | M | 27 | 11–50 | 11–50 | 61 | ||||
| 17 | F | 42 | 0 | 1000+ | 76 | ||||
| 18 | F | 53 | 0 | 1000+ | 341 | ||||
| 19 | M | 23 | 2 | 500+ | 119 | ||||
| 20 | M | 25 | 0 | 11–50 | 476 | ||||
| 21 | F | 36 | 11–50 | 51–100 | 326 | x | |||
| 22 | F | 49 | 11–50 | 51–100 | 382 | ||||
| 23 | F | 26 | 0 | 11–50 | 491 | ||||
| 24 | M | 31 | 0 | 11–50 | 212 | ||||
| 25 | M | 47 | 11–50 | 1000+ | 115 | ||||
| 26 | F | 35 | 8 | 11–50 | 112 | ||||
| 27 | M | 26 | 1000+ | 1000+ | 739 | x | |||
| 28 | F | 54 | 0 | 1000+ | 900 | ||||
| 29 | F | 24 | 0 | 0 | 810 | ||||
| 30 | M | 31 | 10–500 | 7 | 5408 | ||||
| 31 | F | 20 | 1000+ | 11–50 | 950 | ||||
| 32 | F | 50 | 51–100 | 1000+ | 1191 | ||||
| 33 | F | 34 | 0 | 101–500 | 508 | ||||
| 34 | M | 41 | 9 | 1000+ | 577 | ||||
| 35 | M | 43 | 0 | 8 | 661 | ||||
| 36 | F | 62 | 11–50 | 51–100 | 1005 | ||||
| 37 | M | 49 | 0 | 51–100 | 767 | ||||
* Volume below detection level (1 cm3)
Fig. 2.
Flow cytometric analysis of the ratio between CD4 and CD8 cells, and the percentages of different T cell populations in PBMC samples of healthy age- and gender-matched controls. a CD4/CD8 ratio and regulatory T cells as defined by CD4+CD25highCD127low phenotype; b frequencies of activated HLA-DR+ CD4+ and CD8+ cells; c percentages of CD45RO+ memory CD4+ and CD8+ T cells; d percentages of chronically activated CD57+ CD4+ and CD8+ T cells and e percentages of CD27− effector CD4+ and CD8+ T cells
Next, we divided the NF1 patients (n = 37) into four different groups according to the total internal PNF tumor volume as determined by whole-body MRI: The groups show no internal tumors (n = 5), low tumor volume (n = 11), medium tumor volume (n = 9) and high tumor load (n = 11) and associated the frequency of the T cell subpopulations with these groups (Fig. 2). We found no significant differences between NF1 patients and healthy controls or between NF1 patients with different tumor loads for any of the CD16/CD56 NK cell populations investigated (data not shown). In addition, no associations were found between regulatory T cells and the CD4+/HLA-DR+, CD45RO+, CD57+, and CD27− cell subpopulations, as well as the CD8+/HLA-DR+ and CD45RO+ T cell subpopulations (Fig. 2a–e). In contrast, the CD8+/CD57+ and CD8+/CD27− memory and effector T cell populations were significantly and inversely correlated with increased internal tumor burden of NF1 patients (Fig. 2d, e). The levels dropped even below baseline in the NF1 group with the highest tumor load. The association only correlated with internal tumor burden and was independent of age (data not shown). No correlation was found between lymphocyte subpopulations and subcutaneous or cutaneous neurofibromas (data not shown). Although a correlation between the growth rate of plexiform and subcutaneous, but not cutaneous tumors has been described (27), the data may indicate a different role of lymphocytes in internal, subcutaneous and cutaneous tumors as compared to PNFs. Other NF1-related phenotypes were not quantified in the observed healthy and NF1 cohorts, and hence, it was not possible to assess associations with vascular, skeletal or cognitive disease manifestations.
The results indicate that although not significantly different, there is, similar to the significantly different D57+ cells, a tendency to also lower frequency of CD8+/HLA-DR+-activated T cells with increased tumor burden (Fig. 2b). However, for CD8+/HLA-DR+-activated T- cells, we did not observe an increase from samples of patients having no or low tumor volume as observed for CD57+ and CD27− cells (Fig. 2b, d, e). This might be due to different expression kinetics of the respective maturity stages. Interestingly, we did not observe differences in CD45RO+ memory T cell frequencies in relation to tumor burden (Fig. 2c). Thus, we only observe significant differences in frequencies of more terminally differentiated (CD57+/CD27−) T cells, which show the highest functional capacity, indicating an increasing deficit in T cell differentiation with tumor progression.
It is interesting that CD8+CD27− T cells in NF1 patients without tumor are lower than in the low tumor group. However, there is a striking increase in frequencies of CD8+CD27− T cells from patients with no tumor to low tumor, whereas it declines with further tumor progression (Fig. 2d, e). We hypothesize that at the beginning of tumor formation T cell activation and full differentiation are still intact and that the frequencies increase in order to control the developing tumor. However, during further tumor development and at larger tumor volumes, full T cell differentiation is inhibited by so far undefined mechanisms requiring further investigations in a larger investigation.
CD57 expression is a characteristic of functional immune deficiency and replicative senescence [26]. We therefore analyzed the capacity of the CD4+ and CD8+/CD57+ T cells in NF1 patients to secrete inflammatory cytokines upon stimulation. Interestingly, IL-2 and IFN-γ production is significantly increased in CD8+/CD57+ T cells of NF1 patients when compared to healthy controls (p < 0.05) (Fig. 3). No difference was found for CD4+/CD57+ helper T cells. These data suggest that CD8+/CD57+ replicative exhausted memory T cells in NF1 patients react strongly to stimulation and produce pro-inflammatory cytokines at higher levels than control subjects. There was no correlation between tumor load and cytokine secretion (data not shown), which is in agreement with our previous finding of increased IFN-γ and TNF-α serum levels in NF1 patients independently of tumor load [23].
Fig. 3.
Intracellular cytokine staining after PMA stimulation by CD4 and CD8 cells from age- and gender-matched NF1 patients (n = 15) and healthy controls (n = 13). IL2, TNF-α and IFN-γ production was determined by FACS (*p < 0.05). a Comparison of CD4+ and CD8+ fractions; b comparison of CD8+/CD57− and CD8+/CD57+ fractions
NF1 patients with high internal tumor load are at high risk for increased morbidity and for development of malignant peripheral nerve sheath tumors (MPNST). The data suggest that the level of CD8+/CD57+ and CD27− T cells present a useful marker for the internal tumor burden and subsequently the risk to transformation into MPNST. Its clinical use as such a biomarker alone or together with other biomarkers such as insulin-like growth factor binding protein 1 (IGFBP1) [23, 24] will need validation in larger cohorts.
Our findings potentially also indicate that the T cell-mediated immune response in patients with low tumor load is potentially stronger than in patients with high tumor load. Although it would be necessary to substantiate this assumption in a larger cohort, it would suggest that patients with high tumor load only mount a weak cellular immune response, favoring the establishment of a tumor-promoting environment. The data do not distinguish between the alternative hypotheses that aberrant immune cell counts are the consequence rather than a cause of the variable systemic tumor phenotype in NF1 patients. For example, a large tumor burden might increasingly exhaust the existing effector CD57+ T cell reservoir. Interestingly, an increased number of CD8+CD57+ T cells in NF1 patients was also shown in another study [16], although our data confirm such an increase only for the NF1 subgroups with low or medium internal tumor load. It is of note that the CD8+/CD27− populations in the NF1 group without internal tumors were smaller than in any other subgroup, including the healthy control group. The aberrantly low levels of this T cell population were thus not dependent on tumors and caused by the tumor phenotype. Although the group size is too small for statistically useful evaluation, this trend may suggest a general difference in the T cell phenotype of NF1 patients.
In conclusion, our results show that the CD8 memory and effector T cell levels are inversely correlated with tumor burden in NF1 patients. Hence, the ratios of CD8+/CD57+ and CD27− T cells may present a useful surrogate marker for the internal tumor burden of NF1 patients, which justifies further validation in a larger cohort.
Acknowledgments
The study was supported by a Grant from the von Recklinghausen Gesellschaft e.V. and from the Deutsche Krebshilfe (Grants 109713 and 110588). We thank Petra Reinke for her continuing support and encouragement of this work.
Abbreviations
- APC
Allophycocyanin
- CD
Cluster of differentiation
- DMSO
Dimethylsulfoxide
- ECD
Electron coupled dye
- FACS
Fluorescence-activated cell sorting
- FBS
Fetal bovine serum
- FITC
Fluorescein isothiocyanate
- GAP
GTP activating domain
- GTP
Guanosine triphosphate
- HLA
Human histocompatibility leukocyte antigen
- HLA-DR
HLA antigen D related
- IFN
Interferon
- IL
Interleukin
- MRI
Magnetic resonance imaging
- NF1
Neurofibromatosis type 1
- NF1/Nf1
Neurofibromin gene (human/mouse)
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PE
Phycoerythrin
- PE-Cy
PE-cyanin
- PerCP
Peridinin chlorophyll protein
- PMA
Phorbol myristate acetate
- PNF
Plexiform neurofibroma
- STIR
Short-Ti inversion-recovery
- T cell
Thymocyte
- TNF
Tumor necrosis factor
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
S Farschtschi and SJ Park have contributed equally to this work.
References
- 1.Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet. 1989;26:712–721. doi: 10.1136/jmg.26.11.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman JM, Gutmann DH, MacCollin M, Riccardi VM. Neurofibromatosis. Phenotype, Natural History and Pathogenesis. Baltimore: The Johns Hopkins University Press; 1999. pp. 110–118. [Google Scholar]
- 3.Harrisingh MC, Lloyd AC. Ras/Raf/ERK signalling and NF1. Cell Cycle. 2004;3:1255–1258. doi: 10.4161/cc.3.10.1182. [DOI] [PubMed] [Google Scholar]
- 4.Larizza L, Gervasini C, Natacci F, Riva P. Developmental abnormalities and cancer predisposition in neurofibromatosis type 1. Curr Mol Med. 2009;9(5):634–653. doi: 10.2174/156652409788488801. [DOI] [PubMed] [Google Scholar]
- 5.Isaacson P. Mast cells in benign nerve sheath tumors. J Pathol. 1976;119(4):193–196. doi: 10.1002/path.1711190402. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, et al. Serum biomarker in neurofibromatosis type 1. J Dermatol Sci. 2012;67(2):155–158. doi: 10.1016/j.jdermsci.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Nürnberger M, Moll I. Semiquantitative aspects of mast cells in normal skin and in neurofibromas of neurofibromatosis types 1 and 5. Dermatology. 1994;188(4):296–299. doi: 10.1159/000247170. [DOI] [PubMed] [Google Scholar]
- 8.Tucker T, Riccardi VM, Sutcliffe M, Vielkind J, Wechsler J, Wolkenstein P, et al. Different patterns of mast cells distinguish diffuse from encapsulated neurofibromas in patients with neurofibromatosis 1. J Histochem Cytochem. 2011;59:584–590. doi: 10.1369/0022155411407340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riccardi VM. Cutaneous manifestation of neurofibromatosis: cellular interaction, pigmentation, and mast cells. Birth Defects Orig Artic Ser. 1981;17(2):129–145. [PubMed] [Google Scholar]
- 10.Shannon KM, O’Connell P, Martin GA, Paderanga D, Olson K, Dinndorf P, McCormick F. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med. 1994;330(9):597–601. doi: 10.1056/NEJM199403033300903. [DOI] [PubMed] [Google Scholar]
- 11.Miles DK, Freedman MH, Stephens K, Pallavicini M, Sievers EL, Weaver M, Grunberger T, Thompson P, Shannon KM. Patterns of hematopoietic lineage involvement in children with neurofibromatosis type 1 and malignant myeloid disorders. Blood. 1996;88(11):4314–4320. [PubMed] [Google Scholar]
- 12.Staser K, Yang FC, Clapp DW. Mast cells and the neurofibroma microenvironment. Blood. 2010;116(2):157–164. doi: 10.1182/blood-2009-09-242875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang FC, et al. Nf1-dependent tumors require a microenvironment containing Nf1± and c-kit-dependent bone marrow. Cell. 2008;135(3):437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, et al. Neurofibromas in NF1: schwann cell origin and role of tumor environment. Science. 2002;296(5569):920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staser K, Park SJ, Rhodes SD, Zeng Y, He YZ, Shew MA, et al. Normal hematopoiesis and neurofibromin-deficient myeloproliferative disease require Erk. J Clin Invest. 2013;123(1):329–334. doi: 10.1172/JCI66167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasater EA, Li F, Bessler WK, Estes ML, Vemula S, Hingtgen CM, et al. Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. J Clin Invest. 2010;120:859–870. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing B, Li F, et al. Heterozygous inactivation of the Nf1 gene in myeloid cells enhances neointima formation via a rosuvastatin-sensitive cellular pathway. Hum Mol Genet. 2013;22(5):977–988. doi: 10.1093/hmg/dds502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Chen S, He Y, Rhodes SD, Mohammad KS, Li X, Yang X, Jiang L, Nalepa G, Snider P, Robling AG, Clapp DW, Conway SJ, Guise TA, Yang FC. The haploinsufficient hematopoietic microenvironment is critical to the pathological fracture repair in murine models of neurofibromatosis type 1. PLoS One. 2011;6(9):e24917. doi: 10.1371/journal.pone.0024917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons GW, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram DA, Zhang L, McCarthy J, Wenning MJ, Fisher L, Yang FC, Clapp DW, Kapur R. Lymphoproliferative defects in mice lacking the expression of neurofibromin: functional and biochemical consequences of Nf1 deficiency in T-cell development and function. Blood. 2002;100(10):3656–3662. doi: 10.1182/blood-2002-03-0734. [DOI] [PubMed] [Google Scholar]
- 21.Oliver JA, Lapinski PE, Lubeck BA, Turner JS, Parada LF, Zhu Y, King PD. The Ras GTPase-activating protein neurofibromin 1 promotes the positive selection of thymocytes. Mol Immunol. 2013;55(3–4):292–302. doi: 10.1016/j.molimm.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuss DE, Mucha J, Holtkamp N, Müller U, Berlien HP, Mautner VF, et al. Functional MHC Class II Is upregulated in neurofibromin-deficient schwann cells. J Invest Dermatol. 2013;133(5):1372–1375. doi: 10.1038/jid.2012.488. [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Sawitzki B, Kluwe L, Mautner VF, Holtkamp N, Kurtz A. Serum biomarkers for neurofibromatosis type 1 and early detection of malignant peripheral nerve sheath tumors. BMC Med. 2013;11:109. doi: 10.1186/1741-7015-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mashour GA, Driever PH, Hartmann M, Drissel SN, Zhang T, Scharf B, et al. Circulating growth factor levels are associated with tumorigenesis in neurofibromatosis type 1. Clin Cancer Res. 2004;10:5677–5683. doi: 10.1158/1078-0432.CCR-03-0769. [DOI] [PubMed] [Google Scholar]
- 25.Lévy P, Bièche I, Leroy K, Parfait B, Wechsler J, Laurendeau I, et al. Molecular profiles of neurofibromatosis type 1-associated plexiform neurofibromas: identification of a gene expression signature of poor prognosis. Clin Cancer Res. 2004;10:3763–3771. doi: 10.1158/1078-0432.CCR-03-0712. [DOI] [PubMed] [Google Scholar]
- 26.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87(1):107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 27.Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol. 2013;115:939–955. doi: 10.1016/B978-0-444-52902-2.00053-9. [DOI] [PubMed] [Google Scholar]
- 28.Hematology: Clinical Principles and Applications. By Bernadette F. Rodak, George A. Fritsma, Elaine Keohane, ELSEVIER Saunters. 3251 Riverport Ln, St Louis, Missouri 63043, (2012) ISBN 978-1-4377-0692-5
- 29.Plotkin SR, Bredella MA, Cai W, Kassarjian A, Harris GJ, Esparza S, et al. Quantitative assessment of whole-body tumor burden in adult patients with neurofibromatosis. PLoS One. 2012;7(4):e35711. doi: 10.1371/journal.pone.0035711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mautner VF, Asuagbor FA, Dombi E, Fünsterer C, Kluwe L, Wenzel R, et al. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008;10(4):593–598. doi: 10.1215/15228517-2008-011. [DOI] [PMC free article] [PubMed] [Google Scholar]