Abstract
Background and Purpose
New therapies for inflammatory bowel disease (IBD) are highly desirable. As apolipoprotein (apo)A‐I mimetic peptides are beneficial in several animal models of inflammation, we hypothesized that they might be effective at inhibiting murine colitis.
Experimental Approach
Daily injections of 5A peptide, a synthetic bihelical apoA‐I mimetic dissolved in PBS, or PBS alone were administered to C57BL/6 mice fed 3% (w v‐1) dextran sodium sulfate (DSS) in drinking water or healthy controls.
Key Results
Daily treatment with 5A peptide potently restricted DSS‐induced inflammation, as indicated by improved disease activity indices and colon histology, as well as decreased intestinal tissue myeloperoxidase levels and plasma TNFα and IL‐6 concentrations. Additionally, plasma levels of monocyte chemoattractant protein‐1 and the monocyte expression of adhesion‐mediating molecule CD11b were down‐regulated, pro‐inflammatory CD11b+/Ly6chigh monocytes were decreased, and the number of intestinal monocytes was reduced in 5A peptide‐treated animals as determined by intravital macrophage‐related peptide‐8/14‐directed fluorescence‐mediated tomography and post‐mortem immunhistochemical F4/80 staining. Intravital fluorescence microscopy of colonic microvasculature demonstrated inhibitory effects of 5A peptide on leukocyte adhesion accompanied by reduced plasma levels of the soluble adhesion molecule sICAM‐1. In vitro 5A peptide reduced monocyte adhesion and transmigration in TNFα‐stimulated monolayers of human intestinal microvascular endothelial cells. Increased susceptibility to DSS‐induced inflammation was noted in apoA‐I−/− mice.
Conclusions and Implications
The 5A peptide is effective at ameliorating murine colitis by preventing intestinal monocyte infiltration and activation. These findings point to apoA‐I mimetics as a potential treatment approach for IBD.
Abbreviations
- BCECF‐AM
2'7'‐bis‐(2‐carboxyethyl)‐5 carboxyfluorescein acetoxymethylester
- DSS
dextran sodium sulfate
- HIMECs
human intestinal microvasculature endothelial cells
- IBD
inflammatory bowel diseases
- MCP‐1
monocyte chemoattractant protein‐1
- MPO
myeloperoxidase
- sICAM‐1
soluble inter‐cellular adhesion molecule 1
Tables of Links
| LIGANDS | |
|---|---|
| 5A Peptide | IL‐6 |
| ICAM‐1 | MCP‐1 (CCL2) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
The incidence of inflammatory bowel diseases (IBD) is escalating in the industrialized world with current prevalence rates for Crohn's disease and ulcerative colitis exceeding 200 – 250/100.000 (Burisch et al., 2013). As IBD pathogenesis is still incompletely understood, the majority of patients are treated with unspecific immunosuppressive medications, which are often limited by severe side effects, and up to 30% of all affected individuals need to undergo surgery at least once during the course of disease (Annese et al., 2016). Thus, there is an urgent need for novel anti‐inflammatory therapeutic strategies in IBD that would induce and/or maintain long‐term remission.
HDL is a macromolecular complex in plasma formed by structural proteins such as apolipoprotein (apo)A‐I and lipids that protects against atherosclerosis and cardiovascular disease by mediating reverse cholesterol transport – a process, in which HDL shuttles cholesterol from its deposits in the arterial wall to the liver for excretion (Yvan‐Charvet et al., 2010). In addition, both HDL and apoA‐I exhibit multiple anti‐inflammatory properties, which arise from a direct interaction between HDL and various cell types (Yvan‐Charvet et al., 2010; Navab et al., 2011). For instance, HDL interrupts pro‐inflammatory signal transduction cascades involving sphingosine kinase and ERK activation, inhibits NF‐κB signalling and reduces endothelial expression of inflammatory adhesion molecules thereby preventing monocyte recruitment to sites of inflammation (Saemann et al., 2010). Moreover, both HDL and apoA‐I attenuate the T‐cell contact‐activation of macrophages and reduce the accompanying release of pro‐inflammatory cytokines and chemokines (Norata et al., 2012). Clinical studies have revealed increased IL‐6 levels as well as activated monocyte phenotype in subjects with low‐HDL concentrations (Sarov‐Blat et al., 2007; Zuliani et al., 2007). Conversely, administration of reconstituted HDL particles containing apoA‐I to patients with peripheral vascular disease or type II diabetes reduced monocyte activation and decreased plasma levels of soluble inflammatory markers (Patel et al., 2009; Murphy et al., 2011).
To better exploit the anti‐inflammatory properties of HDL and apoA‐I, apoA‐I mimetic peptides have been developed, which do not possess sequence homology to any of the 10 individual helices of apoA‐I, but exhibit similar structural properties with respect to the alternate distribution of charged, hydrophilic and hydrophobic amino acids (Anantharamaiah et al., 2007). Similar to the full‐length protein, apoA‐I mimetic peptides were shown to promote reversed cholesterol transport and to elevate HDL concentrations in plasma and thereby reduce vascular lesion formation in animal models of atherosclerosis (White et al., 2014). In addition, apoA‐I mimetic peptides have proved effective in a wide range of inflammatory conditions in experimental animals including murine models of lupus erythematosus, systemic sclerosis, house dust mite‐induced asthma, influenza A virus infection and sepsis (Van Lenten et al., 2004; Weihrauch et al., 2007; Zhang et al., 2009; Woo et al., 2010; Nandedkar et al., 2011; Yao et al., 2011).
Given the crucial role of monocyte activation and infiltration into intestine mucosa in the pathogenesis of IBD, we hypothesized that apoA‐I mimetic peptides might be effective as a therapeutic approach for colitis. In this study, we showed that administration of 5A peptide, which is a synthetic bihelical apoA‐I mimetic peptide exerting potent anti‐inflammatory effects in vivo and in vitro (Tabet et al., 2010; Yao et al., 2011), attenuates the key manifestations of colitis and limits monocyte infiltration into the inflamed area in the dextran sodium sulfate (DSS)‐induced model of colitis in mice.
Methods
Animals and colitis induction
Female wild‐type C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). Female apoA‐I‐deficient mice (strain B6.129P2‐Apoa1 tm1Unc/J) were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Mice were group‐housed (max. five per cage) at the animal facility of the University Hospital of Münster in a temperature‐controlled room at 22–24°C with 12 h light/dark cycle under pathogen‐free conditions. The mice had free access to a standard rodent chow diet and tap water until reaching the desired weight (20–25 g at 6–9 weeks of age). Assessment of gut microbiota revealed comparable microbial composition in murine strains used in this study (Supporting Information Fig. S1). DSS‐induced colitis represents a standardized model to study inflammatory processes in IBD and for preclinical testing of therapeutic substances, especially in terms of the involvement of the innate immune system (Kawada et al., 2007). The induction of DSS colitis was as has been described elsewhere (Wirtz et al., 2007). Briefly, mice were administered 3% (w v‐1) DSS (MW ~40 000) in drinking water for up to 5 days (days 0 to 5 of the experiment). Non‐colitic control mice received drinking water without DSS. In parallel, mice were injected i.p. with 5A peptide (50.0 μg day−1) dissolved in 200 μL sterile PBS or PBS only. Peptide or PBS was administered throughout the entire experiment from day 0 to euthanasia of mice. All injections were performed at the same time point of each experimental day.
Animals were monitored daily throughout the entire experiment. Mice that lost greater than 20% initial weight or that became moribund (persistently hunched posture, decreased movement, labored breathing, markedly erect coat) were removed from the study and were killed. Animals were killed by CO2 asphyxia, followed by rapid cervical dislocation. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015) and were approved by the Landesamt für Natur, Umwelt und Verbraucherschutz (LANUV; permit 8.87‐50.10.36.08.304) Nordrhein‐Westfalen according to the German Animal Protection Law (Tierschutzgesetz).
Assessment of colitis
The course of inflammation was monitored by daily assessment of weight loss and presence of blood in the stools using a guaiac paper test. At the end of the treatment, animals were killed, and colons were removed and measured. Afterwards, each colon was opened longitudinally, embedded as ‘Swiss rolls’ in optimum cutting temperature formulation (O.C.T. compound) and frozen at −80°C. For histological analysis, cryostat sections from the proximal, medial and distal colon were picked up, stained with haematoxylin and eosin and graded in a blinded fashion using a colitis score as previously described by Dieleman et al. (1998). Briefly, all sections were graded within a range from zero to three with respect to the amount of inflammation (none, slight, moderate, severe) and the extent of injury (none, mucosal, mucosal and submucosal, transmural) and within a range from zero to four with respect to the amount of crypt damage (none, basal 1/3 damaged, basal 2/3 damaged, only surface epithelium intact, entire crypt and epithelium lost). The changes were also quantified as percentage affected by the disease process: (i) 1–25%; (ii) 26–50%; (iii) 51–75%; (4) 76–100%. Each section was then scored separately by calculating the product of the grade and the percentage involved, and all numbers were summed. A mean score was then calculated from proximal, medial and distal sections that were analysed per mouse. Immunohistochemistry was performed as described previously (Kucharzik et al., 2005). Briefly, acetone fixed and frozen sections (4.0 μm) were blocked in 5.0% rat serum and incubated overnight at 4°C with diluted biotinylated primary antibodies (rat‐anti‐mouse F4/80 – a glycoprotein expressed by murine macrophages – or rat‐anti‐mouse Gr‐1 – granulocyte (myeloid)‐differentiation antigen Gr‐1 (Ly6G) – , both 1:500 (v v‐1)). Sections were washed three times in PBS and incubated with streptavidin‐FITC (for F4/80) or streptavidin‐Alexa (for Gr‐1, both 1:100 (v v‐1) in PBS/BSA (0.1% w v‐1) for 1 h at room temperature. Sections were then washed, and contrast staining was performed using DAPI (1:1000 v v‐1). Fluorescence images were acquired using a Zeiss LSM510 confocal microscope. A number of F4/80‐ or Gr‐1‐positive cells were counted per crypt/per view in three colon sections.
Tissue myeloperoxidase assay
For measurements of myeloperoxidase (MPO) levels, colon samples were rinsed with PBS, blotted dry and snap‐frozen for further use. Thawed samples were weighed, homogenized in ready to use sample buffer, sonicated and centrifuged (200× g, 10 min, 4°C).
MPO levels in the homogenate were measured using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions.
Plasma levels of cytokines and adhesion molecules
Heparin‐treated plasma samples collected from mice were assayed for IL‐6, TNFα, monocyte chemoattractant protein‐1 (MCP‐1) and soluble inter‐cellular adhesion molecule 1 (sICAM‐1) using commercially available ELISA kits according to the manufacturers' instructions.
Alarmin S100A8/S100A9 (myeloid‐related protein‐8/14) fluorescence‐mediated tomography
A polyclonal antibody, targeted for murine alarmin S100A9 (myeloid‐related protein‐14), was generated in rabbits and labelled with Cy5.5‐NHS‐ester for optical imaging approaches (Vogl et al., 2014). In brief, purified antibody was dissolved in NaHCO3 buffer (0.1 mmol·L−1, pH 8.3) and incubated with Cy5.5‐NHS‐ester for 1 h. The labelled antibody was purified from unbound precursors by chromatography and resolved in PBS for in vivo application. Colitic mice received the labelled antibody in an amount corresponding to 2.0 nmol Cy5.5 (80 μL antibody solution) i.v. via the tail vein 24 h prior to in vivo imaging. All in vivo imaging experiments were performed using a VisEN 2500 Beta Flurescence‐mediated Tomography (FMT) device (VisEn Medical, Woburn, MAS, USA) for small animal fluorescence imaging. FMT allows for three‐dimensional quantitative fluorescence mapping of dye distribution in vivo. The device was equipped with a Cy5.5‐adapted filter set for excitation and emission. Animals were held under isoflurane inhalation anaesthesia for the time of examination (about 10 min for complete scan) to prevent movement artefacts and additional stress. The imaging data were screened for fluorescence uptake in the abdominal region, and the fluorescence signal was recorded and quantified for the whole abdominal region as well as for distinct segments of the bowel by manual placing of regions of interest (ROI) on the three‐dimensional maps. Data were documented as total amounts of fluorescence signal in the ROI.
Intravital fluorescence microscopy
The surgical preparation, intravital fluorescence microscopy and video analysis were performed as have been described in detail elsewhere (Vowinkel et al., 2004). Briefly, mice were anaesthetized using ketamine hydrochloride (150 mg·kg−1 i.p.) and xylazine (7.5 mg·kg−1 i.p.), and the right jugular vein was cannulated for the infusion of leukocyte labelling dye. Leukocytes were labelled in vivo with 100 μL of rhodamine 6G [0.1 mL rhodamine 6G (20 mg·mL−1) dissolved in 5 mL water] 5 min prior to the microscopy experiments. After laparotomy, animals were placed on the right side, and the proximal large bowel was exteriorized and superfused at 37°C with bicarbonate‐buffered saline solution (pH 7.4). Leukocytes were observed under an inverted microscope (Nikon, Tokyo, Japan) equipped with a 75‐W XBO xenon lamp. The microscopic images were received by a charge‐coupled device video camera (C2400; Hamamatsu Photonics, Hamamatsu, Japan). Five randomly selected postcapillary venules (20–40 μm diameter) in each colon preparation were recorded for 1 min each. Leukocytes were classified according to their interaction with the venular wall as either free flowing or adherent (when cells remained stationary for 30 s or more), and the adherence was expressed as cell number·mm‐2 venular surface.
Static adhesion and transmigration assays
Human intestinal microvasculature endothelial cells (HIMECs) were isolated from surgical specimens obtained from the small intestine or colon as described previously (Heidemann et al., 2007) and cultured in growth medium (Endothelial Cell Growth Medium MV) containing FBS (10% v v‐1). Experiments were performed on cultures between passages 8 and 14 to prevent replicative senescence. For static endothelial‐monocyte adhesion assays, U937 cells (a human monocyte‐like cell line) were labelled by incubating with 2'7'‐bis‐(2‐carboxyethyl)‐5(6) carboxyfluorescein acetoxymethylester (BCECF‐AM) (10.0 μmol·L−1) for 30 min at 37°. Labelled monocytes (2 × 106·mL−1) were added to HIMEC monolayers grown in 96‐well tissue culture dishes and prestimulated for 20 h with TNFα (10 ng·mL−1) in the presence or absence of 5A peptide. After co‐incubation for 30 min, non‐adherent cells were rinsed off with PBS, and plates were centrifuged (5 min, 500× g) and air dried. Fluorescence intensity was measured using a Fluostar (BGM Labtech, Ortenburg, Germany) fluorescence plate reader.
For endothelial transmigration assays, HIMECs grown to confluence on collagen‐coated Transwell® polycarbonate filter inserts (pore size 5 μm) were stimulated with TNFα with or without 5A peptide as indicated. Calcein‐AM‐labelled U937 cells (5 × 106) were added on top and allowed to migrate for 4 h at 37°C. Transmigrated cells (lower well) and cells remaining in the upper well were quantified by fluorescence readings (Heidemann et al., 2007). Equal volumes and counting intervals were applied. Each condition was assessed in triplicate.
Preparation of leukocytes and flow cytometry
Blood was obtained from anaesthetized animals by retroorbital puncture, and leukocytes were isolated using Biocoll according to the manufacturer's instructions. Cells (1 × 106·mL−1) were incubated in FACS buffer with anti‐CD11b or anti‐Ly6C antibodies (1:100 v v‐1 each) for 30 min at 4°C. Cells were analysed by flow cytometry (FACSCalibur, BD Biosciences GmbH, Heidelberg, Germany).
Experimental design and statistical analysis
Group sizes
All experiments were performed in control and treated groups of five animals. Repetitive experiments were performed to confirm the effects observed, or if not, all measurements could be performed in the same animals. Data were pooled for statistical analysis, where applicable. The number of animals included in the statistical analysis for each parameter is specified in each graph or the respective figure legend. Variations in group sizes occurred, if mice had to be excluded from the experiment due to ethical reasons (meeting predefined humane endpoints as described above). An initial control experiment evaluating the effect of the 5A peptide in non‐colitic mice (receiving no DSS) was performed with animals receiving 5A peptide (50.0 μg day−1, i.p.) dissolved in sterile PBS or PBS alone (n = 5 per group). As in non‐colitic mice, the peptide showed no effect on clinical indices (weight, faecal blood excretion), distribution of peripheral blood monocytes and post‐mortem measurements (histology, colon length, mucosal neutrophil and monocyte infiltration); the respective controls were not repeated for all parameters determined in the colitis group for animal ethics reasons.
Randomization and blinding
Mice were randomly assigned to treatment and control groups. Experiments were performed in a blinded set‐up with the investigator being unaware of the respective treatment both during the actual animal experiment and the post hoc analyses including biochemical assays and histological assessment.
Data and statistical analysis
Data were analysed using one‐way or two‐way ANOVA. Homogeneity of variances was tested using the Levene test. Post hoc comparisons were conducted with Student–Newman–Keuls (S.N.K) test, if F achieved P < 0.05 and there was no significant variance in homogeneity. In case of a significant variance inhomogeneity (P < 0.05 in the Levene test), Welch's test was used followed by Games‐Howell post hoc test for multiple comparisons, if F achieved a P < 0.05. In cases of non‐parametric data distribution, the Kruskal–Wallis test was performed. Pairwise comparisons between two groups were performed using Mann–Whitney rank sum test. A P‐value of <0.05 was considered statistically significant. The computer programs spss version 23 (IBM, Armonk, NY, USA) and sigmaplot version 11 (Systat Software Inc., San Jose, CA, USA) were used for statistical analyses. Values are shown as mean ± SEM. Data normalization was not performed. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Materials
The apoA‐I mimetic 5A peptide (DWLKAFYDKVAEKLKEAF‐PDWAKAAYDKAAEKAKEAA) has been designed by Remaley and colleagues, synthesized by a solid‐phase procedure using Fmoc/DIC/HOBt chemistry, purified to greater than 99% by reverse‐phase HPLC and solubilized exactly as described previously (Sethi et al., 2008). DSS was from ICN Biomedicals Inc. (Eschwege, Germany). Faecal occult blood guaiac paper test was from Roche Diagnostics (Mannheim, Germany). O.C.T. compound was from Tissue‐Tek® (Sakura Finetek, Zoeterwoude, The Netherlands). Rat‐anti‐mouse anti‐F4/80 antibody and rat‐anti‐mouse anti‐Gr‐1 antibodies as well as FITC‐conjugated anti‐Ly6C rat‐anti‐mouse antibody and streptavidin‐FITC were from BD Pharmingen (Heidelberg, Germany). Streptavidin‐Alexa565 was purchased from Molecular Probes (Darmstadt, Germany). Anti‐CD11b rat‐anti‐mouse antibody was from Caltec (San Francisco, CA, USA). Biocoll was from Biochrom (Berlin, Germany). DAPI and rhodamine were obtained from Sigma‐Aldrich, (Deisenhoffen, Germany). Calcein‐AM and BCECF‐AM were from Molecular Probes (Eugene, OR, USA). ELISA kits for determination of IL‐6, TNFα, MCP‐1 and sICAM‐1 were from R&D Systems (Wiesbaden, Germany). The ELISA kit for determination of MPO was from Immundiagnostik AG (Bensheim, Germany). Cy5.5‐NHS‐ester was from GE Healthcare (Piscataway, NJ, USA). HIMEC growth medium (Endothelial Cell Growth Medium MV) was obtained from PromoCell (Heidelberg, Germany). Bicoll was from Biochrom AG (Berlin, Germany) and FACS buffer from BD Pharmingen (Heidelberg, Germany). All other chemicals were from Sigma and were of the highest purity available.
Results
5A peptide ameliorates clinical course and improves histology in DSS‐induced colitis in mice
DSS‐induced colitis resembles human ulcerative colitis with respect to weight loss, rectal bleeding, superficial ulceration and mucosal damage and proved useful for examining mechanisms underlying the inflammatory pathology associated with IBD. Therefore, DSS‐treated animals and control animals were administered 5A peptide in a dose of 50 μg per animal day−1, a dose that had previously been shown to exert beneficial effects in house dust mite‐induced asthma (Yao et al., 2011) and the efficacy of which has been confirmed in a preliminary dose‐finding study (Supporting Information Fig. S2.). Decreasing body weight and faecal occult blood were used as clinical indices of inflammation. As shown in Figure 1A, application of DSS led to initial decrease in body weight of all animals tested beginning on day 4, while control animals receiving no DSS (with or without 5A peptide) showed no significant weight loss. Whereas weight loss was most prominent at day 7 and body weight recovered thereafter in mice treated with 5A peptide, mice injected with PBS alone continued to lose weight. In addition, colitic animals treated with 5A peptide showed reduced daily excretion of faecal occult blood (Figure 1B). Colonic shortening is considered a macroscopic indicator of inflammation and inflammation‐induced oedema in the course of colitis. As shown in Figure 1C, post‐mortem measurements of colon length revealed significantly less shortened colon in colitic mice treated with 5A peptide as compared with mice injected with PBS alone.
Figure 1.
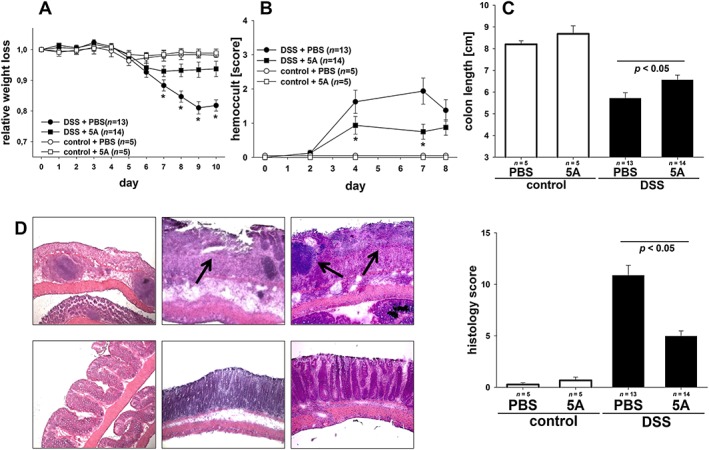
Effect of 5A peptide on clinical parameters and histological injury in the course of DSS‐induced colitis in mice. C57BL/6 mice were given DSS (3% w v‐1) in drinking water for 5 days. Control mice were given drinking water without DSS. The 5A peptide (0.25 mg·mL−1) or PBS was administered daily (days 0 to 10) by i.p. injection. Animals were killed at day 10. Numbers of animals analysed are provided in graphs. The data shown are pooled from three independent experiments in the colitis group and one experiment in the control group. Data were analysed using one‐ or two‐way ANOVA followed by Student–Neuman–Keul or Welch's test followed by Games‐Howell post hoc test. (A) Changes in body weight are presented relative to the initial weight. (B) Blood excretion in faeces as determined by guaiac paper test (haemoccult, 0 = negative, 4 = blood macroscopically). (C) Post‐mortem colon length. (D) Histological injury as assessed by degree of crypt damage and extent of inflammation. Shown are representative histological images [haematoxylin/eosin staining; magnifications ×10 (left panel), ×20 (middle panel) and ×40 (right panel) of colitic mice treated with 5A peptide (lower panels) or PBS alone (upper panels)]. Note the profound destruction of epithelial architecture and inflammatory infiltrates (arrowheads). Bar graph: injury score was determined as described in Methods.
To directly assess large bowel damage in the course of colitis, post‐mortem blinded histological analysis of the colon was performed on day 10 after DSS application. Figure 1D (upper panels) demonstrates inflammatory changes typical for DSS‐induced colitis in mice injected with PBS alone that were mainly confined to the mucosa and characterized by loss of goblet cells, crypt damage, mucosal ulceration and accompanying submucosal oedema. Compared with colitic animals, colons of 5A peptide‐treated mice presented with attenuated morphological damage with largely preserved epithelial architecture and nearly intact intestinal crypts (Figure 1D, lower panels). Quantitative assessment of histological damage using an injury score based on the degree and extent of inflammation, crypt damage and percent involvement revealed that severity of DSS‐induced colitis was substantially attenuated in 5A peptide‐treated in comparison with PBS‐treated colitic mice (Figure 1D, bar graph). Control mice receiving no DSS (with or without 5A peptide) showed no histological damage.
5A peptide ameliorates inflammation in DSS‐induced colitis in mice
As DSS‐induced colitis is associated with infiltration of immune cells into mucosa and submucosa combined with elevated production of pro‐inflammatory cytokines and chemokines, we next attempted to assess the effect of 5A peptide on inflammatory response in DSS‐treated mice. Immunochemical staining for the neutrophil marker Gr‐1 was employed to quantify leukocyte infiltrates in the mucosa. As demonstrated in Figure 2A, colitic animals treated with 5A peptide showed a significantly reduced presence of neutrophils within inflamed crypts on day 10 after DSS application as compared with mice injected with PBS alone while non‐colitic mice (treated or not with 5A peptide) showed no significant neutrophil infiltration. To additionally confirm this effect, colonic MPO levels were determined, which reflect both neutrophil number and inflammatory activity. Figure 2B shows significantly decreased MPO levels in the colon tissue obtained from colitic mice treated with 5A peptide versus animals receiving PBS injections. Finally, plasma TNFα and IL‐6 levels – two major pro‐inflammatory cytokines – were determined to additionally assess the inflammatory response in colitic mice. As shown in Figure 2C and D, similar low concentrations of both cytokines were observed at the end of experiment in control mice irrespective of the treatment with 5A peptide. Colitic mice showed a notable increase in IL‐6. However, IL‐6 levels were significantly reduced in 5A peptide‐treated mice as compared with colitic mice treated with PBS alone (Figure 2C). Similarly, plasma levels of TNFα were significantly elevated in colitic mice treated with PBS alone, while they were decreased to control levels in colitic mice treated with 5A peptide (Figure 2D). Cytokine plasma levels were not significantly different on days 0 and 4 of the experiment in the colitis group (data not shown).
Figure 2.
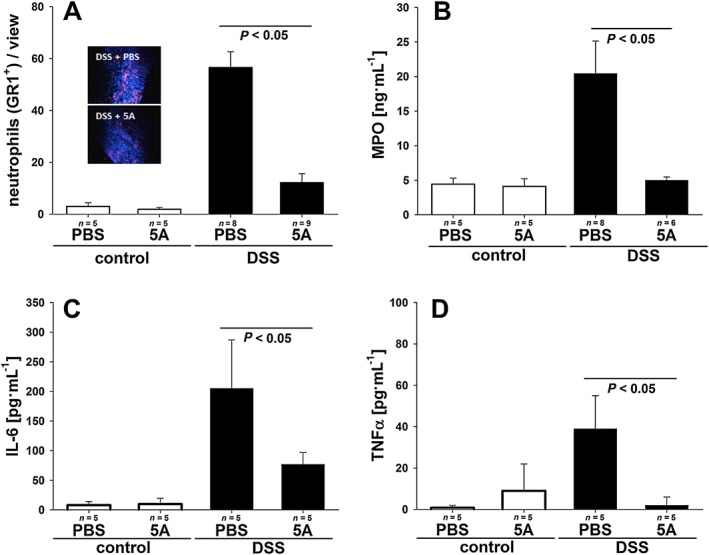
Effect of 5A peptide on intestinal inflammation in DSS‐induced colitis in mice. C57BL/6 on DSS (3% w v‐1) or control mice receiving drinking water without DSS were administered, daily, 5A peptide (0.25 mg·mL−1) or PBS by i.p. injection for 10 days. Numbers of animals analysed are provided in graphs. The data shown are pooled from two independent experiments in the colitis group and one experiment in the control group. Data were analysed using one‐way ANOVA followed by Student–Neuman–Keul or Welch's test followed by Games‐Howell post hoc test or Kruskal–Wallis test. (A) Immunohistochemical visualization of mucosa neutrophil infiltrations. Shown are representative images of anti‐Gr‐1 staining in colitic animals. Bar graph: cell count/view was determined as described in Methods. (B) MPO concentration in colonic tissue of 5A peptide‐ or PBS‐treated mice. (C and D) Levels of pro‐inflammatory cytokines IL‐6 (C) and TNFα (D) as determined in plasma from 5A peptide‐ or PBS‐treated mice.
5A peptide suppresses monocyte recruitment into inflamed mucosa in DSS‐induced colitis in mice
As monocyte recruitment into mucosa and submucosa represents a critical step preceding development of full‐fledged colitic inflammation, we next quantified the monocytic infiltrate in the inflamed intestine as compared with non‐colitic control mice employing immunochemical staining for the monocyte/macrophage marker F4/80. As demonstrated in Figure 3A, colitic animals treated with 5A peptide showed a significantly reduced presence of monocytes/macrophages within inflamed crypts on day 10 after DSS application as compared with mice injected with PBS alone. Non‐colitic mice showed no relevant macrophage infiltration regardless of the 5A peptide treatment. To further examine monocyte recruitment into the mucosa, fluorescence tomography was applied, which utilizes a fluorescence‐labelled antibody against murine alarmin S100A8/S100A9 and allows direct visualization of activated macrophages in living animals. We have recently demonstrated that alarmin S100A8/S100A9 serves as a sensitive local and systemic marker for the detection of even subclinical disease activity in inflammatory processes such as contact dermatitis, collagen‐induced arthritis or experimental leishmaniosis (Vogl et al., 2014). As shown in Figure 3B, colitic mice treated with 5A peptide showed a significantly decreased level of fluorescence comparable with that seen in healthy mice (Vogl et al., 2014) on day 5 after DSS application as compared with PBS‐injected animals indicating early reduction of monocyte penetration and conversion to active macrophages under the influence of 5A peptide treatment. To get further insights into the mechanisms underlying reduced monocyte recruitment into the intestinal mucosa in 5A peptide‐treated mice, we determined th eplasma MCP‐1 concentration – a chemokine critically responsible for the efficient chemoattraction of monocytes to inflamed areas. As shown in Figure 3C, reduced MCP‐1 concentrations in plasma were observed 11 days after the initiation of DSS treatment in mice receiving 5A peptide injections. We next assessed the monocyte surface expression of CD11b, which mediates the endothelial adhesion through an interaction with ICAM‐1 and found that it was reduced in cells obtained from 5A peptide‐treated colitic animals (Figure 3D). Recently, the concept of distinct subpopulations of mouse blood monocytes has been established that differ in their capacity to become recruited to inflammatory sites and to differentiate into pro‐inflammatory macrophages. Therefore, we additionally quantified blood monocytes according to the level of CD11b and Ly6C expressions. As shown in Figure 3E, the induction of colitis by administration of DSS in mice produced a distinct shift towards a higher proportion of inflammatory CD11bhighLy6Chigh monocytes as compared with pre‐experiment conditions, while the total number of monocytes did not differ in both groups (not shown). In contrast, this shift failed to appear in colitic mice treated with 5A peptide.
Figure 3.
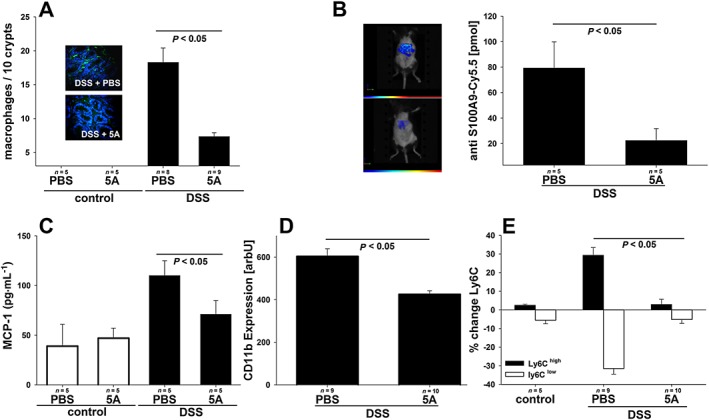
Effect of 5A peptide on monocyte recruitment to intestine mucosa in the course of DSS‐induced colitis in mice. C57BL/6 on DSS (3% w v‐1) and control mice receiving drinking water alone were administered, daily, 5A peptide (0.25 mg·mL−1) or PBS by i.p. injection for 10 days. Numbers of animals analysed are presented in graphs. Data were analysed using one‐way ANOVA followed by Student–Neuman–Keul, Mann–Whitney test or Welch's test followed by Games‐Howell post hoc test. (A) Immunohistochemical visualization of monocyte infiltrations in the mucosa. Representative images of anti‐F4/80 staining in colitic mice are shown. Bar graph: cell count/view was determined as described in Methods. (B) FMT scans in colitic mice administered Cy5.5‐conjugated antibody against murine alarmin S100A8/S100A9. Representative images are shown with colour‐coded fluorescence intensity corresponding to the extent of inflammatory infiltrate. Bar graph: mean fluorescence intensity was determined in ROI, and the amount of bound antibody was calculated as described in Methods. (C) Levels of chemotactic chemokine MCP‐1 were determined in plasma from 5A peptide‐ or PBS‐treated mice. (D and E) Peripheral blood monocytes from colitic mice as well as control mice were analysed by flow cytometry for the expression of CD11b (D) and Ly‐6C on CD11b‐positive cells (E).
5A peptide inhibits monocyte adhesion to endothelial cells and transmigration in DSS‐induced colitis in mice
As inhibition of monocyte recruitment to mucosa probably represents the mechanism accounting for the protective effects of 5A in colitis, we next examined the influence of 5A peptide on monocyte adhesion to endothelial cells and transmigration – two important steps preceding monocyte accumulation at inflammation sites. For this purpose, the microscopic analysis of leukocyte–endothelium interactions in vivo using intravital microscopy and the measurement of soluble adhesion molecule sICAM‐1 in plasma was performed. As shown in Figure 4A, administration of 5A peptide to DSS‐treated mice significantly reduced rolling and adherent leukocytes in postcapillary venules as compared with mice receiving PBS alone. In addition, colitic mice treated with 5A peptide showed significantly lower plasma levels of sICAM‐1 – a molecule mediating interactions between monocytes and endothelial cells (Figure 4B). The effect of 5A peptide on monocyte adhesion was additionally studied in vitro using HIMEC. TNFα stimulation increased the number of U937 monocytes bound to the HIMEC monolayer under static conditions. When HIMEC monolayers were exposed to both TNFα and 5A peptide, the number of adherent cells was significantly decreased. The 5A peptide reduced adhesion in a dose‐dependent manner (Figure 4C). To further examine the effect of 5A peptide on endothelial cells, transendothelial monocyte migration was examined in vitro. Figure 4D demonstrates that TNFα enhanced transendothelial U937 cell migration, as inferred from quantification of transmigrated cells, and this was decreased by 5A peptide.
Figure 4.
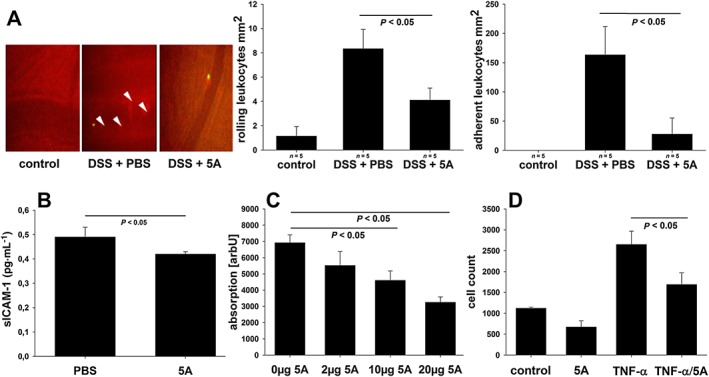
Effect of 5A peptide on leukocyte–endothelium interactions. (A) C57BL/6 mice on DSS (3% w v‐1) were administered, daily, 5A peptide (0.25 mg·mL−1) or PBS by i.p. injection (n = 5). Leukocyte–endothelium interactions were assessed at day 5 using intravital microscopy as described in Methods. Left panel: original snapshots of peritoneal arterioles. Arrowheads indicate fluorescently labelled adherent leukocytes. Bar graphs: number of rolling (middle panel) and firmly adherent (right panel) leukocytes in postcapillary venules of non‐colitic mice and colitic mice treated with PBS or 5A peptide. (B) Levels of soluble adhesion protein ICAM‐1 were determined in plasma from 5A peptide‐ or PBS‐treated mice by ELISA (n = 5). (C) Effect of 5A peptide on adhesion of BCECF‐AM‐labelled U937 monocytes to HIMECs stimulated with TNFα (10 μg·mL−1) was assessed in vitro using a static adhesion assay. The results from two independent experiments are shown, each in pentuplicate. (D) Effect of 5A peptide on transendothelial migration of calcein‐AM‐labelled U937 monocytes across confluent monolayers of HIMEC grown on polycarbonate pore filters and stimulated with TNFα (10 μg·mL−1). The results from two independent experiments are shown, each in triplicate. Data were analysed using one‐way ANOVA followed by Student–Neuman–Keul of Mann–Whitney test.
DSS‐induced colitis is enhanced in apoA‐I−/− mice
To specifically address the potentially protective role of native apoA‐I in colitis, we examined the effect of DSS administration on apoA‐I−/− mice in comparison with wild‐type littermates. As shown in Figure 5A, apoA‐I−/− mice responded to DSS with an altered weight loss course, which was characterized by a greater loss of body weight and a retarded recovery after withdrawal of DSS. In addition, apoA‐I−/− mice showed increased daily excretion of faecal occult blood (Figure 5B). Post‐mortem quantitative histological analysis of the colon revealed a more severe DSS‐induced colitis in apoA‐I−/− mice (Figure 5C).
Figure 5.
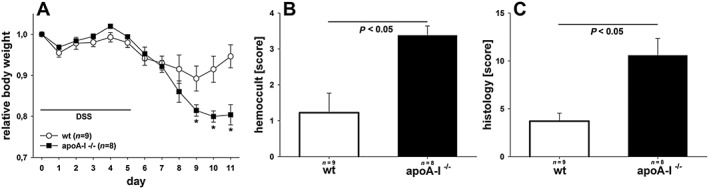
Evaluation of DSS‐induced colitis in apoA‐I−/− mice. C57BL/6 and apoA‐I−/− mice were given DSS (3% w v‐1) in drinking water for 5 days. The results represent pooled data from two independent experiments. Data were analysed using two‐way or one‐way ANOVA followed by Student–Neuman–Keul post hoc test or by Kruskal–Wallis test. (A) Changes in body weight are presented relative to initial weight. (B) Blood excretion in faeces as determined by guaiac paper test (haemoccult, 0 = negative, 4 = blood macroscopically). (C) Histological injury as assessed by degree of crypt damage and extent of inflammation. Histological injury score was determined as described in Methods.
Discussion
Agents with anti‐inflammatory/antioxidant properties hold promise in many chronic conditions including IBD. ApoA‐I mimetic peptides, with a functional profile similar to apoA‐I, have yielded encouraging results in several experimental models of chronic inflammatory diseases and are currently undergoing phase I clinical studies (Weihrauch et al., 2007; Woo et al., 2010; Nandedkar et al., 2011). However, potential salutary effects of apo A‐I mimetics in IBD have not been investigated to date. We, therefore, used apoA‐I mimetic 5A peptide (Tabet et al., 2010; Yao et al., 2011) to assess its effect in DSS‐induced colitis. Although exact processes leading to the development of colitis after DSS exposure are still incompletely understood, this agent is thought to induce a mucosal injury and inflammation through a direct toxic effect on epithelial cells, with subsequent recruitment and activation of inflammatory cells and up‐regulation of inflammatory mediators. In our hands, daily application of 5A peptide significantly ameliorated the course of DSS colitis, as assessed using standard surrogate parameters such as weight loss, colon length or presence of occult blood in faeces. In addition, 5A peptide limited the extent of mucosal injury. Two additional specific indices of inflammation further confirmed the significant anti‐inflammatory effect exerted by 5A peptide in colitis. First, this compound reduced neutrophil accumulation in the inflamed mucosa – a process, which plays a crucial role in DSS‐induced colitis and inhibition of which by administration of anti‐neutrophil serum or blocking the multispecific neutrophil chemokine receptor CXCR2 was found to attenuate colon histological injury (Domek et al., 1995; Farooq et al., 2009). Consistent with this, treatment with 5A peptide decreased the mucosal concentration of MPO, which is liberated in copious amounts by activated neutrophils and which amplifies the inflammatory response by inducing oxidative modification of proteins and lipids. Second, 5A peptide also reduced liberation of pro‐inflammatory cytokines and chemokines including IL‐6, MCP‐1 and TNFα. The latter cytokine represents an important therapeutic target in human Crohn's disease and ulcerative colitis, and TNFα antagonists were reported to reduce inflammatory lesions in DSS‐induced colitis in mice (Kojouharoff et al., 1997; Rutgeerts et al., 2004). Taken together, the present results reveal that 5A peptide exerts protective effects in the murine model of colitis acting as a potent anti‐inflammatory agent.
The 5A peptide is a synthetic apoA‐I mimetic containing two amphipathic α‐helices defined as having opposed polar and non‐polar faces oriented along the long axis of the helix and thereby replicating the secondary structure motive encountered in various protein components of plasma lipoproteins (Anantharamaiah et al., 2007). It is of interest that other apolipoproteins were previously reported to exert beneficial effects in murine models of IBD. For instance, apoA‐IV and COG112 – an apoE‐derived peptide assuming amphipathic helical structure – were both demonstrated to mitigate the severity of clinical disease in the DSS‐ and/or Citrobacter rodentium‐induced colitis as well as in the 5‐fluorouracil‐induced intestinal mucositis and to limit the accompanying inflammatory component, as inferred from the reduced leukocyte infiltration into inflamed areas and the production of pro‐inflammatory cytokines such as TNFα (Vowinkel et al., 2004; Singh et al., 2008; 2011; Azevedo et al., 2012). As 5A peptide, apoA‐IV and COG112 display common secondary structures, but show little or no sequence homology, it may be assumed that anti‐inflammatory effects exerted by these compounds in murine models of colitis are attributable to their amphipathic character rather than to their ability to initiate distinct anti‐inflammatory pathways. Two observations in the present study appear to favour this opinion. Firstly, although dose‐dependent, the effects of the 5A peptide were accomplished at a dose of 2 mg·kg−1 day−1, which is one order of magnitude lower than the 30 mg·kg−1 day−1 dose that was used to promote apoA‐I‐specific reverse cholesterol transport in a murine model of atherosclerosis (Amar et al., 2010). Secondly, while the clinical course of colitis and the histological mucosa injury were markedly aggravated in DSS‐treated apoA‐I‐deficient mice, these animals failed to develop mucosal inflammation spontaneously. As normal plasma contains several amphipathic proteins and peptides (Hortin et al., 2006), a part of which only is derived from apoA‐I, it may be speculated that the amphipathic potential of plasma in apoA‐I‐deficient mice, although reduced, is nevertheless sufficient to secure protection against colitis, albeit at a lower level.
No unequivocal conclusion can be drawn based on the present results as to which mechanism accounts for the protective effects of 5A peptide in DSS‐induced colitis. Previous investigations highlighted the role of endothelial dysfunction and/or disintegration as critical steps preceding leukocyte recruitment to injured areas of intestinal mucosa (Cromer et al., 2011). The present study provides several pieces of evidence pointing to intestinal endothelium as a target of the anti‐inflammatory action of 5A peptide in murine IBD. Administration of this compound to colitic mice significantly reduced plasma concentrations of ICAM‐1, which regulates leukocyte–endothelium interactions in the microcirculation and which is abundantly shed from the surface of dysfunctional endothelial cells. This effect could be separately confirmed in experiments utilizing intravital microscopy, which provided direct evidence for the reduced rolling and adhesion of leukocytes to colonic endothelium in mice receiving 5A peptide. Furthermore, the anti‐inflammatory effects of 5A peptide on endothelium in colitic animals could be replicated under in vitro conditions. Both monocyte adhesion to and transmigration across endothelial cells obtained from intestinal microvasculature (HIMEC) and pre‐activated with TNFα were considerably reduced following incubation with 5A peptide. The present results are in concordance with previous studies showing that penetration of neutrophils and/or monocytes into the wall of large arteries in rodent models of atherosclerosis is attenuated by chronic administration of apoA‐I mimetics (Tabet et al., 2010; Woo et al., 2010). In addition, both 5A peptide and other apoA‐I mimetics were shown to reduce the expression of adhesion molecules including ICAM‐1 in injured arteries of treated animals and to prevent monocyte adhesion to endothelial cells under in vitro conditions (Tabet et al., 2010; Di Bartolo et al., 2011). However, while pointing to the pivotal role of intestinal endothelium in mediating anti‐inflammatory effects of 5A peptide in colitis, the present data do not exclude the possibility that this compound also directly or indirectly affects leukocyte function. Previously, apoA‐I mimetic peptides were shown to directly limit neutrophil activation and to prevent recruitment of neutrophils to the airspace in response to lipopolysaccharide‐ or aluminium hydroxide/ovalbumin‐induced airway inflammation (Dai et al., 2012; Madenspacher et al., 2012; Sharifov et al., 2014). In addition, apoA‐I mimetics ETC‐642 and D‐4F were shown to decrease the expression of CD11b – an ICAM‐1 binding partner – on the surface of monocytes (Smythies et al., 2010; Di Bartolo et al., 2011). The present study has replicated these findings using 5A peptide and extends them to show that diminished CD11b expression on monocytes obtained from treated animals was accompanied by the decreased accumulation of activated (alarmin S100A8/S100A9‐positive) monocytes/macrophages in the intestine and the reduced presence of CD11b+/Ly6chigh monocytes in blood. Macrophage‐derived alarmins were previously found in the inflamed colonic mucosa, in which they provoked up‐regulation of adhesion molecules and chemokines (Foell et al., 2008). CD11b+/Ly6chigh monocytes constitute a subpopulation of aggressive pro‐inflammatory monocytes that home to inflammation sites, give rise to activated macrophages and directly contribute to tissue damage in the course of chronic inflammatory diseases such as atherosclerosis, encephalitis, steatohepatitis or lupus nephritis (Sunderkotter et al., 2004; Swirski et al., 2007; Getts et al., 2008; Deng et al., 2009; Bethunaickan et al., 2011). In both DSS‐ and T‐cell transfer‐induced colitis CD11b+/Ly6chigh monocytes were found to massively infiltrate the small intestine lamina propria and to play pro‐inflammatory functions by producing IL‐12, IL‐23 and TNFα, driving the differentiation of IFNγ‐secreting T cells and promoting eosinophil penetration into inflamed areas (Waddell et al., 2011; Rivollier et al., 2012). Finally, we cannot entirely dismiss the possibility that 5A peptide beneficially influences monocytes/macrophages via indirect mechanisms such as modulation of T‐cell distribution and/or function. In this context, it is worthwhile noting that apoA‐I has been recently demonstrated to attenuate both IFN‐γ and IL‐17 secretion by antigen‐specific T cells and thereby to reduce the severity of rheumatic arthritis in a murine model of this disease (Tiniakou et al., 2015).
There is substantial evidence suggesting that chronic inflammation of the intestine is conducive to the accelerated development of atherosclerosis. An increased incidence of cardiovascular events has been repeatedly observed in IBD patients, who were also reported to present with increased intima‐media thickness of the common carotid artery (Aggarwal et al., 2014; Ozturk et al., 2015), and evidence of microvascular endothelial dysfunction, which precedes the formation of atherosclerotic lesions, was found in IBD patients (Principi et al., 2013). Lower levels of apoA‐I and HDL, which are major plasma‐born atheroprotective factors in humans, are frequent in active IBD, while anti‐inflammatory HDL function seems to be impaired both in the active and the remission phase of the disease (van Leuven et al., 2007; Sappati Biyyani et al., 2010). By demonstrating the amelioration of DSS colitis after administration of apoA‐I mimetics and moderately exacerbated colitis in apoA‐I‐deficient mice, the present study further underscores the existence of pathophysiological links between IBD and atherosclerosis. As apoA‐I mimetics were previously shown to partition into HDL and to partially restore their anti‐inflammatory and anti‐oxidative function, it is conceivable that the improvement in HDL functionality could additionally contribute to the beneficial effects of 5A peptide observed in this study in colitic mice.
In conclusion, the present data demonstrate the potent anti‐inflammatory potential of the apoA‐I mimetic 5A peptide to ameliorate murine colitis by preventing monocyte infiltration and activation in the intestine at an early stage in the inflammatory process. Our results identify apoA‐I mimetics as a potential treatment approach for IBD. Additionally, our results emphasize the therapeutic potential of regulating monocyte infiltration to the gut as a therapeutic concept in IBD, which is also pursued by the anti‐integrin drugs recently introduced to the therapeutic portfolio. Finally, our results point to HDL and apolipoproteins as astonishingly versatile molecules displaying several anti‐inflammatory functions and to their underestimated role in chronic inflammatory disorders such as IBD.
Author contributions
T.M.N. and J.R.N. designed the study, performed experiments, analysed and interpreted data and drafted the article. A.T.R. designed the study, analysed and interpreted data and revised the manuscript. D.B., M.E., T.V.4, F.B., T.V.5, A.L. and J.H. performed experiments, analysed data and revised the manuscript. J.R. and U.J.T. analysed data and revised the manuscript. All authors approved the version to be submitted.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Two figures (comparison of microbiota between genetically different mice obtained from various vendors; Dose dependency of salutary effects of the 5A peptide in murine colitis)
Figure S1. Faecal microbiota composition in apoA‐I‐deficient and wild‐type mice.
Figure S2. Dose‐dependent effect of 5A peptide on clinical parameters and histological injury in DSS‐induced colitis in mice.
Figure S1. Supporting Information.
Acknowledgements
We thank Ms. Sonja Dufentester, Ms. Elke Weber and Ms. Beate Schulte for the excellent technical assistance. This work was supported by Innovative Medizinische Forschung Münster (IMF) (No. 111017 to T.M.N., J.‐R.N. and J.H.).
Nowacki, T. M. , Remaley, A. T. , Bettenworth, D. , Eisenblätter, M. , Vowinkel, T. , Becker, F. , Vogl, T. , Roth, J. , Tietge, U. J. , Lügering, A. , Heidemann, J. , and Nofer, J. ‐R. (2016) The 5A apolipoprotein A‐I (apoA‐I) mimetic peptide ameliorates experimental colitis by regulating monocyte infiltration. British Journal of Pharmacology, 173: 2780–2792. doi: 10.1111/bph.13556.
References
- Aggarwal A, Atreja A, Kapadia S, Lopez R, Achkar JP (2014). Conventional risk factors and cardiovascular outcomes of patients with inflammatory bowel disease with confirmed coronary artery disease. Inflamm Bowel Dis 20: 1593–1601. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar MJ, D'Souza W, Turner S, Demosky S, Sviridov D, Stonik J et al. (2010). 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther 334: 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharamaiah GM, Mishra VK, Garber DW, Datta G, Handattu SP, Palgunachari MN et al. (2007). Structural requirements for antioxidative and anti‐inflammatory properties of apolipoprotein A‐I mimetic peptides. J Lipid Res 48: 1915–1923. [DOI] [PubMed] [Google Scholar]
- Annese V, Duricova D, Gower‐Rousseau C, Jess T, Langholz E (2016). Impact of new treatments on hospitalisation, surgery, infection, and mortality in IBD: a focus paper by the Epidemiology Committee of ECCO. J Crohns Colitis 10: 216–225. [DOI] [PubMed] [Google Scholar]
- Azevedo OG, Oliveira RA, Oliveira BC, Zaja‐Milatovic S, Araujo CV, Wong DV et al. (2012). Apolipoprotein E COG 133 mimetic peptide improves 5‐fluorouracil‐induced intestinal mucositis. BMC Gastroenterol 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y et al. (2011). A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol 186: 4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burisch J, Jess T, Martinato M, Lakatos PL (2013). The burden of inflammatory bowel disease in Europe. J Crohns Colitis 7: 322–337. [DOI] [PubMed] [Google Scholar]
- Cromer WE, Mathis JM, Granger DN, Chaitanya GV, Alexander JS (2011). Role of the endothelium in inflammatory bowel diseases. World J Gastroenterol 17: 578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX et al. (2012). Apolipoprotein A‐I attenuates ovalbumin‐induced neutrophilic airway inflammation via a granulocyte colony‐stimulating factor‐dependent mechanism. Am J Respir Cell Mol Biol 47: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z et al. (2009). Immature myeloid cells induced by a high‐fat diet contribute to liver inflammation. Hepatology 50: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolo BA, Nicholls SJ, Bao S, Rye KA, Heather AK, Barter PJ et al. (2011). The apolipoprotein A‐I mimetic peptide ETC‐642 exhibits anti‐inflammatory properties that are comparable to high density lipoproteins. Atherosclerosis 217: 395–400. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG et al. (1998). Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domek MJ, Iwata F, Blackman EI, Kao J, Baker M, Vidrich A et al. (1995). Anti‐neutrophil serum attenuates dextran sulfate sodium‐induced colonic damage in the rat. Scand J Gastroenterol 30: 1089–1094. [DOI] [PubMed] [Google Scholar]
- Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW (2009). Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate‐induced colitis. J Pharmacol Exp Ther 329: 123–129. [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J et al. (2008). Phagocyte‐specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol 216: 183–192. [DOI] [PubMed] [Google Scholar]
- Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B et al. (2008). Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med 205: 2319–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann J, Ruther C, Kebschull M, Domschke W, Bruwer M, Koch S et al. (2007). Expression of IL‐12‐related molecules in human intestinal microvascular endothelial cells is regulated by TLR3. Am J Physiol Gastrointest Liver Physiol 293: G1315–G1324. [DOI] [PubMed] [Google Scholar]
- Hortin GL, Shen RF, Martin BM, Remaley AT (2006). Diverse range of small peptides associated with high‐density lipoprotein. Biochem Biophys Res Commun 340: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada M, Arihiro A, Mizoguchi E (2007). Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol 13: 5581–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeier F, Mannel DN, Andus T, Scholmerich J et al. (1997). Neutralization of tumour necrosis factor (TNF) but not of IL‐1 reduces inflammation in chronic dextran sulphate sodium‐induced colitis in mice. Clin Exp Immunol 107: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT 3rd, Lugering A, Abbas JA, Bettini M, Lake JG et al. (2005). Acute induction of human IL‐8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 54: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madenspacher JH, Azzam KM, Gong W, Gowdy KM, Vitek MP, Laskowitz DT et al. (2012). Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J Biol Chem 287: 43730–43740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D et al. (2011). Neutrophil activation is attenuated by high‐density lipoprotein and apolipoprotein A‐I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol 31: 1333–1341. [DOI] [PubMed] [Google Scholar]
- Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W et al. (2011). D‐4F, an apoA‐1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res 52: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Reddy ST, Van Lenten BJ, Fogelman AM (2011). HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 8: 222–232. [DOI] [PubMed] [Google Scholar]
- Norata GD, Pirillo A, Ammirati E, Catapano AL (2012). Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 220: 11–21. [DOI] [PubMed] [Google Scholar]
- Ozturk K, Guler AK, Cakir M, Ozen A, Demirci H, Turker T et al. (2015). Pulse wave velocity, intima media thickness, and flow‐mediated dilatation in patients with normotensive normoglycemic inflammatory bowel disease. Inflamm Bowel Dis 21: 1314–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ et al. (2009). Reconstituted high‐density lipoprotein increases plasma high‐density lipoprotein anti‐inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol 53: 962–971. [DOI] [PubMed] [Google Scholar]
- Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P et al. (2013). Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis 7: e427–e433. [DOI] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL (2012). Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209: 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgeerts P, Van Assche G, Vermeire S (2004). Optimizing anti‐TNF treatment in inflammatory bowel disease. Gastroenterology 126: 1593–1610. [DOI] [PubMed] [Google Scholar]
- Saemann MD, Poglitsch M, Kopecky C, Haidinger M, Horl WH, Weichhart T (2010). The versatility of HDL: a crucial anti‐inflammatory regulator. Eur J Clin Invest 40: 1131–1143. [DOI] [PubMed] [Google Scholar]
- Sappati Biyyani RS, Putka BS, Mullen KD (2010). Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol 4: 478–482. [DOI] [PubMed] [Google Scholar]
- Sarov‐Blat L, Kiss RS, Haidar B, Kavaslar N, Jaye M, Bertiaux M et al. (2007). Predominance of a proinflammatory phenotype in monocyte‐derived macrophages from subjects with low plasma HDL‐cholesterol. Arterioscler Thromb Vasc Biol 27: 1115–1122. [DOI] [PubMed] [Google Scholar]
- Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E et al. (2008). Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1‐dependent cholesterol efflux by peptides. J Biol Chem 283: 32273–32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifov OF, Xu X, Gaggar A, Tabengwa EM, White CR, Palgunachari MN et al. (2014). L‐4F inhibits lipopolysaccharide‐mediated activation of primary human neutrophils. Inflammation 37: 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Chaturvedi R, Asim M, Barry DP, Lewis ND, Vitek MP et al. (2008). The apolipoprotein E‐mimetic peptide COG112 inhibits the inflammatory response to Citrobacter rodentium in colonic epithelial cells by preventing NF‐kappaB activation. J Biol Chem 283: 16752–16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Chaturvedi R, Barry DP, Coburn LA, Asim M, Lewis ND et al. (2011). The apolipoprotein E‐mimetic peptide COG112 inhibits NF‐kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem 286: 3839–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M et al. (2010). Apolipoprotein A‐I mimetic 4F alters the function of human monocyte‐derived macrophages. Am J Physiol Cell Physiol 298: C1538–C1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA et al. (2004). Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172: 4410–4417. [DOI] [PubMed] [Google Scholar]
- Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R et al. (2007). Ly‐6Chi monocytes dominate hypercholesterolemia‐associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S et al. (2010). The 5A apolipoprotein A‐I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol 30: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiniakou I, Drakos E, Sinatkas V, Van Eck M, Zannis VI, Boumpas D et al. (2015). High‐density lipoprotein attenuates Th1 and th17 autoimmune responses by modulating dendritic cell maturation and function. J Immunol 194: 4676–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP et al. (2004). D‐4F, an apolipoprotein A‐I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation 110: 3252–3258. [DOI] [PubMed] [Google Scholar]
- van Leuven SI, Hezemans R, Levels JH, Snoek S, Stokkers PC, Hovingh GK et al. (2007). Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn's disease. J Lipid Res 48: 2640–2646. [DOI] [PubMed] [Google Scholar]
- Vogl T, Eisenblatter M, Voller T, Zenker S, Hermann S, van Lent P et al. (2014). Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat Commun 5: 4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowinkel T, Mori M, Krieglstein CF, Russell J, Saijo F, Bharwani S et al. (2004). Apolipoprotein A‐IV inhibits experimental colitis. J Clin Invest 114: 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell A, Ahrens R, Steinbrecher K, Donovan B, Rothenberg ME, Munitz A et al. (2011). Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage‐derived CCL11. J Immunol 186: 5993–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihrauch D, Xu H, Shi Y, Wang J, Brien J, Jones DW et al. (2007). Effects of D‐4F on vasodilation, oxidative stress, angiostatin, myocardial inflammation, and angiogenic potential in tight‐skin mice. Am J Physiol Heart Circ Physiol 293: H1432–H1441. [DOI] [PubMed] [Google Scholar]
- White CR, Garber DW, Anantharamaiah GM (2014). Anti‐inflammatory and cholesterol‐reducing properties of apolipoprotein mimetics: a review. J Lipid Res 55: 2007–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF (2007). Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546. [DOI] [PubMed] [Google Scholar]
- Woo JM, Lin Z, Navab M, Van Dyck C, Trejo‐Lopez Y, Woo KM et al. (2010). Treatment with apolipoprotein A‐1 mimetic peptide reduces lupus‐like manifestations in a murine lupus model of accelerated atherosclerosis. Arthritis Res Ther 12: R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X et al. (2011). 5A, an apolipoprotein A‐I mimetic peptide, attenuates the induction of house dust mite‐induced asthma. J Immunol 186: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan‐Charvet L, Wang N, Tall AR (2010). Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol 30: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF et al. (2009). Apolipoprotein A‐I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol 297: H866–H873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani G, Volpato S, Ble A, Bandinelli S, Corsi AM, Lauretani F et al. (2007). High interleukin‐6 plasma levels are associated with low HDL‐C levels in community‐dwelling older adults: the InChianti study. Atherosclerosis 192: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two figures (comparison of microbiota between genetically different mice obtained from various vendors; Dose dependency of salutary effects of the 5A peptide in murine colitis)
Figure S1. Faecal microbiota composition in apoA‐I‐deficient and wild‐type mice.
Figure S2. Dose‐dependent effect of 5A peptide on clinical parameters and histological injury in DSS‐induced colitis in mice.
Figure S1. Supporting Information.


