Abstract
Background and purpose
Fingolimod (FTY‐720) is the first oral therapeutic drug approved for the relapsing–remitting forms of multiple sclerosis. Neural stem cells (NSCs) are capable of continuous self‐renewal and differentiation. The dentate gyrus of the hippocampus in the adult mammalian brain contains a population of NSCs and is one of the regions where neurogenesis takes place. FTY‐720 has been shown to have neuroprotective effects in several model systems, so we investigated the direct effects of FTY‐720 on NSCs and adult neurogenesis.
Experimental approaches
We assessed the effects of FTY‐720 on the proliferation and differentiation of cultured embryonic hippocampal NSCs using the 5‐bromo‐2‐deoxyuridine incorporation assay, the neurosphere formation assay and western blot analysis. Receptor selective agonists and antagonists were used to identify the mechanisms involved. Neurogenesis in the hippocampus of C57BL/6 mice was also assessed by immunohistochemistry. The Morris water maze and fear conditioning tests were used to detect the learning and memory abilities of mice.
Key results
FTY‐720 promoted the proliferation of embryonic hippocampal NSCs probably via the activation of ERK signalling, Gi/o proteins and S1P1 receptors. However, FTY‐720 did not affect the differentiation of cultured hippocampal NSCs. In vivo, chronic treatment with FTY‐720 promoted hippocampal neurogenesis in adult C57BL/6 mice and enhanced their learning and memory abilities.
Conclusions and Implications
Our results suggest a new target for the activation of NSCs and provide an insight into the therapeutic effects of FTY‐720 in neuropsychiatric disorders, neurodegenerative diseases and age‐related cognitive decline where hippocampal neurogenesis is compromised.
Abbreviations
- FC
fear conditioning
- MWM
Morris water maze
- NSCs
neural stem cells
- S1P
sphingosine‐1‐phosphate
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a, 2015b, 2015c).
Introduction
As a sphingosine‐1‐phosphate (S1P) analogue, fingolimod (FTY‐720) is the first oral therapeutic drug approved for the relapsing–remitting forms of multiple sclerosis (RRMS). S1P is a lipid mediator formed by the metabolism of sphingomyelin and is abundant in serum, blood and many organ systems (Hla, 2004). A family of cell surface GPCRs termed as S1P receptors 1–5 mediates most of its biological actions in multiple biology processes (Kingsbury et al., 2003; Mizugishi et al., 2005). FTY‐720 is phosphorylated in vivo by sphingosine kinase 2 to form the active agent FTY720‐phosphate, which is a sphingosine 1‐phosphate receptor modulator that binds to four of the five known S1P receptors (S1P1, S1P3, S1P4 and S1P5) (Albert et al., 2005).
It is generally accepted that the main therapeutic effect of FTY‐720 in MS is mediated by its ability to act as an immunomodulator (Aktas et al., 2010). However, the fact that FTY‐720 readily crosses the blood–brain barrier and the presence of its receptors within the CNS highlights the importance of evaluating its direct effects on neurons and glial cells (Mullershausen et al., 2007; Miron et al., 2008). More recently, evidence has been obtained showing that the therapeutic effect of FTY‐720 in RRMS is due to its ability to affect both the immune system and CNS (Zhang et al., 2015). Many researches have proposed that FTY‐720 can exert a neuroprotective effect in several CNS disorders, such as stroke, Alzheimer's disease and Parkinson's disease (Hemmati et al., 2013; Vargas‐Medrano et al., 2014).
Neural stem cells (NSCs) are characterized by the capacity to continuously self‐renew and generate a multitude of neuronal and glial lineages (Gage, 2000). Neurogenesis exists not only in the developing nervous system but also in the nervous system of all adult mammalian organisms (Altman and Das, 1965; Altman, 1969). Adult neurogenesis has been demonstrated at two locations under normal conditions: the subventricular zone of the lateral ventricles and the subgranular zone (SGZ) in the dentate gyrus of the hippocampus. It consists of complex processes and is controlled by multiple intracellular and extracellular cues, named as neurogenic niche (Stolp and Molnar, 2015). Adult neurogenesis is important in the maintenance and repair of the adult brain, but its ability is limited, rendering the brain particularly vulnerable to injuries and disease. Numerous studies have proved that a tight relationship exists between adult neurogenesis and brain function in normal and pathological conditions (Song et al., 2002; Mirescu and Gould, 2006).
Although the effects of FTY‐720 on the CNS have been widely studied, the direct effects of FTY‐720 on embryonic NSCs and adult neurogenesis have not been elucidated. Based on the facts that the S1P receptors are expressed on the NSCs (McGiffert et al., 2002) and S1P exerts direct regulatory effects on NSCs (Harada et al., 2004), the goal of the present study was to investigate the possible regulatory influences of FTY‐720 on embryonic NSCs and adult neurogenesis.
Methods
Animal welfare and ethical statement
All the animals were purchased from SLAC Laboratory Animals Co. Ltd. (Shanghai, China). The treatment of the animals and experimental procedures were performed following the rules of the Association for Assessment and Accreditation of Laboratory Animal Care International and approved by the Institutional Animal Care and Use Committee of the Shanghai Institute of Material Medica, Chinese Academy of Sciences. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Animals were housed in environmentally‐controlled conditions (12 h light–dark cycle, temperature 22–26°C, air humidity 40–60%) and had free access to standard laboratory chow and water. The experimental animals were randomly assigned to different groups in each part of the experiment. A total of 60 mice were used in the behavioural experiments as detailed below.
Isolation and culture of NSCs
NSCs were cultured according to a neurosphere formation assay developed by Reynolds and Weiss (1996) and Gritti et al. (1996). Cells were isolated from the hippocampi of E13‐E14 Sprague–Dawley rat embryos and cultured in DMEM/F12 medium (1:1 mixture) supplemented with 20 ng·mL−1 EGF, 20 ng·mL−1 basic FGF (bFGF), 1% N2 and 2% B27. The cells were maintained in a humidified incubator at 37°C with 95% atmospheric air/5% CO2, grown as free‐floating aggregates (neurospheres), and the medium was changed every other day. Neurospheres were passaged every 5–6 days by digestion with the StemPro Accutase Cell Dissociation Reagent for 3–5 min at 37°C and replated in cell culture plates. All the cells used in the in vitro experiments were the second passages.
NSCs proliferation assay
Single‐cell suspensions were plated in 6‐well cell culture plates and cultured in the medium without EGF or bFGF; the density was 3–4 × 105 cells·mL−1. The FTY‐720 [dissolved in 0.1% (v v‐1) of DMSO] was added to the medium at different concentrations (0, 25, 50, 100 or 250 nM) 4 h later. The 5‐bromo‐2‐deoxyuridine (BrdU) incorporation assay and the neurosphere formation assay were used to identify the proliferation of NSCs. In the BrdU incorporation assay, NSCs were cultured with different concentrations of FTY‐720 for 18 h, and then, 10 μM BrdU was added to the medium. The cells were incubated for another 24 h; after that, the neurospheres were digested with the StemPro Accutase Cell Dissociation Reagent for 3–5 min at 37°C and plated on 12 mm glass coverslips pre‐coated with 50 μg·mL−1 poly‐D‐lysine (PDL). After that, the cells were processed for BrdU staining later. In the neurosphere formation assay, NSCs were incubated with different concentrations of FTY‐720 for 5 days, and then, the diameter of the neurospheres was analysed. To determine the mechanisms, receptor‐selective agonists and antagonists were used. During the experiments, the antagonists were added to the medium 15 min prior to the addition of FTY‐720.
NSCs differentiation assay
For the differentiation experiments, the neurospheres were plated on 6‐well culture plates pre‐coated with 50 μg·mL−1 PDL and were cultured in DMEM/F12 medium containing 1% N2, 2% B27 supplements and different concentrations of FTY‐720 (0, 25, 50, 100 or 250 nM) for 3 days. After that, the total cellular proteins were extracted for western blotting.
Immunocytochemistry for NSCs
The cells on the coverslips were washed with PBS, fixed for 30 min with 4% paraformaldehyde and then permeabilized with 0.1% Triton X‐100 for 15 min. For the BrdU staining, cells were treated with 2 M HCl for 20 min to denature DNA and 0.1 M Na2B4O7 for another 15 min to neutralize the HCl before the permeation. The cells were blocked for 1 h in blocking buffer (5% normal goat serum and 5% BSA in PBS) before incubation with the corresponding primary antibodies (mouse anti BrdU, 1:300) at 4°C overnight. The next day, the cells were washed three times with PBS buffer and incubated with Alexa Fluor 488–conjugated secondary antibodies (1:500) at room temperature for 1 h. Cell nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (10 μg·mL−1). Finally, the coverslips were mounted on slide glasses. Fluorescence was detected and photographed with a photomicroscope (Olympus, Tokyo, Japan) (LUCPlanFLN, 20×/0.45Ph1 objective lens) equipped with a camera spot system. Ten random fields in each coverslip were chosen to conduct the cell counting; the cell counting was undertaken using image analysis software image‐pro‐plus 5.1 (Media Cybernetics, Inc., Warrendale, USA).
Protein extraction and western blotting
The cell lysates of NSCs were collected, and the concentrations of protein were determined with a bicinchoninic acid (BCA) protein assay kit. In the ERK stimulating experiments, cells were starved in D‐Hank's buffer for 1 h before treatment with the compounds. Samples were run on a 15, 10 or 8% SDS‐PAGE and transferred onto a PVDF membrane (0.45 or 0.22 μm). After blocking the non‐specific protein binding sites with 5% non‐fat milk powder in Tris buffered saline with Tween 20 buffer for 1 h at room temperature, the membranes were incubated with primary antibodies in blocking buffer at 4°C overnight. The following day, the membranes were incubated with the HRP‐conjugated secondary antibodies (1:500); eventually, an enhanced chemiluminescent (ECL) kit was used to detect the immunoreactive proteins. Primary antibodies were directed against the following: rabbit anti‐TuJ1 antibody (1:1000), rabbit anti‐S100β antibody (1:1000), rabbit anti‐PDGF receptor α (PDGFRα) antibody (1:1000), rabbit anti‐p‐ERK1/2 antibody (1:1000), rabbit anti‐total ERK1/2 antibody (1:1000) and mouse anti‐β‐actin antibody (1:15 000).
FTY‐720 treatment and BrdU labelling in adult mice
Eight‐week‐old male C57BL/6 mice were randomly allocated to three groups and received daily i.p. injections of normal saline (NS), 2 or 5 mg·kg−1 of FTY‐720 for 7 days. After that, the mice received an i.p. injection of 50 mg·kg−1 BrdU. Two BrdU injection paradigms were used. To label the proliferation cells in the dentate gyrus, 24 h after the last NS or FTY‐720, the mice received one dose of BrdU and were killed 4 h later. To assess the influence of FTY‐720 on the differentiation of newborn cells in the dentate gyrus, the mice received three injections of BrdU (24 h after the last NS or FTY‐720) each at a 12‐h interval and were killed 1 month after the last injection of BrdU.
Immunohistochemistry
The mice were anaesthetized by an i.p. injection of 450 mg·kg−1 chloral hydrate and perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were then removed, placed in the same fixation for 48 h at 4°C for post‐fixation and transferred to the 30% sucrose solution until they sank. The coronal sections (20 μm thickness) of the brain were obtained with a freezing microtome purchased from Leica, the Pathology Company (Wetzlar, Germany). Free‐floating sections were rinsed in PBS and then permeabilized with 0.1% Triton X‐100 for 15 min. For BrdU staining, the sections were incubated in 2 M HCl at 37°C for 30 min to denature DNA and 0.1 M Na2B4O7 for another 15 min to neutralize the HCl. After a 1 h incubation at room temperature in the blocking buffer (5% BSA plus 5% goat serum), the sections were incubated with the corresponding primary antibodies at 4°C overnight. The following day, the sections were washed with PBS buffer for three times before being incubated in another solution containing secondary antibodies (Alexa Fluor 555‐conjugated donkey anti‐mouse IgG, 1:500, or Alexa Fluor 488‐conjugated donkey anti‐rabbit IgG, 1:500) at room temperature for 1 h. Finally, the specimens were mounted on slide glasses. All sections were processed using the same standardized conditions. The immunolabelled sections were imaged with an Olympus FV 1000 laser scanning confocal microscope (Olympus). The brain slices were observed with a 20× objective lens (UPLSAPO, 20×, NA: 0.75). Primary antibodies were directed against the following: mouse anti‐BrdU (1:300), rabbit anti‐Ki67 (1:500) and rabbit anti‐NeuN (1:800).
Quantification of BrdU and NeuN labelling
For BrdU, Ki67 and BrdU/NeuN quantification in the dentate gyrus, we used the method developed previously with some modifications (Jiang et al., 2005; Morales‐Garcia et al., 2012); 20 μm sections were cut coronally throughout the dentate gyrus starting at a random location in the hippocampus region (bregma −2.56 to −3.30). For each mouse, only one in every five sections throughout the hippocampus was used for further immunohistochemistry. The entire six sections of each mouse were analysed all from the similar bregma levels of the animals, to make sure the comparison of results was appropriate. We counted every single BrdU‐labelled, Ki67‐labelled or BrdU/NeuN‐labelled cell in the entire granule cell layer, SGZ, and the hilus in each brain slice. All the BrdU‐positive cells in the dentate gyrus were analysed to obtain the proportion of BrdU/NeuN double‐labelling cells to BrdU‐stained cells. The results from all six sections were collected from each mouse, and the mean of the six sections of each mouse is taken as n = 1. The data are presented as the total number of cells in each section. The cell counting was undertaken using the image analysis software image‐pro‐plus 5.1 (Media Cybernetics, Inc.). During the procedure of the cell counting, we used the histogram‐based threshold method to separate the foreground and the background, and the threshold number is 95–255. To avoid mistaking cell clusters as a single cell, we set the diameter as a filter condition: the diameter of a single cell was 0–10 μm, so the spots with a 20–30 μm diameter were counted as two cells; the spots with a diameter above 30 μm were counted as three cells. The method was applied when quantifying both the BrdU and Ki67 results.
Behavioural tests
The spatial learning and memory abilities of mice were evaluated using the Morris water maze (MWM) at the fourth week after the last injection of NS or FTY‐720. The methods used were as described previously (Morris, 1984; Jia et al., 2015; Yook et al., 2015). In brief, each mouse was trained to find a white platform (10 cm diameter) in a pool of opaque water (120 cm in diameter × 32 cm deep, maintained at 23°C). The platform was submerged 1 cm below the water surface. The maze was placed in a room with dim light; black geometric shapes on the walls surrounding the maze served as visual cues. A video on the top of the maze linked to the analysis system was used to track the swimming paths of each subject. The equipment and analysis systems were purchased from Shanghai Jiliang Software Technology Co. Ltd. (Shanghai, China). The mice were subjected to three trials with a 20–30 min interval each day for five consecutive days. The mice were randomly put into the water at one of the three start points – the three start points were in the other three quadrants, which did not have a separate platform. The mice were allowed 60 s to swim until they landed on the platform. If a mouse failed to locate the platform within 60 s, it was manually guided to the platform and removed 10 s later. For each trial, the escape latency (time to find the hidden platform) was videotaped and analysed using image tracking software. On day 6, the platform was removed from the water; a 60 s probe test was conducted in the morning. The entrance point was in the opposite quadrant from which the distance to the platform was the longest. The swimming path, crossing times across the virtual platform, time spent in each quadrant and the swimming speed of each mouse were recorded and analysed. Only when the centre of a mouse's body completely crossed the circle of the platform position was it counted as one crossing.
We conducted two kinds of fear conditioning (FC) tests to test the fear learning and memory abilities: contextual FC and cued FC. We performed the FC tests as described previously with some modifications (Raybuck and Lattal, 2011; Wang et al., 2011). The tests took place in a chamber (50 × 40 × 18 cm) located in a sound‐attenuated box; the box was put in a quiet test room. The chamber had clear Plexiglas walls and a ceiling with holes to allow ventilation. The floor consisted of steel rods, spaced 1.3 cm apart, which were wired to a shock source and solid‐state grid scrambler for the delivery of footshock. To record the behaviour of the animals, a video camera was suspended above the chamber. A programmable audio generator was located in the left corner of one wall, and the same wall was equipped with a single house light in the top to illuminate the chamber. For contextual FC assay, 2 days after the probe test of the MWM, the mice were trained and tested for 2 days: conditioning day and testing day. In the conditioning day, the mice were placed in the chamber and allowed to explore freely for 5 min. The habituation procedure was introduced to make sure the mice were completely familiarized with the stimuli of the environment and prevented any other uncontrolled stimuli interference during the experiments. After the habituation procedure, the mice received 20 consecutive electric footshocks (0.5 mA, 2 s) at 60 s intervals. The mice were left in the conditioning chamber for 30 s after the termination of the procedure and then returned to their home cage. Twenty‐four hours after the training, the mice were returned to the same context without the shock and scored for freezing behaviour for 3 min. The next day, the mice were trained and tested for another 2 days for cued FC tests. During the training day, the mice were placed in the chamber and allowed to explore freely for 5 min, after which the mice received a tone conditioned stimulus (8000 Hz white noise) for 20 s co‐terminated with a 2 s footshock (0.5 mA). This conditioning was repeated 20 times each with a 1 min interval. The mice were left in the conditioning chamber for 30 s after the termination of the procedure and then returned to their home cage. Twenty‐four hours later, the mice were placed in a modified environment (black Plexiglas walls, plastic floor over the grid bars) for 6 min with the tone presented continuously for the last 3 min, and the freezing behaviour was monitored. Freezing was defined as the complete absence of movement, except for respiratory movement. Data are presented as the percentage of the total time in which freezing behaviour was presented. The equipment and analysis software were from the Sophisticated Life Science Research Instrumentation (TSE, Homburg, Germany).
Materials
DMEM/F12 medium (CAT#12 500 062), EGF (CAT#PHG0313), bFGF (CAT#PHG0021), N2 (CAT#17 502 048), B27(CAT#17 504 044), StemPro Accutase Cell Dissociation Reagent (solution of proteolytic and collagenolytic enzymes) (CAT#A1110501), goat serum (CAT#16 210–072), Alexa Fluor 488‐conjugated donkey anti‐mouse IgG (CAT#A21202), Alexa Fluor 555‐conjugated goat anti‐mouse IgG (CAT#A21422) and Alexa Fluor 488‐conjugated donkey anti‐rabbit IgG (CAT#A21206) were purchased from Invitrogen Corp. (Carlsbad, USA).
FTY‐720 (CAT# SML0700), BrdU (CAT#B5002), PDL (CAT#P7280), W146 (CAT#W1020), U0126 (CAT#U120), SEW2871 (CAT# S3944), AUY954 (CAT# 297 712), BSA (CAT#B2064), DAPI (CAT#D9542), β‐actin antibody (CAT#A1978) and BrdU antibody (CAT#B2531) were purchased from Sigma (St Louis, USA).
W123 (CAT#10 010 992) was bought from Cayman Chemicals (Ann Arbor, USA).
Suramin (CAT#574 625), and pertussis toxin (PTX) (CAT# AG723) were purchased from EMD Bioscience (San Diego, CA, USA).
BCA protein assay kit (CAT#23 227) and the ECL kit (CAT#34 080) were purchased from Thermo Fisher Scientific (Waltham, USA).
PVDF membrane (0.45 μm: CAT#IPVH00010, 0.22 μm: CAT#ISEQ00010), HRP‐conjugated secondary antibodies (CAT#12–349), Goat anti‐rabbit IgG antibody, HRP‐conjugate (CAT#12–348) and NeuN antibody (CAT#ABN78) were purchased from Merck Millipore (Darmstadt, Germany).
TuJ1 antibody (CAT#5568), PDGFRα antibody (CAT#3174), p‐ERK1/2 antibody (CAT#9101) and total ERK1/2 antibody (CAT#9102) were purchased from Cell Signaling Technology (Boston, USA).
Ki67 (CAT#ab15580), S100β antibodies (CAT# ab41548) were purchased from Abcam (Cambridge, UK).
Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
All data are presented as means ± SEM. Group differences in the NSCs differentiation assay and the escape latency in the MWM training task were analysed with two‐way ANOVA. One‐way ANOVA was used in other experiments. The statistical analysis was conducted with an Instat statistical programme, graphpad Software (San Diego, CA, USA). All the in vitro experiments were performed at least five times independently. The differences with P values <0.05 were considered significant.
Results
FTY‐720 induced the proliferation of cultured embryonic hippocampal NSCs but did not affect their differentiation
We examined the effects of FTY‐720 on the proliferation of embryonic hippocampal NSCs using the BrdU incorporation assay and the neurosphere formation assay. The cultured embryonic NSCs were incubated with different concentrations of FTY‐720 (25, 50, 100 or 250 nM) without mitogen growth factors bFGF or EGF. In the BrdU incorporation assay, the proliferated NSCs were labelled with BrdU, a thymidine analogue that is incorporated into the dividing cells during the DNA synthesis phase. As shown in Figure 1B, FTY‐720 promoted NSCs' proliferation in a concentration‐dependent manner and this effect was significant at 100 and 250 nM (F 4,279 = 42.91), which was later confirmed by the neurosphere formation assay shown in Figure 1D (F 4,1240 = 47.57). A lower concentration (100 nM) of FTY‐720 was used during the next experiments. To examine the influence of FTY‐720 on the differentiation of NSCs, the Western blot of specific antibodies against the markers of neurons, astrocytes and oligodendrocytes (Tuj‐1, S100β and PDGFRα respectively) were used, as shown in Figure 1E, F. These data suggest that FTY‐720 significantly induced the proliferation of the cultured embryonic hippocampal NSCs at 100 and 250 nM and exerted no significant effects on their differentiation.
Figure 1.
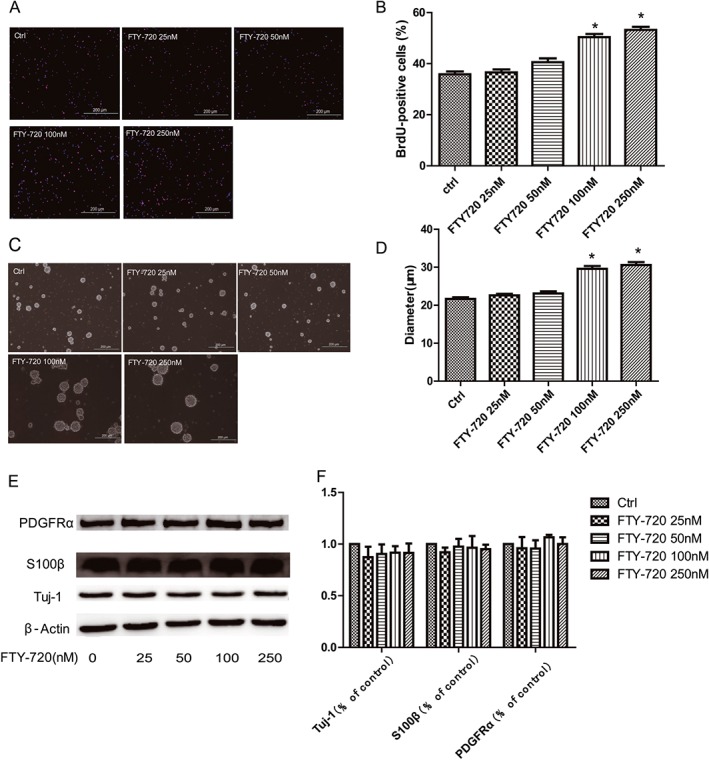
Effects of FTY‐720 on the proliferation and differentiation of cultured embryonic hippocampal NSCs. Cells were treated with different concentrations of FTY‐720 (25, 50, 100 or 250 nM) without mitogen growth factors bFGF or EGF. (A) Representative images of BrdU immunoreactivity (red). DAPI staining (blue) was used as a nuclear marker. Scale bar = 200 μm. (B) The BrdU incorporation analysis showed that FTY‐720 significantly increased the cell proliferation at 100 and 250 nM. (C) Representative phase‐contrast micrographs showing the size of neurospheres after 5 days in the presence of FTY‐720. Scale bar = 200 μm. (D) The analysis of the diameter of the neurospheres in the neurosphere formation test. (E) Representative images of western blot analysis. (F) The analysis of western blots showed that FTY‐720 did not affect the differentiation of the cultured embryonic NSCs. Data shown are mean values ± SEM, n = 5 independent experiments/cultures.
ERK signalling and Gi/o proteins were involved in FTY‐720‐induced NSCs proliferation
We examined the intracellular signalling to elucidate the probable mechanisms involved in the effects of FTY‐720 on NSCs proliferation. MAP kinases play an important role in neural progenitor cell proliferation (Learish et al., 2000), and FTY‐720 is known to activate these kinases in multiple systems (Rutherford et al., 2013; Xie et al., 2015). Very recently, FTY‐720 has been shown to activate ERK1/2 in cultured subventricular zone NSCs (Blanc et al., 2015). Here, we studied whether FTY‐720 could activate ERK1/2 in embryonic hippocampal NSCs and its associationwith proliferation. Our data show that the phosphorylation of phosphate‐ERK1/2 (p‐ERK1/2) during the first 1 h after the FTY‐720 application dramatically changed at specific time points, as shown in Figure 2A, reached the peak level at 15 min (F 7,32 = 19.08) and was significant at 50, 100 and 250 nM (F 4,31 = 35.80) (Figure 2B). In the next experiments, the 15 min time point was used in p‐ERK1/2 stimulation experiments. The significant increase in p‐ERK1/2 implied a possible involvement of ERK signalling in the FTY‐720‐induced proliferation of NSCs. This hypothesis was improved by further experiments with a specific inhibitor of the ERK signalling, U0126, which completely blocked FTY‐720‐induced phosphorylation of p‐ERK1/2 (F 3,16 = 57.64) (Figure 2C) and proliferation of NSCs (F 3,200 = 25.77) (Figure 2D). Furthermore, PTX, a selective blocker of Gi/o protein, also completely blocked the effects of FTY‐720 on NSCs, including the phosphorylation of p‐ERK1/2 (F 3,16 = 18.99) (Figure 2E) and the proliferation (F 3,197 = 15.14) (Figure 2F). These data altogether suggest that FTY‐720‐promoted NSCs' proliferation was dependent on the activation of ERK signalling and Gi/o proteins.
Figure 2.
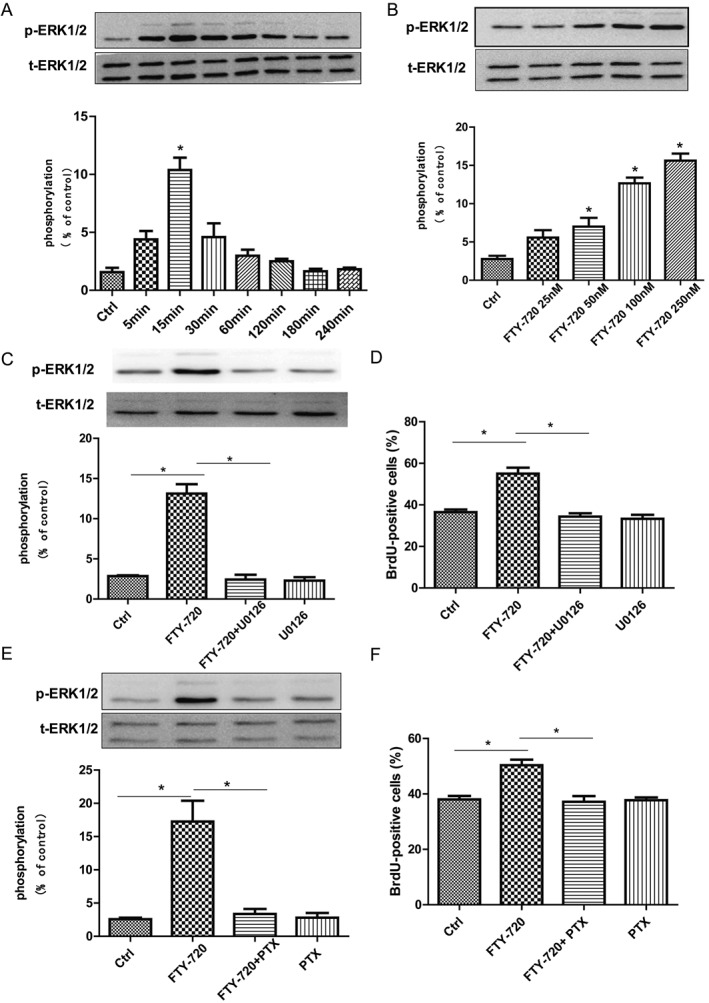
Effects of FTY‐720 on ERK signalling and Gi/o protein of cultured embryonic hippocampal NSCs. (A) Western blot analysis showed that 100 nM FTY‐720 rapidly induced phosphorylation of p‐ERK1/2 in a time‐dependent way. At 15 min, phosphorylation of p‐ERK1/2 reached the peak level. (B) FTY‐720 induced the phosphorylation of p‐ERK1/2 in a dose‐dependent way 15 min after FTY‐720 application. (C) Application of U0126 (10 μM), the ERK signalling inhibitor, blocked the enhanced effect of 100 nM FTY‐720 on the phosphorylation of p‐ERK1/2 in NSCs. (D) BrdU incorporation assay analysis showed that U0126 (10 μM) antagonized the promoting effects of 100 nM FTY‐720 on NSCs proliferation. (E) Application of the Gi/o protein inhibitor PTX (100 ng·mL−1) blocked the promoting effect of 100 nM FTY‐720 on phosphorylation of p‐ERK1/2 in NSCs. (F) BrdU incorporation assay analysis showed that addition of PTX blocked the promoting effects of 100 nM FTY‐720 on NSCs' proliferation. Data shown are mean values ± SEM, n = 5 independent experiments/cultures.
S1P1 receptors mediated the effects of FTY‐720 on the cultured embryonic hippocampal NSCs
The active agent of FTY‐720 in vivo, FTY720‐phosphate, binds with high affinity to four of the five known S1P receptors (S1P1, S1P3, S1P4 and S1P5). Embryonic NSCs express S1P1, S1P2, S1P3 and S1P5 receptors (McGiffert et al., 2002; Harada et al., 2004). We performed experiments using the receptor selective agonists and antagonists to investigate which receptor mediated the effects of FTY‐720 on NSCs. SEW2871 and GNF‐AC‐1 (AUY954) are S1P1 selective agonists, W123 and W146 are S1P1 selective antagonists and suramin is a dual antagonist for S1P3 and S1P5 receptors. As shown in Figure 3A and B, both SEW2871 (5 μM) and AUY954 (1 μM) increased the phosphorylation of p‐ERK1/2 (F 4,16 = 24.76) and promoted NSCs' proliferation (F 3,277 = 29.99). The S1P1 receptor selective antagonists, W123 (5 μM) and W146 (5 μM), blocked FTY‐720‐induced NSCs proliferation (F 5,25 = 29.82) and the phosphorylation of the p‐ERK1/2 (F 5,308 = 23.78) (Figure 3C,D). Furthermore, suramin did not block the effects of FTY‐720 on NSCs, including the phosphorylation of the p‐ERK1/2 (F 3,16 = 21.94) and NSCs' proliferation (F 3,215 = 15.55) (Figure 3E, F). These data suggest that the FTY‐720‐induced proliferation and phosphorylation of the ERK1/2 in NSCs are mediated by S1P1 receptors.
Figure 3.
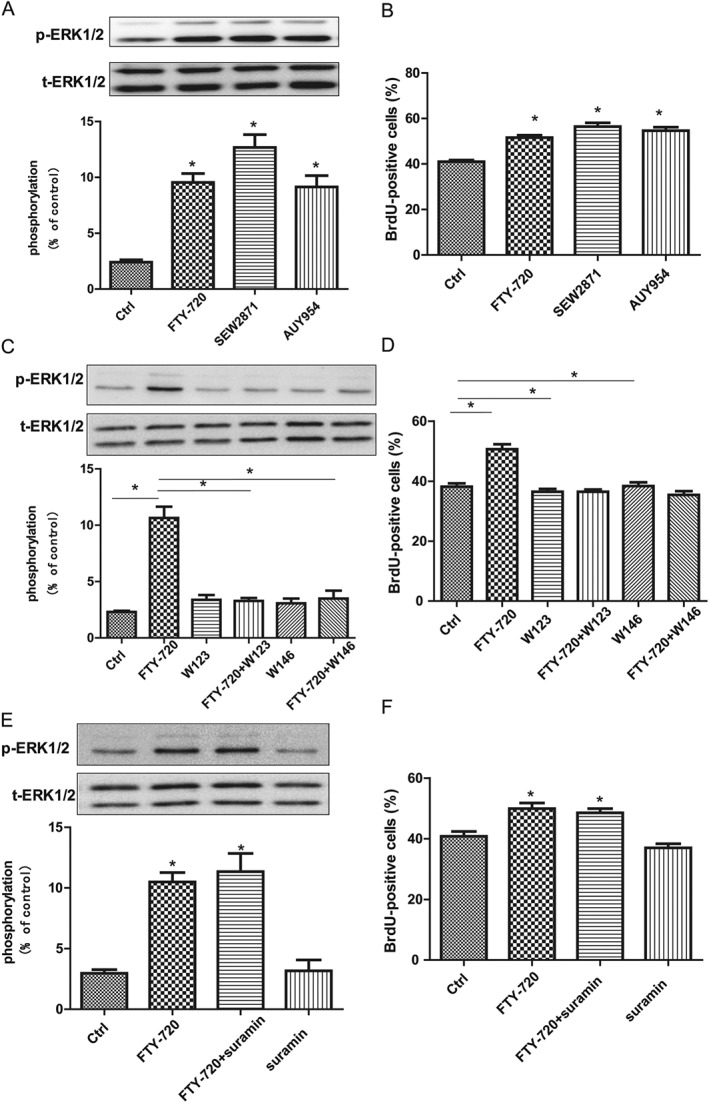
Influences of S1P receptor agonists and antagonists on the effects of 100 nM of FTY‐720 on cultured embryonic hippocampal NSCs. (A) Western blot analysis showed SEW2871 (5 μM) and GNF‐AC‐1 (1 μM) significantly induced the phosphorylation of p‐ERK1/2 at 15 min after its application like FTY‐720 did. (B) Data analysis of BrdU incorporation assay showed that S1P1 receptor agonists SEW2871 (5 μM) and GNF‐AC‐1 (1 μM) significantly promoted the proliferation of NSCs like FTY‐720 did. (C) Western blot analysis showed W123 (5 μM) and W146 (5 μM) blocked FTY‐720‐mediated increase in p‐ERK1/2 phosphorylation. (D) Data analysis of BrdU incorporation assay showed that S1P1 receptor antagonists W123 (5 μM) and W146 (5 μM) blocked FTY‐720‐induced proliferation of NSCs. (E) Western blot analysis showed suramin (1 μM) did not block FTY‐720‐mediated increase in p‐ERK1/2 phosphorylation. (F) Data analysis of BrdU incorporation assay showed that S1P3 and S1P5 receptor‐selective antagonist suramin (1 μM) did not block FTY‐720‐induced proliferation of NSCs. Data shown are mean values ± SEM, n = 5 independent experiments/cultures.
FTY‐720 treatment increased the proliferation of hippocampal NSCs in adult C57BL/6 mice
Considering our work with FTY‐720 in vitro and the participation of S1P signalling in adult neurogenesis (Alfonso et al., 2015), we hypothesized that FTY‐720 could enhance adult neurogenesis in vivo. The 8‐week‐old C57BL/6 mice were randomly allocated to three groups, received daily i.p. injections of NS, 2 or 5 mg·kg−1 of FTY‐720 for 7 days and then received BrdU i.p. 24 h after the last FTY‐720 to label the dividing cells. The quantification of BrdU‐stained cells showed that FTY‐720 caused a significant dose‐dependent increase in cell proliferation in adult hippocampal dentate gyrus (F 2,27 = 13.13) (Figure 4B). To verify the results of the BrdU analysis, we used immunohistochemistry to detect the expression of Ki67 – a nuclear protein expressed in active dividing cells, often used as a proliferation marker (Kee et al., 2002). The data were comparable with the results of the BrdU analysis (F 2,27 = 11.66; Figure 4D). These results indicate that FTY‐720 promotes the proliferation of hippocampal dentate gyrus NSCs in adult mice.
Figure 4.
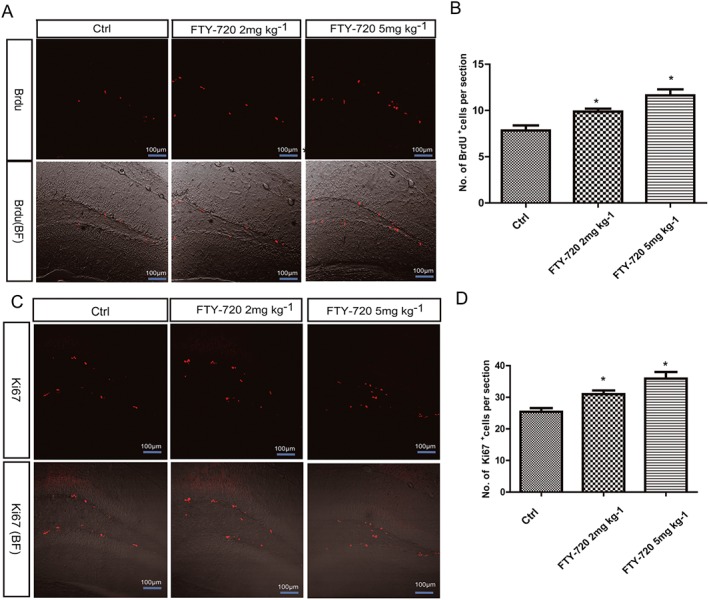
Effects of FTY‐720 treatment on the cell proliferation in the hippocampus dentate gyrus (DG) in adult C57BL/6 mice. (A) Representative microphotographs of the DG showed BrdU‐positive cells in the control and treated animals brain slices. Scale bar = 100 μm. (B) Data analysis of BrdU‐positive cell numbers showed FTY‐720 treatment dose‐dependent significantly promoted the cell proliferation in the DG. (C) Representative microphotographs of the DG showed Ki67‐positive cells in control and treated animals. Scale bar = 100 μm. (D) Data analysis of Ki67‐positive cell numbers showed FTY‐720 treatment dose‐dependent significantly promoted the cell proliferation in the DG. Data shown are mean values ± SEM, n = 10 mice in each group.
FTY‐720 treatment increased the newborn hippocampal neurons in adult C57BL/6 mice
The newborn cells in the hippocampal dentate granules can survive for weeks and stably integrate into the granule cell layer (Kempermann et al., 2003). To determine the final quantity of newly‐generated neurons in the hippocampal dentate gyrus, 8‐week‐old mice were treated with NS or FTY‐720 (2 and 5 mg·kg−1, i.p.) for 7 days. Twenty‐four hours after the last injection of FTY‐720 or NS, the adult mice received three BrdU (50 mg·kg−1, i.p.) injections each at a 12‐h interval and were killed 4 weeks later. We performed the double staining of BrdU and NeuN proteins to examine the newborn neurons. The majority of the BrdU‐labelled newborn cells migrated and dispersed into the dentate granule cell layer and co‐localized with NeuN in the three groups of mice (Figure 5A). The number of BrdU/NeuN double‐positive cells in the dentate gyrus was significantly increased in a dose‐dependent manner by FTY‐720 (Figure 5B) (F 2,27 = 42.78). FTY‐720 treatment also significantly increased the proportion of BrdU/NeuN double‐labelling cells to the total BrdU single‐labelled cells (F 2,27 = 8.683) (Figure 5C), suggesting that FTY‐720 increased the neuronal differentiation of the newborn cells.
Figure 5.
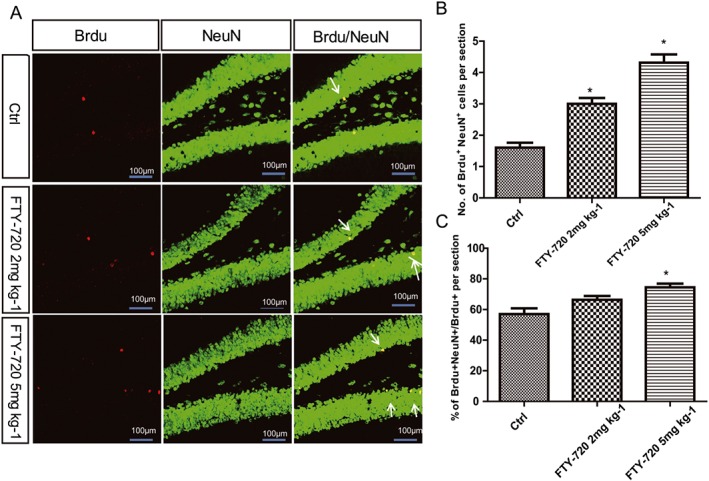
Effects of FTY‐720 on the newborn hippocampal neurons in adult C57BL/6 mice brain. (A) Confocal microscopic images depict double‐staining BrdU (red) and NeuN (green) neurons in the hippocampus dentate gyrus. Scale bar = 100 μm. (B) Data analysis showed FTY‐720 treatment increased the number of BrdU/NeuN double‐stained cells in the dentate gyrus significantly in a dose‐dependent way. (C) Data analysis showed FTY‐720 treatment significantly increased the proportion of cells doubly stained with BrdU and NeuN to the total cells singly stained with BrdU. Data shown are mean values ± SEM, n = 10 mice in each group.
FTY‐720 treatment promoted the learning and memory abilities of adult mice in MWM and FC
The hippocampal formation is the main structure involved in learning and memory (Bailey and Kandel, 1993). Neurons developing in the adult SGZ migrate into the granule cell layer of the dentate gyrus and become dentate granule cells, which are important for learning and memory (Deng et al., 2010). To investigate whether the FTY‐720 treatment can improve the learning and memory abilities, we evaluated the behavioural performance of the mice in the MWM and FC tests. In the MWM, the mice were trained to find the hidden platform in the water‐maze for 5 days with three trials per day, and we analysed the time spent to find the platform. The two‐way repeated‐measures ANOVA (group × day) showed a main effect of day (F 4,285 = 61.27, P < 0.0001) and group (F 2,285 = 4.24, P = 0.0192) on escape latency (Figure 6A), and on the fifth day, the difference in the times the mice spent to find the platform between the control and FTY‐720‐treated groups (5 mg·kg−1) was significant (P < 0.05). In the probe test, the crossing times across the virtual platform of the FTY‐720‐treated mice were significantly increased (F 2,57 = 5.279; Figure 6C). There were no significant differences among the three groups in the time spent in the target quadrant (F 2,57 = 0.9411; Figure 6D), but compared with the control group (F 3,76 = 1.288), the FTY‐720‐treated mice (2 mg·kg−1 F 3,76 = 6, 5 mg·kg−1 F 3,76 = 3.741) spent more time in the target quadrant than in the other three quadrants, showing significant differences (Figure 6E). There were no significant differences in the swimming speeds of the mice from the three groups (Figure 6F). Figure 6B shows a representative image of the swimming trials of the three groups in the probe test. After the MWM tests, we performed another hippocampal‐dependent task, contextual FC. In the contextual FC test, FTY‐720 treatment increased the time of freezing and this was significant at 5 mg·kg−1 (F 2,57 = 6.714) (Figure 6G). These data indicate that FTY‐720 treatment enhanced the hippocampal‐dependent learning and memory abilities in adult mice. As FTY720 has wide effects on the CNS, we investigated another hippocampal‐independent task: cued FC. As shown in Figure 6H, FTY‐720 treatment increased the time of freezing, significantly at 5 mg·kg−1 (F 2,57 = 3.595). Taken together, these data suggest that FTY‐720 promotes the learning and memory abilities of adult mice, both in hippocampal‐dependent and ‐independent tasks.
Figure 6.
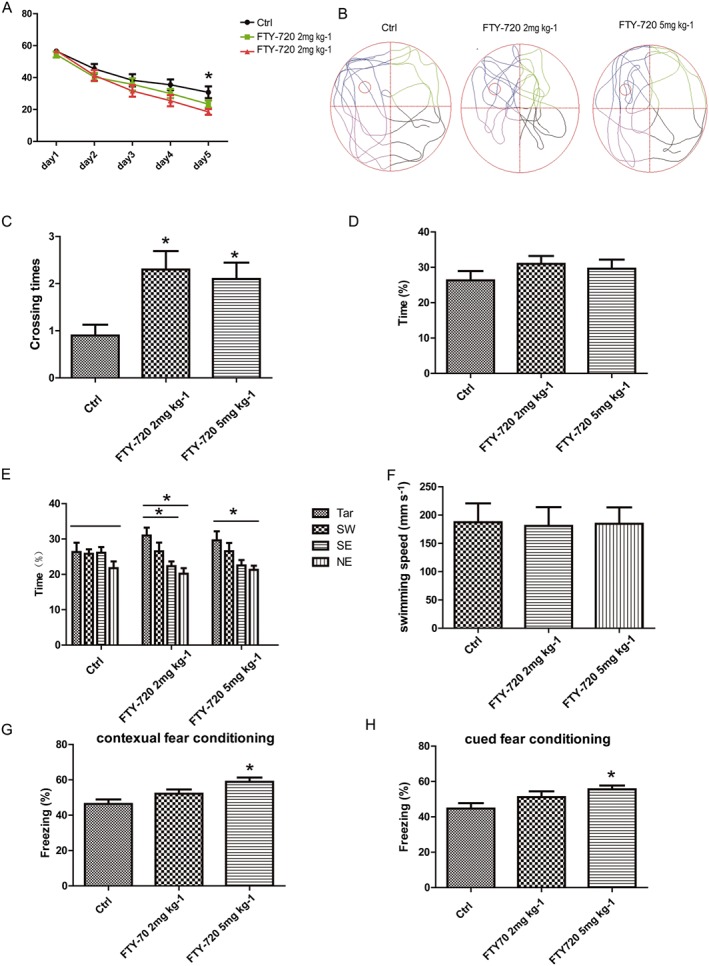
Effects of FTY‐720 treatment on behavioural performance of adult C57BL/6 mice in MWM and FC. Eight‐week‐old mice were treated with NS or FTY‐720 (2 and 5 mg·kg−1, i.p.) for 7 days and were trained in MWM and FC 4 weeks later. (A) The diagram represents the escape latency of mice to find the platform during the training. At the fifth day, the difference in the time spent to find the platform between the control and the FTY‐720‐treated groups (5 mg·kg−1) was significant. (B) Representative image of swimming trials of three groups during the probe day. (C) FTY‐720 treatment significantly increased the crossing times across the target platform during the probe test. (D) In the probe test, there was no significant difference in the time spent in the target quadrant among the three groups. (E) In the probe test, FTY‐720‐treated mice spent more time in the targeted quadrant than the other three quadrants compared with the control group. (F) In the probe test, there was no difference in swimming speeds of mice among the three groups. (G) FTY‐720 treatment (5 mg·kg−1) significantly increased freezing time in the contextual fear conditioning tests. (H) FTY‐720 treatment (5 mg·kg−1) significantly increased freezing time in the cued fear conditioning tests. Data shown are mean values ± SEM, n = 20 mice in each group.
Discussion
In our study, in vitro and in vivo experiments were carried out to demonstrate the possible effects of FTY‐720 on NSCs and adult neurogenesis. We demonstrated that FTY‐720 promoted the proliferation of embryonic hippocampus NSCs without significant effects on their differentiation in vitro. We also proved that the mechanisms involved ERK signalling, Gi/o proteins, and these effects were mediated by S1P1 receptors. During the in vivo experiments, we found that FTY‐720 improved hippocampal neurogenesis and the learning and memory abilities of adult C57BL/6 mice.
Our results confirmed that FTY‐720 promotes the proliferation of NSCs prepared from rat embryonic hippocampus in a dose‐dependent way. These results indicate that in the presence of FTY‐720, a higher proportion of NSCs undergo self‐renewal, resulting in the expansion of the stem cell pool, and this is in agreement with previous findings (Harada et al., 2004). Under our experimental conditions, FTY‐720 had no significant effects on NSCs' differentiation, which is consistent with a very recent research with NSCs from neonatal subventricular zone (Blanc et al., 2015). But the effects of FTY‐720 on NSCs may vary and be dependent on the method used to isolate the NSCs and the expression of S1P receptors in different stem cells, so we cannot exclude the possibility that FTY‐720 affects the differentiation of NSCs under other conditions.
ERK signalling plays important roles in various cell events, including cell proliferation. Previous work has shownthat S1P can stimulate the proliferation of NSCs in a manner sensitive to the MEK inhibitor U0126 (Harada et al., 2004). We found that FTY‐720‐promoted proliferation of NSCs was also dependent on the activation of ERK signalling and probably involved Gi/o proteins. This finding is in agreement with the previous findings that PTX‐sensitive GPCRs mediated multiple cellular responses to S1P and FTY‐720 (Sorensen et al., 2003), and most of the effects of FTY‐720 on the CNS are mediated by ERK signalling (Osinde et al., 2007; Blanc et al., 2015). We further identified the receptor subtype that mediated the effects of FTY‐720 in the present study. FTY‐720P is a known full agonist for S1P1, S1P4 and S1P5 receptors and a partial agonist for S1P3 receptors but has no activity on S1P2 receptors. S1P1, S1P2, S1P3 and S1P5 receptors are expressed in hippocampal NSCs (Harada et al., 2004). Of the different S1P receptor subtypes, S1P1 and S1P5 are preferentially coupled to PTX‐sensitive G proteins such as Gi/o proteins (Windh et al., 1999). As we showed that the effects of FTY‐720 on NSCs depended on the activation of Gi/o protein, we hypothesized that the S1P receptors accounting for the proliferation effects of FTY‐720 were either S1P1 or S1P5. In addition, S1P1 receptors are expressed in the embryonic mouse brain including the hippocampus, and their expression is temporally and spatially coincident with neurogenesis (McGiffert et al., 2002), which strongly suggests that S1P1 receptors might be important during brain development and mediate the proliferation effects of FTY‐720. Moreover, S1P1 receptors have been shown to mediate numerous effects of FTY‐720 in both in vitro and in vivo studies (Mullershausen et al., 2007; Hasegawa et al., 2010; Choi et al., 2011). The direct evidence in our experiment for the involvement of S1P1 receptors came from the selective S1P1 receptor agonists SEW2971 and GNF‐AC‐1 (AUY954), which induced the proliferation and increased ERK1/2 phosphorylation in NSCs. In addition, the two S1P1 receptor antagonists W123 and W146 blocked the effects of FTY‐720 on NSCs. Suramin is a S1P3 and S1P5 receptor dual antagonist and did not affect the effects of FTY‐720 on NSCs. Taken together, we suggest that S1P1 receptors are critical in mediating the effects of FTY‐720 on NSCs.
The hippocampal dentate gyrus is one of the two major neurogenic niches in the adult brain and contains a population of NSCs that are capable of self‐renewal and differentiating into granule cells. Our data showed that FTY‐720 treatment significantly increased cell proliferation in the hippocampal dentate gyrus of adult mice, which consequently resulted in more newborn mature neurons in the granule cell layer. But unlike the results in vitro, FTY‐720 treatment promoted an increase in the final amount of newly‐generated neurons. These different results may be due to the different neurogenic niches and different pharmacology in in vitro and in vivo environments. It remains unclear how FTY‐720 could alter the complex cascade of neurogenesis occurring in vivo. Considering that FTY‐720 plays an important role in angiogenesis, which is a critical component in adult neurogenesis, neurovascularization may be an important participant. Additional studies are needed to further define the mechanisms whereby FTY‐720 promotes adult neurogenesis.
Following the observation that FTY‐720 treatment increased adult neurogenesis in the dentate gyrus, we investigated the functional significance of these FTY‐720‐induced newly generated neurons. Adult hippocampal neurogenesis has been suggested to be involved in memory formation. Adult neurogenesis‐depleted mice showed poor performances in hippocampal‐dependent memory tasks (Deng et al., 2010). We hypothesized that FTY‐720‐induced hippocampal neurogenesis might also be correlated with learning and memory abilities. Our subsequent experiments after 1 month of FTY‐720 treatment, using hippocampal‐dependent learning tasks: MWM and contextual FC, supported this hypothesis. As shown previously, FTY‐720 inhibits HDACs and enhances the histone acetylation and gene expression programmes associated with memory (Hait et al., 2014), so the FTY‐720‐induced learning and memory enhancement may not be merely induced by the up‐regulation of adult neurogenesis. In our experiments, we used the classic MWM test to assess the enhanced adult neurogenesis‐related learning and memory abilities, but this is not always effective (Garthe et al., 2014). Further studies may include ablation strategies (e.g. irradiation) combined with FTY‐720 administration or a refined MWM (Garthe et al., 2009; Garthe and Kempermann, 2013) to define the direct relationship between the FTY‐720‐induced enhancement of adult neurogenesis and increases in learning and memory abilities. In addition, we were interested in finding out whether FTY‐720 had an effect in another, hippocampal‐independent learning task, the cued FC test; FTY‐720 also enhanced the performance in this test. The cued FC is an amygdala‐dependent fear learning task; therefore, the results imply that FTY‐720 can exert a wide effect on behavioural performance tasks, not just on hippocampal‐dependent ones. This is not unexpected considering that FTY720 has been shown to have a wide variety of effects in the CNS including antidepressant effects (di Nuzzo et al., 2015).
In the present study we have demonstrated that FTY‐720 enhances the proliferation of NSCs in vitro and hippocampal adult neurogenesis in vivo. The identification of factors that promote the proliferation and differentiation of NSCs can be used to manipulate and enlarge the NSC pool, and the production of new neurons, and so help to elucidate the mechanisms that maintain neural circuits in the CNS. One important finding of this study is that the activation of S1P1 receptors is required for the observed effects of FTY‐720 on NSCs in vitro. These results offer a new target for manipulating NSCs' proliferation. Adult neurogenesis is critical for hippocampal functions, including specific types of learning and memory, the hippocampal‐dependent stress response and mood regulation (Perera et al., 2007; Lagace et al., 2010; Petrik et al., 2012). It is proposed that dysregulation of neurogenesis contributes to a range of disorders, such as disease‐related or age‐related memory deficits, addiction and depression. A therapeutic strategy for these disorders is to regenerate adult neurogenesis and shift the balance from neurodegeneration to neurogenesis and neuronal plasticity, so compounds/mechanisms that enhance adult neurogenesis are in the spotlight. Environmental enrichment or physiological stimuli such as voluntary running can enhance adult neurogenesis (Kempermann et al., 1998; van Praag et al., 1999); however, such operations may not always be easily manipulated in aged or diseased individuals. Thus, it is important to find pharmacological agents that can enhance neurogenesis. In the present study, we found that FTY‐720 enhanced adult neurogenesis and promoted the learning and memory abilities of adult mice. Compared with other small molecules that have been shown to promote adult neurogenesis (Pieper et al., 2010; Wurdak et al., 2010), FTY‐720 has the advantage of being safe as it has already been approved by the FDA. Very recently, the antidepressant activity of FTY‐720 in mice has been correlated with an enhancement of adult neurogenesis in the hippocampal dentate gyrus (di Nuzzo et al., 2015). Although in this research FTY‐720 treatment did not affect the adult hippocampal neurogenesis in normal mice or their learning and memory abilities, the different aims and experimental designs may have contributed to these discrepancies.
Thus, our studies provide evidence for the stimulating effect of FTY‐720 on the proliferation of NSCs and adult hippocampal neurogenesis. These studies reveal a novel role of S1P1 receptors in NSCs' proliferation and FTY‐720 in adult neurogenesis and may represent a potential therapeutic strategy for stem cell activation in the treatment of brain disorders involving neurogenesis deficits.
Author contributions
L.F. conceived and designed the experiments; Y.S., F.H. and L.Z. performed the experiments; L.F. and Y.S. analysed the data and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by grants from the Scientific Innovation Project of the Chinese Academy of Sciences (XDA 01040304) and National Natural Science Foundation of China (81123004).
Sun, Y. , Hong, F. , Zhang, L. , and Feng, L. (2016) The sphingosine‐1‐phosphate analogue, FTY‐720, promotes the proliferation of embryonic neural stem cells, enhances hippocampal neurogenesis and learning and memory abilities in adult mice. British Journal of Pharmacology, 173: 2793–2807. doi: 10.1111/bph.13557.
References
- Aktas O, Kury P, Kieseier B, Hartung HP (2010). Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol 6: 373–382. [DOI] [PubMed] [Google Scholar]
- Albert R, Hinterding K, Brinkmann V, Guerini D, Muller‐Hartwieg C, Knecht H et al. (2005). Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem 48: 5373–5377. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J, Penkert H, Duman C, Zuccotti A, Monyer H (2015). Downregulation of sphingosine 1‐phosphate receptor 1 promotes the switch from tangential to radial migration in the OB. J Neurosci 35: 13659–13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137: 433–457. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER (1993). Structural changes accompanying memory storage. Annu Rev Physiol 55: 397–426. [DOI] [PubMed] [Google Scholar]
- Blanc CA, Grist JJ, Rosen H, Sears‐Kraxberger I, Steward O, Lane TE (2015). Sphingosine‐1‐phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral‐induced demyelination. Am J Pathol 185: 2819–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K et al. (2011). FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1‐phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Nuzzo L, Orlando R, Tognoli C, Di Pietro P, Bertini G, Miele J et al. (2015). Antidepressant activity of fingolimod in mice. Pharmacol Res Perspect 3: e00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH (2000). Mammalian neural stem cells. Science (New York, NY) 287: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G (2009). Adult‐generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4: e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Huang Z, Kaczmarek L, Filipkowski RK, Kempermann G (2014). Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav 13: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Kempermann G (2013). An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A, Parati EA, Cova L, Frolichsthal P, Galli R, Wanke E et al. (1996). Multipotential stem cells from the adult mouse brain proliferate and self‐renew in response to basic fibroblast growth factor. J Neurosci 16: 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Wise LE, Allegood JC, O'Brien M, Avni D, Reeves TM et al. (2014). Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci 17: 971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Foley M, Moskowitz MA, Waeber C (2004). Sphingosine‐1‐phosphate induces proliferation and morphological changes of neural progenitor cells. J Neurochem 88: 1026–1039. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH (2010). Activation of sphingosine 1‐phosphate receptor‐1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 41: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati F, Dargahi L, Nasoohi S, Omidbakhsh R, Mohamed Z, Chik Z et al. (2013). Neurorestorative effect of FTY720 in a rat model of Alzheimer's disease: comparison with memantine. Behav Brain Res 252: 415–421. [DOI] [PubMed] [Google Scholar]
- Hla T (2004). Physiological and pathological actions of sphingosine 1‐phosphate. Semin Cell Dev Biol 15: 513–520. [DOI] [PubMed] [Google Scholar]
- Jia Z, Geng L, Xie G, Chu Q, Zhang W (2015). Sevoflurane impairs acquisition learning and memory function in transgenic mice model of Alzheimer's disease by induction of hippocampal neuron apoptosis. Int J Clin Exp Med 8: 15490–15497. [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G et al. (2005). Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic‐ and antidepressant‐like effects. J Clin Invest 115: 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002). The utility of Ki‐67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115: 97–105. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Brandon EP, Gage FH (1998). Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol 8: 939–942. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003). Early determination and long‐term persistence of adult‐generated new neurons in the hippocampus of mice. Development 130: 391–399. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J (2003). Non‐proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci 6: 1292–1299. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O et al. (2010). Adult hippocampal neurogenesis is functionally important for stress‐induced social avoidance. Proc Natl Acad Sci U S A 107: 4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learish RD, Bruss MD, Haak‐Frendscho M (2000). Inhibition of mitogen‐activated protein kinase kinase blocks proliferation of neural progenitor cells. Brain Res Dev Brain Res 122: 97–109. [DOI] [PubMed] [Google Scholar]
- McGiffert C, Contos JJ, Friedman B, Chun J (2002). Embryonic brain expression analysis of lysophospholipid receptor genes suggests roles for s1p(1) in neurogenesis and s1p(1‐3) in angiogenesis. FEBS Lett 531: 103–108. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E (2006). Stress and adult neurogenesis. Hippocampus 16: 233–238. [DOI] [PubMed] [Google Scholar]
- Miron VE, Schubart A, Antel JP (2008). Central nervous system‐directed effects of FTY720 (fingolimod). J Neurol Sci 274: 13–17. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL (2005). Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Garcia JA, Luna‐Medina R, Alonso‐Gil S, Sanz‐Sancristobal M, Palomo V, Gil C et al. (2012). Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci 3: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R (1984). Developments of a water‐maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Craveiro LM, Shin Y, Cortes‐Cros M, Bassilana F, Osinde M et al. (2007). Phosphorylated FTY720 promotes astrocyte migration through sphingosine‐1‐phosphate receptors. J Neurochem 102: 1151–1161. [DOI] [PubMed] [Google Scholar]
- Osinde M, Mullershausen F, Dev KK (2007). Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology 52: 1210–1218. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C et al. (2007). Antidepressant‐induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci 27: 4894–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ (2012). The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology 62: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM et al. (2010). Discovery of a proneurogenic, neuroprotective chemical. Cell 142: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM (2011). Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One 6: e15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1996). Clonal and population analyses demonstrate that an EGF‐responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol 175: 1–13. [DOI] [PubMed] [Google Scholar]
- Rutherford C, Childs S, Ohotski J, McGlynn L, Riddick M, MacFarlane S et al. (2013). Regulation of cell survival by sphingosine‐1‐phosphate receptor S1P1 via reciprocal ERK‐dependent suppression of Bim and PI‐3‐kinase/protein kinase C‐mediated upregulation of Mcl‐1. Cell Death Dis 4: e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH (2002). Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci 5: 438–445. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ et al. (2003). Common signaling pathways link activation of murine PAR‐1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol 64: 1199–1209. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Molnar Z (2015). Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266–270. [DOI] [PubMed] [Google Scholar]
- Vargas‐Medrano J, Krishnamachari S, Villanueva E, Godfrey WH, Lou H, Chinnasamy R et al. (2014). Novel FTY720‐based compounds stimulate neurotrophin expression and phosphatase activity in dopaminergic cells. ACS Med Chem Lett 5: 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang F, Yang YJ, Hu ZL, Long LH, Fu H et al. (2011). The flavonoid baicalein promotes NMDA receptor‐dependent long‐term potentiation and enhances memory. Br J Pharmacol 162: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR (1999). Differential coupling of the sphingosine 1‐phosphate receptors Edg‐1, Edg‐3, and H218/Edg‐5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem 274: 27351–27358. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Min KH, Aimone L, Lairson LL, Watson J et al. (2010). A small molecule accelerates neuronal differentiation in the adult rat. Proc Natl Acad Sci U S A 107: 16542–16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Bao X, Yu J, Chen W, Wang L, Zhang Z et al. (2015). Disruption and inactivation of the PP2A complex promotes the proliferation and angiogenesis of hemangioma endothelial cells through activating AKT and ERK. Oncotarget 6: 25660–25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook JS, Okamoto M, Rakwal R, Shibato J, Lee MC, Matsui T et al. (2015). Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol Nutr Food Res 60: 589–599. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang ZG, Li Y, Ding X, Shang X, Lu M et al. (2015). Fingolimod treatment promotes proliferation and differentiation of oligodendrocyte progenitor cells in mice with experimental autoimmune encephalomyelitis. Neurobiol Dis 76: 57–66. [DOI] [PubMed] [Google Scholar]


