ABSTRACT
Nogo receptor (NgR) is common in myelin-derived molecules, i.e., Nogo, MAG, and OMgp, and plays important roles in both axon fasciculation and the inhibition of axonal regeneration. In contrast to NgR’s roles in neurons, its roles in glial cells have been poorly explored. Here, we found a dynamic regulation of NgR1 expression during development and neuronal injury. NgR1 mRNA was consistently expressed in the brain from embryonic day 18 to postnatal day 25. In contrast, its expression significantly decreased in the spinal cord during development. Primary cultured neurons, microglia, and astrocytes expressed NgR1. Interestingly, a contusion injury in the spinal cord led to elevated NgR1 mRNA expression at the injury site, but not in the motor cortex, 14 days after injury. Consistent with this, astrocyte activation by TGFβ1 increased NgR1 expression, while microglia activation rather decreased NgR1 expression. These results collectively suggest that NgR1 expression is enhanced in a milieu of neural injury. Our findings may provide insight into the roles of NgR1 in glial cells.
Key Words: NgR1, Spinal cord injury, astrocytes
INTRODUCTION
Neuronal axons in the central nervous systems (CNS) of adult mammals barely regenerate after injuries. This is due to neurons’ low intrinsic capacity for regeneration and to the emergence of inhibitors.1) These inhibitors can be categorized into three classes: (1) guidance molecules, e.g., ephrins and semaphorins, (2) myelin-derived molecules, i.e, Nogo, MAG (myelin-associated glycoprotein), and OMgp (oligodendrocyte myelin glycoprotein), and (3) chondroitin sulfate and keratan sulfate proteoglycans.1-5) Among these, historically, myelin-derived molecules have been extensively studied. Nogo, MAG, and OMgp share the common receptor Nogo receptor (NgR).6, 7) NgR is a glycophosphatidylinositol-linked protein8) with three isotypes: NgR1, 2, and 3.9, 10) These three are differentially expressed in CNS, but NgR1 is the most prominent one.11) NgR1 has been implicated in axon fasciculation.12) Thus, neutralizing antibody to NgR1 increases fasciculation and decreases the branching of cultured dorsal root ganglion neurons. This phenomenon is also observed in antibody to Nogo or Nogo knockout, suggesting that the Nogo-NgR1 axis suppresses axonal fasciculation.
Therefore, the functions of NgR1 have been extensively studied in neurons. However, NgR1 may be also expressed in other cell types. Here, we highlight NgR1 expressed in glial cells.
MATERIALS AND METHODS
Primary culture
Primary cultured neurons were prepared from the cerebellar granule neurons (CGNs) of postnatal day 7 Sprague Dawley WT rats. The meninges of the brain were carefully removed with fine forceps, and the remaining tissues were minced and digested using a Papain Dissociation System (Worthington, Lakewood, NJ, USA). Dissociated cells were applied to a 35/60% two-step Percoll gradient and centrifuged at 3000×g for 15 min. Cerebellar granule neurons at the interface were collected. Cells were suspended in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 % B27 (Invitrogen), 2 mM glutamine, an additional 20 mM KCl, 50 U/ml penicillin, and 50 μg/ml streptomycin.13) Primary cultured glial cells were prepared from the cortexes of postnatal day 1 WT rats. The whole brain was removed aseptically from the skull and the meninges were excised carefully under a dissecting microscope. The brain was strained through sterile mesh, and small pieces of tissues were cultured in flasks in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, then incubated at 37 ºC in a humidified atmosphere containing 5% CO2. The culture medium was renewed every 7 days. The mixed glia cultured for 3 weeks was shaken at 120 rpm on a gyratory shaker for 6 hours. More than 95% of adhering cells were glial fibrillary acidic protein (GFAP)-positive astrocytes. The detached cells were reseeded in fresh culture dishes, and any contaminated oligodendrocyte progenitors were removed by changing the DMEM after 15 min incubation. More than 95% of the cells were found to be CD11b-positive.14) Astrocytes (7×105 cells/ml) and microglia (7×105 cells/ml) was additionally cultured for 24 h in serum-free DMEM. Astrocytes were stimulated with TGF-β (Peprotech, London, UK) 5 ng/ml, 10 ng/ml, and 20 ng/ml and with EGF (Peprotech) 10 ng/ml and 20 ng/ml. After stimulation with cytokines for 48 hr, cells were collected. Microglia were stimulated with LPS (100 ng/ml).
Immunocytochemistry
The primary cultured cells were fixed for 1 min with 4% paraformaldehyde in 0.1 M phosphate buffer (Wako, Osaka, Japan) at room temperature. After several washes, they were solubulized in PBS with 0.1% Triton X-100 and incubated overnight at room temperature with the following primary antibodies: mouse monoclonal anti-Neuronal ClassIII β-Tubulin (Tuj1) antibody (1:500; Covance, Princeton, NJ, USA), mouse monoclonal anti-CD11b antibody (1:500; BD bioscience, Franklin Lakes, NJ, USA), mouse monoclonal anti-GFAP antibody (1:200; Sigma-Aldrich, St. Louis, MO, USA), and rabbit polyclonal anti-Nogo receptor (NgR1) antibody (1:200; abm, Richmond, BC, Canada). After three rinses with PBS, the cells were incubated for 60 min at room temperature with the following secondary antibodies: Alexa Fluor 594-conjugated goat anti-rabbit (Invitrogen), and Alexa Fluor 488-conjugated donkey anti-mouse (Invitrogen). The cells were rinsed in PBS, mounted with Fluorsave (Calbiochem, San Diego, CA, USA), and observed under a BZ-9000 fluorescence microscope (Keyence, Osaka, Japan).
RT-PCR and Quantitative RT-PCR
Total RNA was extracted from the rat brain, spinal cord, and primary cultured cells using an RNeasy Lipid Tissue kit and RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s recommendations. cDNA was prepared from 1 μg of total RNA by using a Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Mannheim, Germany) following the standard protocols. The cDNA products were used for reverse-transcription polymerase chain reaction (PCR) and quantitative real-time PCR (QRT-PCR). QRT-PCR was performed on M×3000P (Agilent Technologies, Santa Clara, CA, USA) using synthetic primers and SYBR Green (Agilent Technologies). Samples were subjected to 45 cycles of amplification at 95˚C for 15 s and 60˚C for 30 s after holding 68˚C for 15 s and 95˚C for 1 min. Relative expression was calculated using the 2-(Ct experimental sample-Ct internal control sample (GAPDH)) method. The primer sequences used are listed in Table 1.
Table 1.
Primer sequences using Q-PCR
| The primer sequences using quantitative RT-PCR (5' → 3') | |||
|---|---|---|---|
| GAPDH | CTCATGACCACAGTCCATGC | Neurocan | CAGGACACACAGGACACCAC |
| TTCCAGTAGGGTCTCGACTT | CCTCYGTAAGGAGCCTAGTT | ||
| Tuj1 | TGCAGGCAGTCACAATTCTC | CD86 | CAGATCAAGGACACGGGCTT |
| TGAACACTCTCCTCCGGAGT | TAGCGGTTGAAGTCACTTGGA | ||
| Iba1 | GGATGGGATCAACAAGCACT | TNF-α | ATGGGCTCCCTCTCATCAGT |
| CTTCGCTTACGACCTCTTTG | CAGCATCGTTTGGTGGTTCG | ||
| GFAP | AGAAAACCGCATCACCATTC | IFN-γ | AGGAACTGGCAAAAGGACGG |
| CTACCGCTCCAGTAATTCCT | ACAGTAGCTTAGCGTGGACT | ||
| NgR1 | CAACCGTATCCCCAGTGTTC | ||
| TGGACAAACGGTTGTTGGAG | |||
Animal Surgery
Adult female Sprague Dawley (SD) rats weighing 200–230 g were used in the study of spinal cord injuries (SCI). The animals were anesthetized with an intraperitoneal injection of Somnopentyl (Kyoritsu Seiyaku, Tokyo, Japan). After Th 9 laminectomy, we exposed the dura mater and induced injury using a force of 200 kdyn using a commercially available SCI device (Infinite Horizon Impactor; Precision Systems and Instrumentation, Lexington, KY, USA) that provided a consistent degree of spinal cord contusion injury. All injuries included the dorsal CST and dorsal gray matter. After SCI was induced, the muscles and skin were closed in layers. The bladder was compressed by manual abdominal pressure twice daily until bladder function was restored. Food was provided on the cage floor, and the rats had no difficulty reaching their water bottles. All animals were given antibiotics in their drinking water [1.0 ml of Bactrim (Roche, Basel, Swiss Confederation) in 500 ml of acidified water] for 2 weeks after SCI. We excluded the rats without complete paraplegia on the next day after operation in all groups, as they were inappropriate for further evaluation. Animal care and experimental procedures were approved by the Animal Experimentation Committee of the Nagoya University Graduate School of Medicine and were conducted according to the Nagoya University Regulations for Experiments.
Statistical analysis
We performed statistical analysis using SPSS software (SPSS, Chicago, IL, USA).
Our analysis used the unpaired two-tailed Student’s t-test for single comparison and one-way ANOVA with a post hoc Bonferroni test for multiple comparisons. In all statistical analyses, significance was accepted at p<0.05.
RESULTS
NgR1 expression during development
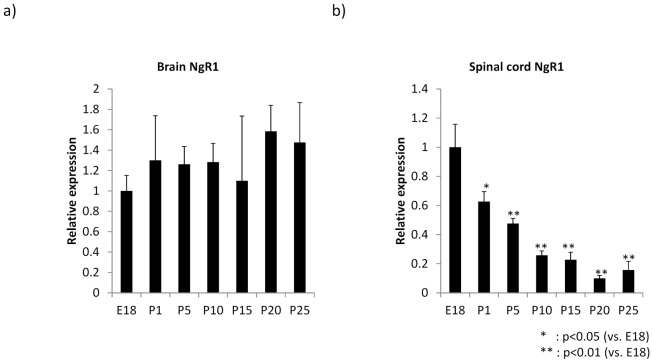
NgR1 mRNA expression was estimated by quantitative RT-PCR. NgR1 mRNA was consistently expressed in the brain from embryonic day 18 to postnatal day 25 (Fig. 1A). In contrast, the spinal cord showed a striking decrease in NgR1 expression during development (Fig. 1B). Thus, NgR1 expression is differentially regulated in the brain and spinal cord during development.
Fig. 1.
Temporal regulation of NgR1 mRNA as revealed by Q-PCR in the developing rat total brain and spinal cord. NgR1 mRNA expression in the developing rat spinal cord was decreased significantly more than E18, although there was no remarkable change in the brain.
a), b) The graph represents NgR1 mRNA expression in the brain and spinal cord. a); total brain, b); spinal cord (n=3) E; embryo, P; postnatal
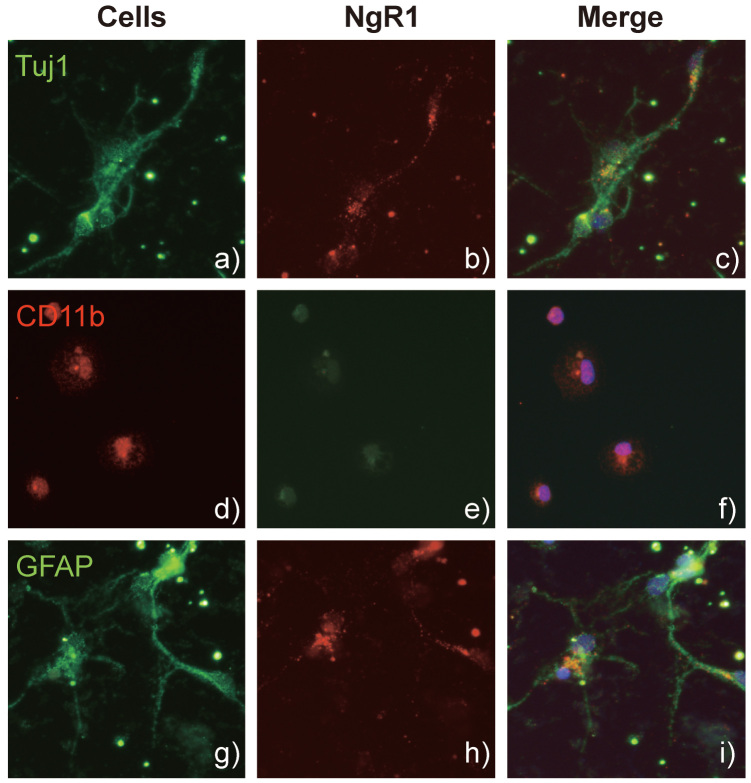
To identify the source of NgR1 expression, we primary cultured cerebellar granule neurons from P7 rats, and astrocytes and microglia from the brain cortex of P1 rats. As shown in Fig. 2, all these cells expressed NgR1. These results suggest that not only neurons but also glial cells could be the source of NgR1 expression.
Fig. 2.
NgR1 expression pattern in the primary culture neurons, microglia, and astrocytes from intact adult rat brain by immunocytochemistry.
NgR1 was expressed in each cell. a), b), c) Neurons were stained with anti-Neuronal ClassIII β-Tubulin (Tuj1) antibody and anti-Nogo receptor (NgR1) antibody. d), e), f) Microglia were stained with anti-CD11b antibody and anti-NgR1. g), h), i) Astrocytes were stained with anti-glial fibrillary acidic protein (GFAP) antibody and anti-NgR1.
NgR1 expression after SCI
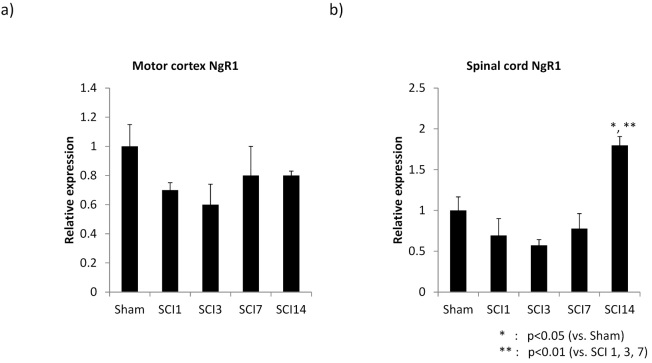
To address NgR1 expression regulation in a pathological condition, we employed a model of SCI. A contusion injury was made at thoracic level 9. Since NgR1 expressed in neurons plays an important role in the inhibition of axonal regeneration/sprouting, we expected that the SCI might influence its expression in the upper motor neurons. To address this question, the motor cortex area was removed after the SCI and subjected to analysis. However, the injury did not affect NgR1 expression in the motor cortex (Fig. 3A). We then examined the spinal cord, removing the injury site (5 mm length) for analysis. Interestingly, NgR1 mRNA expression at the injury site was elevated at 14 days after SCI (Fig. 3B).
Fig. 3.
NgR1 mRNA expression changes in the motor cortex and spinal cord after spinal cord injury.
a), b) The graphs show NgR1 mRNA expression changes in the motor cortex and injured site after spinal cord injury compared to Sham. At 14 days after surgery, NgR1 mRNA expression was significantly higher at the injury site than in Sham, but this expression pattern was not seen in the motor cortex.
a): motor cortex, b): spinal cord (n=3), SCI: spinal cord injury
Enhanced NgR1 expression in activated astrocytes
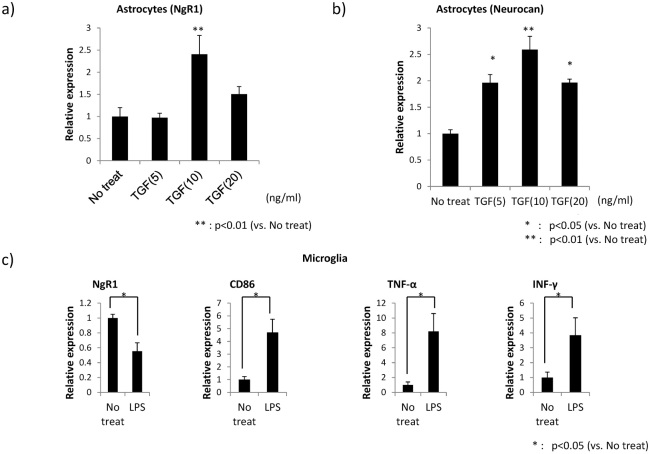
Elevated NgR1 expression at the injury site 14 days after SCI suggests that NgR1 expression may be regulated by glial activation. To address this hypothesis, we employed in vitro activation models of glial cells. TGFβ1 increased NgR1 expression in primary cultured astrocytes (Fig. 4A). The expression of neurocan, a hallmark of astrocyte activation, was also enhanced by TGFβ1 (Fig. 4B). In contrast, microglia activation induced by LPS rather decreased NgR1 expression, while the other markers, CD86, TNF-α, and IFN-γ were increased in this activation (Fig. 4C).
Fig. 4.
NgR1 mRNA expression changes in primary culture astrocytes and microglia received stimulation by lipopolysaccharide (LPS) and cytokine (Transforming growth factor-β=TGF-β, Epidermal Growth Factor=EGF).
a), b) Astrocytes were stimulated with TGF-β and EGF. NgR1 mRNA expression by Q-PCR was significantly elevated in activated astrocytes stimulated with TGF-β.
c) NgR1 mRNA in activated microglia stimulated with LPS was expressed at a significantly lower level compared to that without stimulation.
DISCUSSION
We found that NgR1 is differentially expressed in the brain and the spinal cord during development. Thus, while NgR1 is consistently expressed in the brain, its expression is significantly downregulated in the spinal cord as development proceeds. Therefore, NgR1 expression is at its lowest level in the adult spinal cord. However, SCI enhances its expression at the injury site 14 days after injury. In contrast, NgR1 mRNA expression is not affected in the motor cortex after SCI. These results collectively suggest that SCI influences NgR1 expression in neural cells including glial cells but not in upper motor neurons. Consistent with this idea, in vitro activation of astrocytes enhances NgR1 expression. But microglial activation rather decreases its expression. Therefore, our data collectively suggest that glial cells should be taken into consideration if we address the roles of NgR1 in the nervous system.
The axis of Nogo, MAG, Omgp, and NgR1 has been extensively studied with regard to the inhibition of axonal regeneration/sprouting. Also, NgR1 has been implicated in axon fasciculation. Thus, the functions of NgR1 have been attributed to NgR1 expressed in neurons. Our present study highlighted NgR1 expressed in glial cells, particularly astrocytes. Although its biological roles during neural injuries and/or their recovery processes remain to be further investigated, NgR1 expressed in activated astrocytes should be examined. In this context, it is noteworthy that NgR1 is expressed in immune cells, i.e., B cells, T cells, and monocytes, in multiple sclerosis.15) Although Nogo does not influence the proliferation or the cytokine production of these cells, myelin containing Nogo reduces adhesion and enhances motility. In this way, NgR1 on immune cells may be involved in pathogeneses under the condition of immune cell activation, such as multiple sclerosis.15) In addition, a slow induction of NgR1 (14 days after SCI) was unexpected. Although we could not investigate whether this expression was transient or continue to express thereafter, this question is important to be addressed in a future study.
Our findings of NgR1 expression during development are consistent with previous reports. Thus, it was reported that, although NgR1 mRNA estimated by in situ hybridization is diffusely expressed in the fetal mouse brain and spinal cord, its expression is not detected in the adult mouse spinal cord.11) NgR1 mRNA expression is also not detected in the spinal cord of adult rat and human.11, 16) We also found that, although TGFβ1 increased NgR1 expression, EGF, another activator of astrocytes, did not enhance NgR1 expression in astrocytes (data not shown). This suggests that a specific signaling mediated by some insults, such as TGFβ1, can activate astrocytes and enhance NgR1 expression. Regarding intracellular signaling through NgR1 in a milieu of neural injury, it is known that upon binding of myelin-derived molecules (MAG, Nogo and OMgp) or CSPG, NgR1 makes a complex with p75 and Lingo-1 on neurons. The NgR1 complex activates Rho-GTPase and consequently inhibit axon regeneration.3) In addition to this intracellular signaling in neurons, NgR1 also contribute to oligodendrocyte differentiation where Myocilin, a glycoprotein secreted by astrocytes, binds to NgR1/Lingo-1 complex and suppresses Rho-GTPase in oligodendrocytes.17) However, little is known about the significance of NgR1 expression in astrocytes. Our study demonstrate that astrocytes express NgR1 at least in vitro. Considering that intercellular cross-talk between glial cells and neurons plays a pivotal role in neuronal network reconstitution and functional recovery after SCI, NgR1 expressed in astrocytes would be an important subject for future studies.
ACKNOWLEDGEMENT
This work was supported in part by Grants-in-Aid (Nos. 23110002 to K.K.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
CONFLICT OF INTEREST
The authors have no financial conflicts of interest.
REFERENCES
- 1).Kadomatsu K, Sakamoto K. Mechanisms of axon regeneration and its inhibition: roles of sulfated glycans. Arch Biochem Biophys, 2014; 558: 36–41. [DOI] [PubMed]
- 2).Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol, 2001; 11: 89–94. [DOI] [PubMed]
- 3).Yamashita T, Fujitani M, Yamagishi S, Hata K, Mimura F. Multiple signals regulate axon regeneration through the Nogo receptor complex. Mol Neurobiol, 2005; 32: 105–111. [DOI] [PubMed]
- 4).Ito Z, Sakamoto K, Imagama S, Matsuyama Y, Zhang H, Hirano K et al. N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury. Journal of Neuroscience, 2010; 30: 5937–5947. [DOI] [PMC free article] [PubMed]
- 5).Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z et al. Keratan sulfate restricts neural plasticity after spinal cord injury. Journal of Neuroscience, 2011; 31: 17091–17102. [DOI] [PMC free article] [PubMed]
- 6).Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science, 2002; 297: 1190–1193. [DOI] [PubMed]
- 7).Wang KC, Kim JA, Sivasankaran R, Segal R, He ZG. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature, 2002; 420: 74–78. [DOI] [PubMed]
- 8).Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature, 2001; 409: 341–346. [DOI] [PubMed]
- 9).Lauren J, Airaksinen MS, Saarma M, Timmusk T. Two novel mammalian Nogo receptor homologs differentially expressed in the central and peripheral nervous systems. Molecular and Cellular Neuroscience, 2003; 24: 581–594. [DOI] [PubMed]
- 10).Pignot V, Hein AE, Barske C, Wiessner C, Walmsley AR, Kaupmann K et al. Characterization of two novel proteins, NgRH1 and NgRH2, structurally and biochemically homologous to the Nogo-66 receptor. Journal of Neurochemistry, 2003; 85: 717–728. [DOI] [PubMed]
- 11).Josephson A, Trifunovski A, Widmer HR, Widenfalk J, Olson L, Spenger C. Nogo-receptor gene activity: Cellular localization and developmental regulation of mRNA in mice and humans. Journal of Comparative Neurology, 2002; 453: 292–304. [DOI] [PubMed]
- 12).Petrinovic MM, Duncan CS, Bourikas D, Weinman O, Montani L, Schroeter A et al. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development, 2010; 137: 2539–2550. [DOI] [PubMed]
- 13).Hatten ME. Neuronal Regulation of Astroglial Morphology and Proliferation Invitro. Journal of Cell Biology, 1985; 100: 384–396. [DOI] [PMC free article] [PubMed]
- 14).Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis, 2013; 4: e525. [DOI] [PMC free article] [PubMed]
- 15).Pool M, Niino M, Rambaldi I, Robson K, Bar-Or A, Fournier AE. Myelin regulates immune cell adhesion and motility. Experimental Neurology, 2009; 217: 371–377. [DOI] [PubMed]
- 16).Funahashi S, Hasegawa T, Nagano A, Sato K. Differential expression patterns of messenger RNAs encoding Nogo receptors and their Ligands in the rat central nervous system. Journal of Comparative Neurology, 2008; 506: 141–160. [DOI] [PubMed]
- 17).Kwon HS, Nakaya N, Abu-Asab M, Kim HS, Tomarev SI. Myocilin is involved in NgR1/Lingo-1-mediated oligodendrocyte differentiation and myelination of the optic nerve. J Neurosci, 2014; 34: 5539–5551. [DOI] [PMC free article] [PubMed]