ABSTRACT
Alzheimer’s disease (AD) is a progressive disorder leading to cognitive impairment and neuronal loss. Cerebral extracellular accumulation and deposition of amyloid ß plaques is a pathological hallmark of AD. Chondroitin sulfate (CS) is an extracellular component abundant in the brain. CS is a sulfated glycosaminoglycan covalently attached to a core protein, forming chondroitin sulfate proteoglycan. The structure of CS is heterogeneous with sulfation modification and elongation of the chain. The structural diversity of CS allows it to play various roles in the brain. Increasing evidence has shown that CS promotes aggregation of amyloid ß peptides into higher-order species such as insoluble amyloid ß fibrils. Difficulties in the structural analysis of brain CS, as well as its heterogeneity, limit the study of potential roles of CS in AD pathology. Here we established a microanalysis method with reversed-phase ion-pair high performance liquid chromatography and found that CS in the brains of Tg2576 AD model mice show a lower molecular size and an increased ratio of CS-B motif di-sulfated disaccharide. Our findings provide insight into the structural changes of cerebral CS upon Alzheimer’s pathogenesis.
Key Words: chondroitin sulfate, Alzheimer’s disease, neurodegeneration, HPLC
INTRODUCTION
Chondroitin sulfate (CS) is a linear polysaccharide found in the extracellular space. One or more CS chains are covalently attached to a core protein, comprising chondroitin sulfate proteoglycan.1) CS is a family of glycosaminoglycans consisting of repeating disaccharide units of glucuronic acid and N-acetylgalactosamine. Modification by sulfation and elongation of these disaccharides is governed by Golgi-resident enzymes, conferring the chains structural diversity.2) It is well known that these modifications are varied in physiological and pathological sets. CS is abundant in the brain as well as in skeletal tissues and plays an important role in neural development, plasticity, and regeneration after injury.3-6) These evidence have lead to investigation of the role of CS in neurodegenerative diseases.
Alzheimer’s disease (AD) is one of the most intensively studied neurological disorders.7) AD is a progressive neurodegenerative disease leading to cognitive impairment, neuronal loss, and brain atrophy. Cerebral extracellular deposition of amyloid plaques is a pathological hallmark of AD.7) The plaques are composed of the self-aggregated amyloid ß (Aß) peptide and other extracellular molecules including sulfated glycosaminoglycans.8) Amyloid ß (Aß) peptides form soluble Aß oligomers and larger insoluble Aß fibrils. Robust neurotoxic activity of Aß requires its aggregation in oligomer forms.7) A long-standing debate is whether the aggregative state of Aß in fibrils is toxic or protective. Studies have shown that Aß fibrillogenesis is a neural defense mechanism, acting by immobilizing and concealing neurotoxic soluble Aß.9) CS with sulfation modifications to the size of either disaccharide or polysaccharide promotes fibril formation of Aß peptides in vitro.10, 11) Additionally, CS attenuated Aß cytotoxicity in culture neurons.12, 13) Interestingly, a recent study revealed that low molecular weight CS possesses protective effects on Aß-induced damage both in vitro and in vivo.14) Although increasing evidence has indicated that CS plays a pivotal role in AD pathogenesis, structural features of CS in the AD brain are not yet fully understood. Here, we used a microanalysis method to examine brain CS in Tg2576 mice, an AD transgenic mouse model, and present evidence that changes in CS molecular size with a lower molecular weight and CS composition with an increased ratio of di-sulfated disaccharide units occur in Alzheimer’s pathogenesis.15)
MATERIALS AND METHODS
Materials
The following commercial materials were used: Chondroitinase ABC from Proteus vulgaris, hyaluronidase from Streptomyces hyalurolyticus, chondroitin sulfate-A (whale cartilage) and chondroitin sulfate-C (shark cartilage) were from Seikagaku Corporation (Tokyo, Japan); chondroitin disaccharide sodium salt 0S (ΔDi-0S, 2-acetamide-2-deoxy-3-O-(ß-D-gluco-4-enepyranosyluronic acid)-D-galactose), 6S (ΔDi-6S, 2-acetamide-2-deoxy-3-O-(ß-D-gluco-4-enepyranosyluronic acid)-6-O-sulfo-D-galactose), 4S (ΔDi-4S, 2-acetamide-2-deoxy-3-O-(ß-D-gluco-4-enepyranosyluronic acid)-4-O-sulfo-D-galactose), 2S (ΔDi-2S, 2-acetamide-2-deoxy-3-O-(2-O-sulfo-ß-D-gluco-4-enepyranosyluronic acid)-D-galactose), DiSB (ΔDi-diSB(2,4), 2-acetamide-2-deoxy-3-O-(2-O-sulfo-ß-D-gluco-4-enepyranosyluronic acid)-4-O-sulfo-D-galactose), DiSD (ΔDi-diSD(2,6), 2-acetamide-2-deoxy-3-O-(2-O-sulfo-ß-D-gluco-4-enepyranosyluronic acid)-6-O-sulfo-D-galactose), DiSE (ΔDi-diSE(4,6), 2-acetamide-2-deoxy-3-O-(ß-D-gluco-4-enepyranosyluronic acid)-4,6-bis-O-sulfo-D-galactose), and TriS (ΔDi-triS(2,4,6), 2-acetamide-2-deoxy-3-O-(2-O-sulfo-ß-D-gluco-4-enepyranosyluronic acid)-4,6-bis-O-sulfo-D-galactose), and tetra-n-butylammonium hydrogen sulfate were from Sigma; 2-cyanoacetamide was from Wako (Osaka, Japan); Nanosep Centrifugal Devices 3K was from Nihon Pall (Tokyo, Japan); COSMOSIL 5C18 MS-II was from Nacalai Tesque (Kyoto, Japan); HiLoad 16/60 Superdex 200 pg was from GE Healthcare.
Animals
C57BL/6 mice were from SLC (Hamamatsu, Japan). Tg2576 mice bearing the human amyloid precursor protein with Swedish (K670N, M671L) mutation15) were purchased from Taconic Biosciences (Hudson, NY). Animals were kept under controlled environmental conditions with standard food and water supply. The Nagoya University Institutional Animal Care and Use Committee approved the animal studies.
Tissue preparation
Anesthetized mice were transcardially perfused with 25 mL of ice-cold phosphate-buffered saline (PBS). The brains were dissected out and sagittally divided into sections. Regional sections of hemi-brains, including olfactory bulb, cortex, hippocampus, thalamus, cerebellum, and brainstem, were snap-frozen separately and stored at –80 °C.
Preparation and structural analysis of brain CS
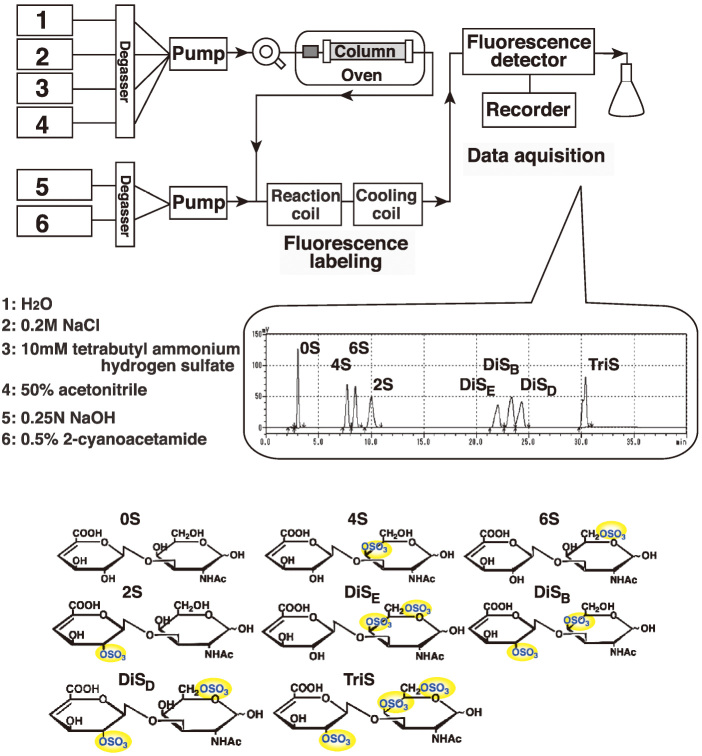
Brain CS was isolated as described previously for heparan sulfate and keratan sulfate with slight modifications.8, 16, 17) Snap-frozen mouse cerebral cortex (25 mg), hippocampus (15 mg) and cerebellum (35 mg) were prepared from 20-month-old non-transgenic (n=5) and Tg2576 (n=7) mice. The frozen tissues were suspended in 2 ml of 0.2 N NaOH and incubated overnight at room temperature. The samples were neutralized with 4 N HCl, as phenol red for a pH indicator, and then treated with DNase I and RNase A (0.04 mg/ml each; Roche) in 50 mM Tris-HCl, pH 8.0, 10 mM MgCl2 for 3 h at 37 °C. Subsequently, the samples were treated with actinase E (0.04 mg/ml; Kaken Pharmaceutical, Tokyo, Japan) overnight at 37 °C. The supernatant was collected by centrifugation at 5,000 × g at 4 °C for 10 min after heat inactivation of the enzyme, and then mixed with the same volume of 50 mM Tris-HCl, pH 7.2. The supernatant was collected as crude CS-containing solutions. The collected samples were subjected to DEAE-Sepharose column chromatography. The samples were applied onto the column, and then washed with 0.2 M NaCl. Most of the hyaluronic acid is washed out by this step. The bound materials were eluted with 2 M NaCl. The resulting elution fractions were added to 3 volumes of ethanol at –80 °C for 30 min. CS was precipitated by centrifugation at 10,000 × g for 15 min, and then rinsed with 70% ethanol. The purified CS was dried, suspended with water, and then mixed with 3 volumes of ethanol containing 1.3% potassium acetate at –80 °C for 30 min. CS was precipitated by centrifugation at 10,000 × g at 4 °C for 30 min. The purified CS solution was treated with chondroitinase ABC. Yielded CS disaccharides were separated by filtering the mixture with a Nanosep 3K filter. The disaccharide compositions of CS were determined by reversed-phase ion-pair high performance liquid chromatography (HPLC) with post-column fluorescent labeling as shown in Fig. 1.
Fig. 1.
Schematic diagram of the reversed-phase ion-pair HPLC with post-column labeling used for chondroitin sulfate (CS) structural analysis.
Schematic diagram of the HPLC used to analyze CS structures is indicated. Solvent 1, 2, 3, and 4, and reaction solution 5 and 6 are displayed. A representative chromatogram with an elution profile of authentic CS disaccharides, 0S, 4S, 6S, 2S, DiSE, DiSB, DiSD, and TriS, and their structures are shown.
Size exclusion chromatography
Purified CS from the cerebral cortex (100 mg) was obtained from 17-month-old non-transgenic (n=3) and Tg2576 (n=3) mice as described above. The samples were treated with hyaluronidase. A Superdex 200 16/60 column was used to separate the CS by size fractionation. The Superdex 200 column was equilibrated with a buffer of 0.2 M NaCl, 20 mM Tris-HCl and run at a flow rate of 1 ml/min. One ml fractions were collected. The collected fractions were lyophilized, and then dissolved in a buffer. The samples were digested with chondroitinase ABC. Yielded disaccharides were separated by filtering the mixture with a Nanosep 3K filter. Post-column labeling reversed-phase ion-pair HPLC was performed to analyze the fractions. The level of CS content was determined by summing the amounts of all disaccharides detected in each sample.
Statistical analysis
All data are presented as mean ± SD. The values were analyzed by unpaired Student’s t-test using Prism software (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered to be significant.
RESULTS
Total amount of CS is reduced in the cerebral cortex of Tg2576 mice
Parenchymal amyloid plaque deposition starts to occur at 11–13 months of age in Tg2576 mice. The amyloid plaque formation in Tg2576 mice was found in the cerebral cortex and hippocampus, which are important for cognitive functioning, while amyloidogenesis was not detected in the cerebellum.8) To investigate whether the CS level is changed in the brain upon Alzheimer’s pathogenesis, we collected cerebral cortices of 20-month-old Tg2576 mice and their non-transgenic (Non-Tg) littermates. The CS was purified from the tissues and analyzed by reversed-phase ion-pair HPLC (Fig. 1). We found that the total amount of CS in the cortex of Tg2576 mice was reduced to 56% of the level in Non-Tg littermates (Fig. 2).
Fig. 2.
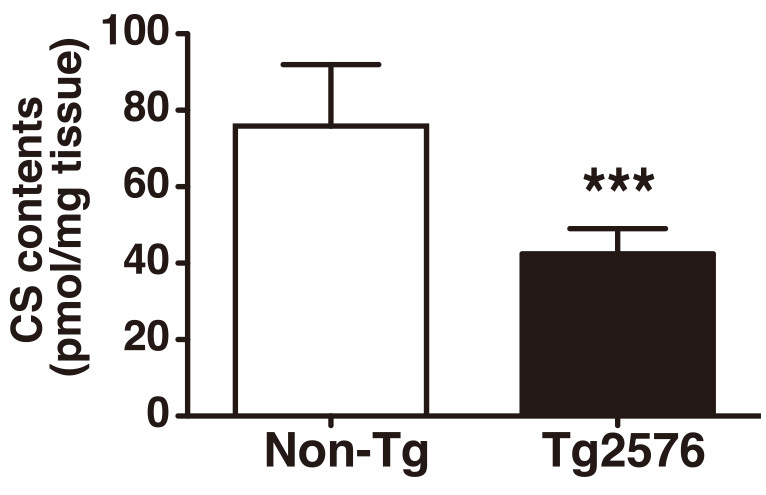
Decreased CS content in the cerebral cortex of Tg2576 Alzheimer’s disease model mice.
CS was prepared from cerebral cortices of age-matched 20-month-old Non-Tg (n=5) and Tg2576 (n=7) mice. CS disaccharide composition was analyzed by the HPLC system. Total contents of CS were calculated. ***P < 0.001.
CS molecular size is reduced in the cerebral cortex of Tg2576
We then investigated whether the molecular size of brain CS is altered with Alzheimer’s pathology. To address this question, we extracted the CS from the cerebral cortex of 17-month-old Tg2576 mice and their Non-Tg littermates. The CS was subjected to size exclusion chromatography using the Superdex 200 column. Cerebral cortex CS in both Non-Tg and Tg2576 mice showed one single peak (Fig. 3). A shift to a later retention time value was observed in the peak of the Tg2576 mice CS. Molecular mass of the peak position was 68 kDa for CS extracted from Non-Tg mice, while that of CS from Tg2576 mice was 58 kDa (Fig. 3).
Fig. 3.

Reduced molecular size of CS in the cortex of Tg2576 Alzheimer’s disease model mice.
CS was prepared from cerebral cortices of age-matched 17-month-old Non-Tg (n=3) and Tg2576 (n=3) mice and separated with a Superdex 200 column. Arrows indicate elution positions of the standard dextran. Molecular mass corresponding to the peak position was estimated (dashed lines). Representative profile for each genotype is shown.
Di-sulfated disaccharide components are increased in CS of Tg2576 brain
A widely used approach to determine CS components is to treat CS with the bacterial endo-type enzyme, chondroitinase ABC, and then analyze the yielded disaccharides by HPLC. To determine whether structural changes in the brain CS occurs in Alzheimer’s pathology, we analyzed CS disaccharide composition in the cortex, hippocampus, and cerebellum of 20-month-old Tg2576 mice and their Non-Tg littermates. In all tissues analyzed, we found that the 4S mono-sulfated disaccharide was a major CS component with 87 to 96% of total disaccharides, and that the level of the 2S mono-sulfated disaccharide was under the detection level in both genotypes (Fig. 4). Percentage of DiSE di-sulfated disaccharide is significantly up-regulated 1.4- to 1.6-fold in the cortex, hippocampus, and cerebellum of Tg2576 mice compared to that of Non-Tg littermates (Fig. 4 and Table 1). In the cortex and hippocampus of Tg2576 mice, where amyloid plaque deposition is abundant, percentage of DiSB di-sulfated disaccharide was significantly up-regulated 1.3- to 1.7-fold. These up-regulations were not observed in the cerebellum of Tg2576 mice, in which amyloid plaque deposition is absent (Fig. 4 and Table 1). The level of the 6S mono-sulfated disaccharide was reduced to 78% of the level in Non-Tg mice in the hippocampus of Tg2576 mice.
Fig. 4.
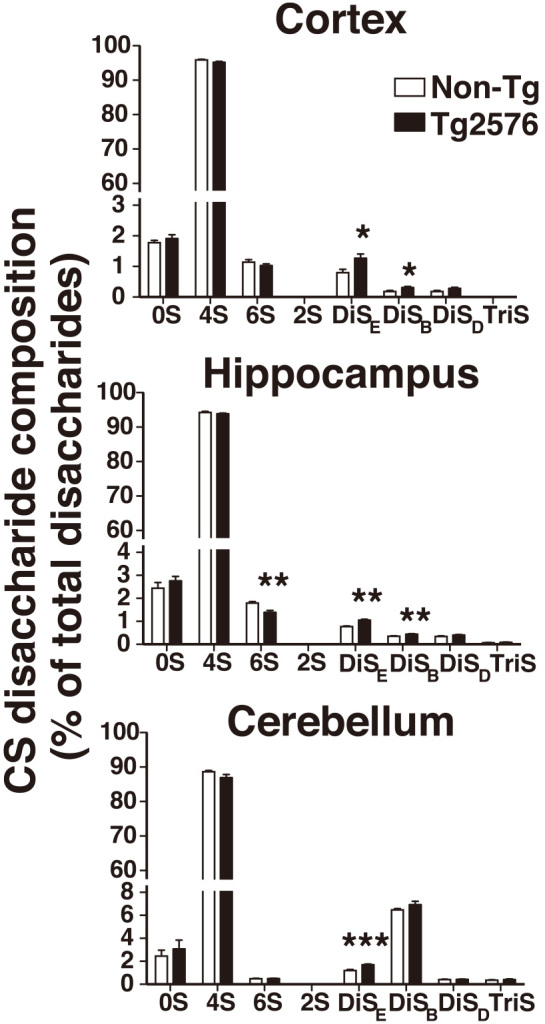
Changes in CS composition with increased di-sulfated disaccharide structures in the brain of Tg2576 Alzheimer’s disease model mice.
CS was prepared from the cortex, hippocampus, and cerebellum of aged-matched 20-month-old Non-Tg (n=5) and Tg2576 (n=7) mice. After chondroitinase ABC treatment, composition of each CS disaccharide was calculated as the percentage of total disaccharides. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
Composition of chondroitin sulfate (CS) disaccharides quantified by reversed-phase ion-pair HPLC
| Unsaturated disaccharidea | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0S | 4S | 6S | 2S | DiSE | DiSB | DiSD | TriS | ||
| Cortex | Non-Tg | 1.77b | 95.89 | 1.13 | ND | 0.80 | 0.19 | 0.20 | ND |
| Tg2576 | 1.91 | 95.20 | 1.02 | ND | 1.27* | 0.32* | 0.28 | ND | |
| Hippocampus | Non-Tg | 2.44 | 94.23 | 1.79 | ND | 0.77 | 0.35 | 0.35 | 0.07 |
| Tg2576 | 2.76 | 93.86 | 1.39** | ND | 1.06** | 0.44** | 0.40 | 0.09 | |
| Cerebellum | Non-Tg | 2.45 | 88.62 | 0.48 | ND | 1.20 | 6.48 | 0.42 | 0.36 |
| Tg2576 | 3.09 | 86.95 | 0.49 | ND | 1.71*** | 6.92 | 0.42 | 0.42 | |
a Percentage of total disaccharides
b The values are means of data shown in Fig. 4 (Non-Tg, n=5; Tg2576, n=7).
ND, not detected.
*P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
In the present study, we found that total amount and average molecular size of CS were significantly reduced in the cerebral cortex of Tg2576 mice. Moreover, in the cerebral cortex and hippocampus but not in the cerebellum, the component of DiSB, the unsaturated form of the CS-B motif di-sulfated disaccharide, was up-regulated in Tg2576 mice. These results clearly indicate that CS structures are altered upon Alzheimer’s pathogenesis.
Cortical areas abundant in CS proteoglycans are less susceptible to cytoskeletal changes in AD patients.18) It is likely that CS may have a protective effect on AD pathology. Reduction in the amount of CS might cause the mice to be more affected by Aß-induced damage. Although the mechanisms underlying down-regulation of cortical CS in Tg2576 mice are currently unknown, potential roles of CS, especially in an ameliorative way, should be explored with administration of CS or genetic manipulation of CS synthesis in AD model mice. Recent findings revealed that low molecular weight CS is protective against Aß-induced neurotoxicity in vivo.14) Our finding that the molecular size of CS in Tg2576 mice was lower than that in Non-Tg littermates could be interpreted as a defense mechanism response. Since CS is a linear polysaccharide, it is presumed that the length of the CS chain in the cortex of Tg2576 mice is shorter than that in Non-Tg littermates. It has previously been reported that sulfation modification by the Golgi-resident GalNAc4S-6-sulfotransferase acts as a terminating signal for CS chain elongation.19) It is conceivable that GalNAc4S-6-sulfotransferase activity or expression level of the sulfotransferase gene might be changed upon Alzheimer’s pathogenesis. Further investigation is needed to determine the expression levels of CS sulfotransferases and elucidate their mechanisms of action on cerebral CS.
Disaccharide constituents of CS from the cortex of AD and control patients has previously been reported.20) The levels of 0S, 4S, and 6S were unchanged in AD patients.20) This is consistent with the findings of our present study using Tg2576 AD model mice, in which the level of 0S, 4S, or 6S disaccharide was unaltered. Our method is capable of determining the levels of di-sulfated and tri-sulfated disaccharide components, as well as the levels of non- and mono-sulfated disaccharides. DiSB and DiSE di-sulfated disaccharide components were found to be up-regulated in Tg2576 mice.
Since up-regulation of DiSB was found in the cortex and hippocampus, but not in cerebellum, where amyloid plaques are absent, it is plausible that the increase in the DiSB level may correlate with amyloid plaque formation and deposition. Endogenous CS rich in the CS-B disaccharide structure, the saturated disaccharide form of DiSB, has been shown to promote the fibril formation of Aß peptides. The Aß fibrils, whose assembly was facilitated by CS-B-rich CS, became stable and reduced their cytotoxicity.21) The increased level of DiSB in Tg2576 mice might be a response to neurotoxic Aß species and might act to reduce their neurotoxicity in the cortex and hippocampus.
DiSE di-sulfated disaccharide was up-regulated in all three tissues. It is possible that the increase in DiSE di-sulfated disaccharide might be associated with AD progression within the whole brain, rather than just in amyloid-specific regions. The CS that contains the CS-E disaccharide structure, the saturated disaccharide form of DiSE, has been shown to be a side chain covalently attached to appican, the proteoglycan form of the amyloid precursor protein.22) Although the mechanisms underlying the up-regulation of DiSE di-sulfated disaccharide are unknown in Tg2576 mice, it is possible that the Tg2576 transgene products, which are human amyloid precursor proteins, could be modified by CS-E-containing CS chains. Up-regulation of DiSE in Tg2576 mice could be dependent on the expression of the transgene product. CS is present as side-chains covalently bound to scaffold proteins in tissues. It is likely that reduction in the total amount of CS reflects changes in the protein-bound form of CS. Application of the assays described in this study to identify and quantify core proteins will enhance our understanding of the potential roles of CS. Furthermore, analysis of human postmortem AD brains with our established method will likely help to clarify the mechanisms underlying the up-regulation of CS and its potential mitigating role in Alzheimer’s pathogenesis.
ACKNOWLEDGMENTS
We thank Tomomi Hosono-Fukao for assistance and support. This work was supported by grants from the Japanese Health and Labour Sciences Research (Comprehensive Research on Aging and Health H19-001 and H22-007 to K.U.), Grants-in-Aid from the Ministry of Education, Science, Sports and Culture (24590349, and 15K08265 to K.U., 23110002 to K.K.), and in part from the Takeda Science Foundation (K.U.), the Kobayashi International Scholarship Foundation (K.U.), and the State Scholarship Fund of the China Scholarship Council (Z.Z.).
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest with respect to the authorship and publication of this article.
REFERENCES
- 1).Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci, 2004; 5: 195–208. [DOI] [PubMed]
- 2).Miyata S, Kitagawa H. Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulphate. J Biochem, 2015; 157: 13–22. [DOI] [PubMed]
- 3).Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature, 1997; 390: 680–683. [DOI] [PubMed]
- 4).Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science, 2002; 298: 1248–1251. [DOI] [PubMed]
- 5).Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev, 2007; 54: 1–18. [DOI] [PubMed]
- 6).Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci, 2012; 15: 414–422. [DOI] [PubMed]
- 7).Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med, 2012; 2: a006338. [DOI] [PMC free article] [PubMed]
- 8).Hosono-Fukao T, Ohtake-Niimi S, Hoshino H, Britschgi M, Akatsu H, Hossain MM, et al. Heparan sulfate subdomains that are degraded by Sulf accumulate in cerebral amyloid ss plaques of Alzheimer’s disease: evidence from mouse models and patients. Am J Pathol, 2012; 180: 2056–2067. [DOI] [PubMed]
- 9).Roher AE, Baudry J, Chaney MO, Kuo YM, Stine WB, Emmerling MR. Oligomerizaiton and fibril asssembly of the amyloid-beta protein. Biochim Biophys Acta, 2000; 1502: 31–43. [DOI] [PubMed]
- 10).Castillo GM, Lukito W, Wight TN, Snow AD. The sulfate moieties of glycosaminoglycans are critical for the enhancement of beta-amyloid protein fibril formation. J Neurochem, 1999; 72: 1681–1687. [DOI] [PubMed]
- 11).Fraser PE, Darabie AA, McLaurin JA. Amyloid-beta interactions with chondroitin sulfate-derived monosaccharides and disaccharides. implications for drug development. J Biol Chem, 2001; 276: 6412–6419. [DOI] [PubMed]
- 12).Woods AG, Cribbs DH, Whittemore ER, Cotman CW. Heparan sulfate and chondroitin sulfate glycosaminoglycan attenuate beta-amyloid(25–35) induced neurodegeneration in cultured hippocampal neurons. Brain Res, 1995; 697: 53–62. [DOI] [PubMed]
- 13).Pollack SJ, Sadler, II, Hawtin SR, Tailor VJ, Shearman MS. Sulfated glycosaminoglycans and dyes attenuate the neurotoxic effects of beta-amyloid in rat PC12 cells. Neurosci Lett, 1995; 184: 113–116. [DOI] [PubMed]
- 14).Zhang Q, Li J, Liu C, Song C, Li P, Yin F, et al. Protective effects of low molecular weight chondroitin sulfate on amyloid beta (Abeta)-induced damage in vitro and in vivo. Neuroscience, 2015; 305: 169–182. [DOI] [PubMed]
- 15).Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 1996; 274: 99–102. [DOI] [PubMed]
- 16).Patnode ML, Yu SY, Cheng CW, Ho MY, Tegesjo L, Sakuma K, et al. KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology, 2013; 23: 381–394. [DOI] [PMC free article] [PubMed]
- 17).Hoshino H, Foyez T, Ohtake-Niimi S, Takeda-Uchimura Y, Michikawa M, Kadomatsu K, et al. KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J Histochem Cytochem, 2014; 62: 145–156. [DOI] [PMC free article] [PubMed]
- 18).Bruckner G, Hausen D, Hartig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience, 1999; 92: 791–805. [DOI] [PubMed]
- 19).Ohtake-Niimi S, Kondo S, Ito T, Kakehi S, Ohta T, Habuchi H, et al. Mice deficient in N-acetylgalactosamine 4-sulfate 6-o-sulfotransferase are unable to synthesize chondroitin/dermatan sulfate containing N-acetylgalactosamine 4,6-bissulfate residues and exhibit decreased protease activity in bone marrow-derived mast cells. J Biol Chem, 2010; 285: 20793–20805. [DOI] [PMC free article] [PubMed]
- 20).Jenkins HG, Bachelard HS. Glycosaminoglycans in cortical autopsy samples from Alzheimer brain. J Neurochem, 1988; 51: 1641–1645. [DOI] [PubMed]
- 21).Bravo R, Arimon M, Valle-Delgado JJ, Garcia R, Durany N, Castel S, et al. Sulfated polysaccharides promote the assembly of amyloid beta(1–42) peptide into stable fibrils of reduced cytotoxicity. J Biol Chem, 2008; 283: 32471–32483. [DOI] [PubMed]
- 22).Tsuchida K, Shioi J, Yamada S, Boghosian G, Wu A, Cai H, et al. Appican, the proteoglycan form of the amyloid precursor protein, contains chondroitin sulfate E in the repeating disaccharide region and 4-O-sulfated galactose in the linkage region. J Biol Chem, 2001; 276: 37155–37160. [DOI] [PubMed]