Abstract
Background and Purpose
The colonic surface epithelium produces acetylcholine, released after the binding of propionate to GPCRs for this short‐chain fatty acid (SCFA). This epithelial acetylcholine then induces anion secretion via stimulation of acetylcholine receptors. The key enzyme responsible for acetylcholine synthesis, choline acetyltransferase, is known to be unselective as regards the fatty acid used for esterification of choline. As the colonic epithelium is permanently exposed to high concentrations of different SCFAs produced by bacterial fermentation, we investigated whether choline esters other than acetylcholine, propionylcholine and butyrylcholine, are produced by the colonic epithelium, too, and whether these ‘atypical’ esters are able to stimulate the acetylcholine receptors involved in the regulation of colonic ion transport.
Experimental Approach
Desorption electrospray ionization mass spectroscopy (DESI‐MS), Ussing chamber and Ca2+‐imaging experiments were performed on rat distal colon.
Key Results
DESI‐MS analyses revealed the production of acetylcholine, propionylcholine and butyrylcholine in the surface epithelium. Relative expression rates were 2–3% in comparison with acetylcholine. In Ussing chamber experiments, both atypical choline esters caused a concentration‐dependent increase in short‐circuit current, that is, stimulated anion secretion. Inhibitor experiments in the absence and presence of the submucosal plexus revealed the involvement of neuronal and epithelial acetylcholine receptors. While butyrylcholine obviously stimulated both nicotinic and muscarinic receptors, propionylcholine predominantly acted on muscarinic receptors.
Conclusions and Implications
These results suggest a novel pathway for communication between intestinal microbes producing SCFA and the host via modification of epithelial production of choline esters involved in the paracrine regulation of the colonic epithelium.
Abbreviations
- DESI‐MS
desorption electrospray ionization mass spectrometry
- Isc
short‐circuit current
- SCFA
short‐chain fatty acid
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Ligand‐gated ion channels b |
| ACh receptors (muscarinic) | Nicotinic ACh receptors |
| M1 receptor | |
| M3 receptor |
| LIGANDS | |
|---|---|
| Acetylcholine | Mecamylamine |
| Atropine | Pirenzepine |
| Bumetanide | Telenzepine |
| Darifenacin | Tetrodotoxin |
| Hexamethonium |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
In the gastrointestinal tract, acetylcholine is known not only to be synthesized and released by submucosal and myenteric neurons, but also by colonic epithelial cells (Klapproth et al., 1997, Bader et al., 2014), which are part of the so‐called non‐neuronal cholinergic system. This system consists mainly of cells with barrier and defence functions such as immune cells or epithelial cells (Wessler et al., 2003). Acetylcholine is released from colonic epithelial surface cells after the binding of propionate to the short‐chain fatty acid receptor GPR41 (FFA3) and/or GPR43 (FFA2) (Yajima et al., 2011b). Consequently, acetylcholine binds to epithelial acetylcholine receptors and induces anion secretion (Yajima et al., 2011a).
The key enzyme for acetylcholine synthesis, choline acetyltransferase, which is also expressed in rat colonic surface epithelium (Klapproth et al., 1997, Bader et al., 2014), catalyses the synthesis of acetylcholine from the precursor substances choline and acetyl coenzyme A (acetylCoA). This enzyme has a high specificity for choline, whereas its selectivity for different acyl groups is quite poor. In rat brain, it has been shown that choline acetyltransferase exhibits the same affinity for butyrylCoA and propionylCoA as for acetylCoA (Rossier, 1977), leading to the production of propionylcholine and butyrylcholine, respectively, in the presence of the respective precursor substances.
The colonic epithelium, however, is permanently exposed to high concentrations of acetate, propionate and butryrate, which are produced by bacterial fermentation of carbohydrates within the colonic lumen. The total concentration of short‐chain fatty acids in the large intestinal lumen is in the range of 100 mM (Bugaut, 1987), and these substrates are rapidly absorbed by the colonic epithelium (Karasov and Douglas, 2013). So it is possible that epithelial choline acetyltransferase might also produce propionyl‐ and/or butyrylcholine, in addition to acetylcholine. Thus, in the present study, we analysed the presence of these ‘atypical’ choline esters within the surface epithelium from rat distal colon using desorption electrospray ionization mass spectrometry and investigated, whether these esters (like native acetylcholine) might be able to alter epithelial functions such as Cl− secretion. In addition to muscarinic receptors of the M1 and the M3 subtype (Haberberger et al., 2006, Bader and Diener, 2015), the colonic epithelium also expresses different subunits of nicotinic receptors (α2, α4, α5, α6, α7, α10 and β4); hence, the sensitivity of the presumed changes in ion transport to muscarinic and nicotinic receptor blockers was also tested.
Methods
Animals
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Female and male Wistar rats were used and weighed 160–250 g. The animals were bred and housed at the Institute of Veterinary Physiology and Biochemistry of the Justus‐Liebig‐University and had free access to food and water until the time of experiment. Animals were housed in macrolone type IV cages with 3 animals per cage at 22°C, 50% air humidity and a light: dark cycle of 12 h : 12 h. Animals killed by exsanguination after being stunned by a blow to the head. Experiments were approved by the named animal welfare officer of the Justus Liebig University (administrative number 487_M) and performed according to the German and European animal welfare law.
Solutions
If not indicated differently (as e.g. in ion substitution experiments), all Ussing chamber experiments were carried out in a bathing solution containing (in mmol·L−1): 107 NaCl, 4.5 KCl, 25 NaHCO3, 1.8 Na2HPO4, 0.2 NaH2PO4, 1.25 CaCl2, 1 MgSO4 and 12.2 glucose. The solution was gassed with 5% (v v‐1) CO2 and 95% (v v‐1) O2 at 37°C and had a pH of 7.4 (adjusted by addition of NaHCO3/HCl). For the Cl−‐free buffer, NaCl and KCl were equimolarly substituted by Na gluconate (NaGluc) and K gluconate (KGluc), respectively. For Ca2 +‐imaging experiments, a HEPES‐buffered Tyrode solution was used containing (in mmol·L−1): 135 NaC1, 5.4 KC1, 1 CaC12, 1 MgCl2, 12.2 glucose and 10 HEPES; buffered at pH 7.4 with NaOH.
Tissue preparation
The rat distal colon, which is a well‐established model for studying epithelial anion secretion, was quickly removed and placed in ice‐cold Ussing chamber bathing solution. The large intestine was carefully flushed several times before it was mounted on a thin plastic rod. A circular incision was made near the distal end with a blunt scalpel. The serosa and muscularis propria were stripped off by hand in order to obtain a mucosal–submucosal preparation. This preparation was either directly used for Ussing chamber experiments or for preparation of the mucosa. For the latter, the mucosa–submucosa was opened along the mesenteric border and placed onto a glass plate with the mucosal surface upwards. The proximal end of the tissue was clamped with a clip between a microscope slide and the upper end of the glass plate. The distal end of the colon was fixed with a blunt object slide. With a fresh and sharp glass slide, the mucosa was carefully separated from the submucosal layer in order to obtain a mucosal preparation. Two colonic segments were prepared from each animal; in general, one segment from each location served as control to study the response to a choline ester in the absence of putative inhibitors and the other segment to study the response in the presence of putative inhibitors. Segments were randomly distributed by a technician; blinding did not seem to be appropriate for these in vitro experiments.
Ussing chamber experiments
Both the mucosal and mucosal–submucosal preparations were fixed in a modified Ussing chamber, bathed with a volume of 3.5 mL on each side. The tissue was incubated at 37°C and short‐circuited by a computer‐controlled voltage‐clamp device (Ingenieur Büro für Mess‐ und Datentechnik Mussler, Aachen, Germany), corrected for solution resistance. The exposed surface of the tissue was 1 cm2.
The short‐circuit current (I sc) was continuously recorded, and tissue conductance (G t) was measured every minute by applying a current pulse of ±50 μA·cm−2 with a duration of 200 ms. I sc is expressed as μEq·h−1·cm−2, that is, the flux of a monovalent ion per time and area with 1 μEq·h−1·cm−2 = 26.9 μA·cm−2. Substances were administered after an equilibration period of about 60 min. The maximal increase in I sc evoked by an agonist is given as difference to the baseline just before administration of the drug. When a drug was administered repeatedly to the same tissue (as indicated in the Results), the compartment to which the substance had been administered was washed three times with 5x the chamber volume, before the drug was administered again.
Imaging experiments at submucosal ganglionic cells
Relative changes in the cytosolic Ca2 + concentration of neurons in intact submucosal ganglia were measured using the Ca2 +‐sensitive fluorescent dye fura‐2 as described previously (Bell et al., 2015). The isolated submucosae were transferred to an experimental chamber mounted on an inverted microscope (IX‐50; Olympus, Hamburg, Germany) equipped with an epifluorescence set‐up and an image analysis system (Till Photonics, Martinsried, Germany). The emission above 420 nm was measured from several regions of interest, each the size of one cell. The cells were excited alternatively at 340 and 380 nm, and the ratio of the emission signal at both excitation wavelengths was calculated.
Data were sampled at 0.2 Hz. The submucosal specimens were loaded for 60 min with 6·10−6 mol·L−1 fura‐2 acetoxymethylester (fura‐2 AM; Molecular Probes, Eugene, USA) in the presence of 0.012 g·L−1 Pluronic®‐F127 (Molecular Probes) in the dark at room temperature. Fura‐2 AM which was not taken up by the cells was washed out. All experiments were carried out at room temperature.
The baseline of the fluorescence ratio of fura‐2 was measured for several minutes before butyrylcholine or acetylcholine was superfused. The rate of superfusion was 1.5 mL·min−1. The administration of the choline esters was followed by a washing period and a viability control with KCl (30 mM), which also served to distinguish neurons from glia cells within the submucosal ganglia. A response to a choline ester was accepted by definition when two conditions were fulfilled simultaneously: (i) the amplitude of the change exceeded the 4‐fold standard deviation of the scattering in the fura‐2 ratio during the control period just prior to addition of the drug. (ii) The amplitude of the change in the fura‐2 ratio exceeded an absolute value of 0.1.
Desorption electrospray ionization (DESI)‐MS experiments
For DESI‐MS measurements, mucosal preparations from rat distal colon were fixed on glass slides (with the mucosal side orientated upwards) using cyanoacrylate adhesive. DESI‐MS experiments were carried out with a home‐built DESI ion source coupled to an orbital trapping mass spectrometer (Exactive, Thermo Fisher Scientific GmbH, Bremen, Germany). The DESI ion source was operated at 4 kV spray voltage, a nitrogen pressure of 6 x 105 Pa and a solvent (acetonitrile) flow rate of 5 μL·min−1. The sample‐to‐sprayer distance was about 2 mm. The distance between sprayer and MS inlet was about 3–5 mm and the spray angle was 65° to the sample surface. The source was equipped with a computer‐controlled moving stage manufactured by Danaher Precision Systems (Salem, USA). The stage was operated by servo design kit software V 5.22 (Galil Motion Control, Rocklin, USA), which also triggered the mass spectrometric measurement.
The mass spectrometric analysis was performed in positive ion mode in a mass‐to‐charge range of 150 to 500. The mass resolution was set to 50 000 at m/z 200, and the mass accuracy obtained was better than ±4 ppm. For spatially resolved analysis of the mucosa preparations, a line‐scan was performed over the sample surface from one side to the other side (with a velocity of about 200 μm·s−1). Mucosal samples from four independent experiments were analysed in triplicate; for statistical analysis, the mean of each triplicate was considered as one data point. Accurate masses were calculated from the chemical composition for choline (m [M+] = 104.10699), acetylcholine (m [M+] = 146.11756), propionylcholine (m [M+] = 160.13321) and butyrylcholine (m [M+] = 174.14886); spectra were analysed in a mass window of ±5 ppm.
Drugs
Bumetanide and mecamylamine were dissolved in ethanol (final maximal concentration 2.5 mL·L−1). Darifenacin and J104129 (Tocris, Bristol, UK) were dissolved in DMSO (final maximal DMSO concentration 2.5 mL·L−1). Tetrodotoxin was dissolved in 20 mM citrate buffer. Atropine, butyrylcholine, hexamethonium, pirenzepine, propionylcholine and telenzepine were dissolved in distilled water. If not indicated differently, drugs were purchased from Sigma, Taufkirchen, Germany..
Statistics
Results are given as mean ± SEM of the number (n) of investigated tissues. For all Ussing chamber experiments, a group size of n = 6 was designed; in some experimental series, it was elevated up to eight if there was a larger variability. For the comparison of two groups either Student's t‐test or Mann–Whitney‐U‐test was applied. An F‐test was used to decide which test method should be used. Both paired and unpaired two‐tailed Student's t‐tests were applied as appropriate. When means of more than two groups had to be compared, an ANOVA was performed. If there was no significant variance in homogeneity and an F‐test indicated that variances between the groups were significantly larger than within the groups, a least‐significance difference post‐hoc test was performed. P < 0.05 was considered to be statistically significant. Statistical comparisons were performed using the statistical software winstat 2012.1 (R. Fitch Software, Bad Krozingen, Germany). The concentrations necessary to evoke an increase in 50% of the maximal I sc response (EC50) were calculated by fitting the concentration response data to a Hill equation using graphpad prism 5 (Graphpad Software, La Jolla, USA). The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
Atypical choline esters are locally synthesized by colonic epithelial cells
In order to determine whether choline acetyltransferase catalyses the synthesis of propionylcholine and butyrylcholine in colonic epithelial cells, in addition to ACh, DESI‐MS experiments were performed on mucosa preparations from rat distal colon. DESI utilizes a spray of electrically charged droplets applied under high pressure (a so called electrospray) to extract, desorb and ionize analytes from the sample (i.e. the colonic epithelium). Afterwards, the desorbed ions were sucked into the mass spectrometer and analysed. A spatially resolved analysis of the sample was achieved by scanning over the sample surface.
High signal intensities were – as expected – found for choline (present in high amounts in biological membranes), within the region of the mucosa fixed to the glass slide (Figure 1, 1st row). Acetylcholine was detected in the same region. In addition, distinct signals for propionylcholine and butyrylcholine were found in the mucosa (Figure 1). Peak intensities for each substance in Figure 1 are shown as relative abundance (in %) in relation to the most intense signal observed for each m/z window. Comparing the three choline esters, mean DESI ion signal intensities across the line scan were propionylcholine 2.6 ± 0.64% (n = 4) and butyrylcholine 2.3 ± 0.54% (n = 4), both normalized to the mean signal intensity of acetylcholine (100%). In other words, the two atypical esters were produced by the colonic surface epithelium, but the concentration of the ‘classical’ choline ester, acetylcholine, within the epithelium was about 40 times higher, assuming an equal desorption/ionization yield for the three esters. Because we did not evaluate ionization efficiencies, the relative quantification seen is only a rough estimate.
Figure 1.
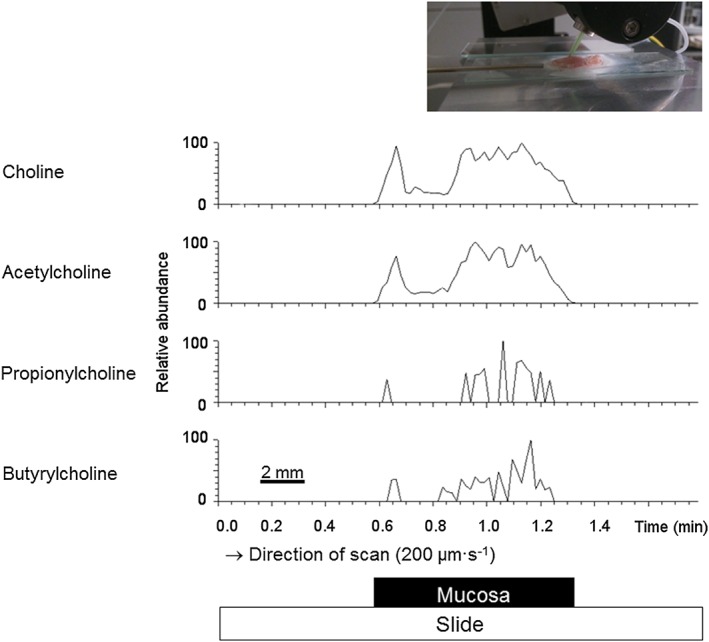
Detection of choline and choline esters in colonic epithelium by DESI‐MS. DESI‐MS ion signals for choline (1st row, m [M+] = 104.10699), acetylcholine (2nd row, (m [M+] = 146.11756), propionylcholine (3rd row, m [M+] = 160.13321) and butyrylcholine (4th row, m [M+] = 174.14886) in mucosal preparations of rat distal colon. A spatially resolved analysis of the sample was achieved by performing a line‐scan over the surface of the sample (inset). Relative signal intensities of the analytes are displayed for a mass window of ±5 ppm, after normalization to the highest signal intensity for each substance, set to 100%. All analytes were detected from the mucosa sample only. Data shown were a selected, representative set out of four independent experiments.
Concentration‐dependent increase in Isc induced by atypical choline esters
Despite their lower local concentration within the epithelium in comparison with acetylcholine, the DESI‐MS data clearly indicate that propionylcholine as well as butyrylcholine are produced in colonic epithelium. Therefore, we investigated whether these choline esters might stimulate acetylcholine receptors in the colonic wall. The response to acetylcholine is mediated by epithelial ACh receptors as well as by ACh receptors on secretomotor neurons innervating the epithelium (Diener et al., 1989). Therefore, two preparations from rat distal colon were used, that is, mucosal–submucosal preparations with an intact submucosal plexus and mucosal preparations, where the submucosa has been ablated. All tissues had a positive basal I sc.
Propionylcholine as well as butyrylcholine (both administered to the serosal side) evoked a concentration‐dependent increase in I sc in mucosal–submucosal as well as in mucosal preparations from rat distal colon (Figure 2). Because desensitization was observed after administration of these agonists at concentrations above 50 μM, the choline esters were administered only once to an individual tissue, when they were used in concentrations ≥100 μM).
Figure 2.
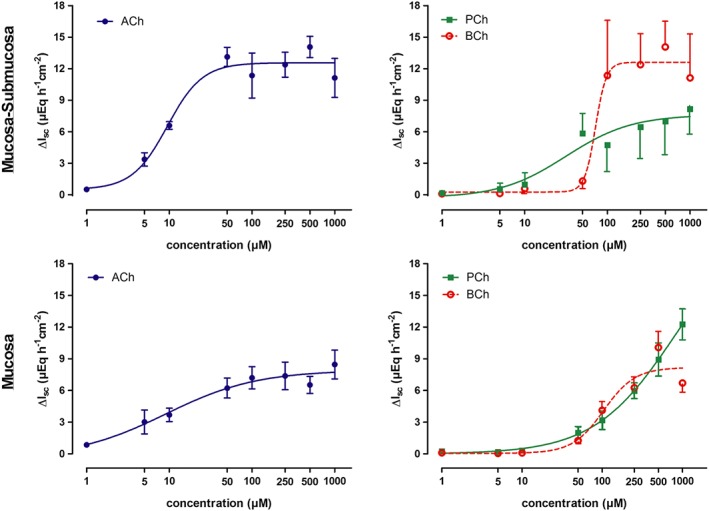
Concentration‐dependent increase in I sc across mucosal–submucosal preparations (1st row) and mucosal preparations (2nd row) from rat distal colon induced by serosal administration of acetylcholine, propionylcholine or butyrylcholine. Because of the desensitization of the I sc response at higher concentrations, all three choline esters were only administered once to an individual tissue when used in concentrations ≥100 μM, whereas for lower concentrations, they could be administered repeatedly to the same tissue with a washing step between the individual administrations (Methods). The curves show fits to a Hill equation. Hill coefficients were in the range of 0.8–2.2 with the exception of the response to butyrylcholine in the mucosal–submucosal preparation, where the Hill coefficient was 6.6 (for EC50 values, see text). Values are given as increase in I sc (ΔI sc) above baseline just prior administration of the respective agonist concentration and are means (symbols) ± SEM (vertical lines). n = 5–8. ACh, acetylcholine; BCh, butyrylcholine; PCh, propionylcholine.
In comparison with native acetylcholine, about 10 times higher concentrations of the atypical esters were needed to evoke a response comparable with acetylcholine. The concentrations needed to evoke an increase of 50% of the maximal I sc response (EC50), was 9.5 ± 1.2 μM for acetylcholine, 31.7 ± 1.5 μM for propionylcholine and 72.0 ± 1.1 μM for butyrylcholine in the mucosal–submucosal preparations. In the mucosal preparations, the EC50 was 9.1 ± 2.9 μM for acetylcholine, 208 ± 1.2 μM for propionylcholine and 105 ± 1.2 μM for butyrylcholine. This clearly indicates a lower affinity of the atypical esters for ACh receptors. However, the maximal increase in I sc was in the same range as that evoked by acetylcholine indicating that they act as full agonists. The increase in I sc was concomitant with an increase in tissue conductance (G t), which did, however, not reach statistical significance (Table 1). Baseline G t was significantly lower in the mucosal–submucosal preparations compared with the mucosal preparations (P < 0.05, Table 1).
Table 1.
Tissue conductance (Gt) under baseline conditions and maximal value observed in the presence of propionylcholine (500 μM at the serosal side) or butyrylcholine (500 μM at the serosal side) in mucosal–submucosal (left) or mucosal preparations (right) of rat distal colon
| G t (mS·cm−2) | |||
|---|---|---|---|
| Baseline | Ester | n | |
| Propionylcholine | |||
| Mucosa–submucosa | 9.3 ± 0.76 | 11.0 ± 1.0 | 8 |
| Mucosa | 13.7 ± 1.6a | 16.6 ± 2.0 | 6 |
| Butyrylcholine | |||
| Mucosa–submucosa | 11.8 ± 1.1 | 12.9 ± 1.2 | 6 |
| Mucosa | 16.4 ± 1.4a | 17.8 ± 1.3 | 6 |
Values are means ± SEM.
P < 0.05 versus baseline G t in mucosal–submucosal preparations.
Desensitization
Repeated administration of the atypical choline esters in higher concentrations led to a desensitization of the tissues. The strongest desensitization was observed with propionylcholine (500 μM at the serosal side) in the mucosal–submucosal preparation, whereas for example, the desensitization against butyrylcholine (500 μM at the serosal side) in the mucosal preparation was only modest (Figure 3, Table 2) and did not reach statistical significance in the ANOVA.
Figure 3.
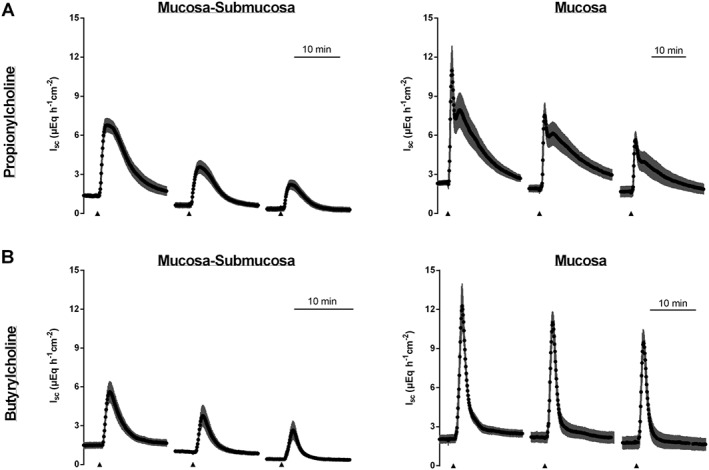
Propionylcholine (500 μM at the serosal side, upper row) or butyrylcholine (500 μM at the serosal side, lower row) were repeatedly administered to mucosal–submucosal (left column) or mucosal (right column) preparations of rat distal colon. The serosal compartment was washed three times with five times the chamber volume before the next administration of the respective choline ester. Values are given as means (black symbols) ± SEM (grey area), n = 6 for propionylcholine and n = 8 for butyrylcholine. Line interruptions are caused by omission of time intervals in order to synchronize the tracings of individual records to the administration of the respective agonists.
Table 2.
Effect of butyrylcholine (500 μM at the serosal side) or propionylcholine (500 μM at the serosal side) on Isc across mucosal‐submucosal or mucosal preparations of rat distal colon after repeated application to the tissue
| Butyrylcholine | ||||
|---|---|---|---|---|
| Mucosa–submucosa | Mucosa | |||
| ΔI sc (μEq·h−1·cm−2) | n | ΔI sc (μEq·h−1·cm−2) | n | |
| 1st administration | 4.34 ± 0.77a | 8 | 11.62 ± 1.49a | 8 |
| 2nd administration | 2.94 ± 0.82a | 8 | 10.28 ± 1.03a | 8 |
| 3rd administration | 2.49 ± 0.82a | 8 | 8.85 ± 0.83a | 8 |
| Propionylcholine | ||||
|---|---|---|---|---|
| Mucosa–submucosa | Mucosa | |||
| ΔI sc (μEq·h−1·cm−2) | n | ΔI sc (μEq·h−1·cm−2) | n | |
| 1st administration | 5.65 ± 0.65a | 7 | 9.69 ± 1.60a | 6 |
| 2nd administration | 3.02 ± 0.55b | 7 | 5.87 ± 0.90b | 6 |
| 3rd administration | 2.03 ± 0.40b | 7 | 4.17 ± 0.44b | 6 |
Butyrylcholine and propionylcholine, respectively, was administered three times to the same tissue, after each administration the tissue was washed three times. Values are means ± SEM and are given as difference to the baseline just before administration of the respective agonist (ΔI sc). Statistical homogeneous groups are indicated by the same letter a, b; ANOVA followed by least‐significance difference post hoc test.
Due to the lower production rate (Figure 1) and the lower affinity of atypical choline esters to ACh receptors (Figure 2), it seems unlikely that they play a physiological role as agonists in regulating epithelial transport. However, they might well act as modulators of cholinergic signalling due to desensitization of the receptors. In order to test this hypothesis, the I sc response evoked by acetylcholine was measured after prior desensitization against an atypical choline ester. For these experiments, propionylcholine was selected because it evoked a stronger desensitization than butyrylcholine (Figure 3). Indeed, after prior desensitization against propionylcholine (administered three times to the serosal side in a concentration of 500 μM with a washing step between each administration), acetylcholine (100 μM at the serosal side) only induced an increase in I sc of 7.64 ± 1.32 μEq·h−1·cm−2 (n = 6) in comparison to 11.51 ± 0.68 μEq·h−1·cm−2 (n = 6) in a control group which had not been pretreated with the atypical choline ester (P < 0.05, Figure 4).
Figure 4.
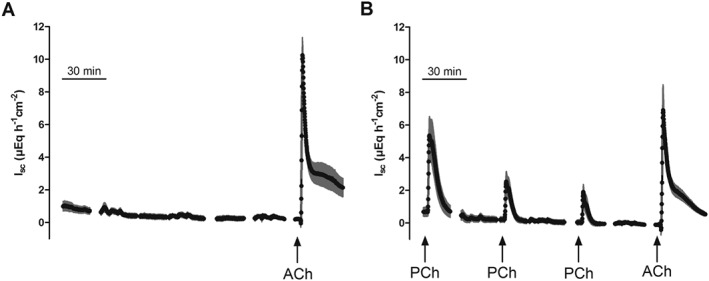
Repeated administration of propionylcholine (PCh; 500 μM at the serosal side; B) leads to desensitization against the subsequent administration of acetylcholine (ACh; 100 μM at the serosal side) in comparison with an untreated control group (A). Values are means (symbols) ± SEM (lines), n = 6. For statistics, see text.
Sensitivity of the Isc response evoked by atypical choline esters to tetrodotoxin
In order to test the involvement of secretomotor neurons in the I sc evoked by atypical choline esters, tissues were pretreated with tetrodotoxin (10 μM at the serosal side), that is, a neurotoxin, which suppresses the propagation of action potentials by blocking voltage‐dependent neuronal Na+ channels (Catterall, 1980). In mucosal–submucosal preparations, pre‐incubation with tetrodotoxin significantly inhibited the increase in I sc evoked by propionylcholine (500 μM at the serosal side) from 4.86 ± 0.90 μEq·h−1·cm−2 (n = 6) in the absence to 1.86 ± 0.70 μEq·h−1·cm−2 in the presence of this neurotoxin (n = 6, P < 0.05 vs. control response n = 6, Figure 5, upper row). However, in mucosal preparations, that is, in the absence of the submucosal plexus, tetrodotoxin was ineffective, as the response to propionylcholine (500 μM at the serosal side) amounted to 9.44 ± 1.29 μEq·h−1·cm−2 in the absence (n = 6) and 8.01 ± 0.70 μEq·h−1·cm−2 (n = 6) in the presence of tetrodotoxin (10 μM at the serosal side, difference not significant).
Figure 5.
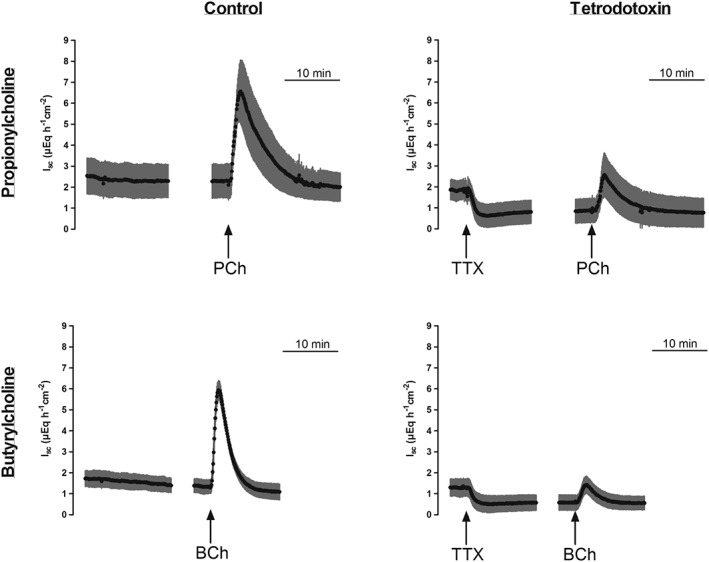
Effect of propionylcholine (500 μM on the serosal side; upper row) or butyrylcholine (500 μM =on the serosal side; lower row) in the absence (left column) or presence (right column) of tetrodotoxin (1 μM at the serosal side). Values are given as means (black symbols) ± SEM (grey area), n = 6. Line interruptions are caused by omission of time intervals in order to synchronize the tracings of individual records to the administration of the respective agonist. BCh, butyrylcholine; PCh, propionylcholine; TTX, tetrodotoxin.
A similar pattern was observed for butyrylcholine. In mucosal–submucosal preparations, a pretreatment with tetrodotoxin (1 μM at the serosal side) significantly inhibited the response in I sc evoked by butyrylcholine (500 μM at the serosal side) from 4.63 ± 0.58 μEq·h−1·cm−2 under control conditions (n = 6) to 0.91 ± 0.17 μEq·h−1·cm−2 (P < 0.05 vs. control response, n = 6, Figure 5). In mucosal preparations, tetrodotoxin had no effect on the butyrylcholine‐induced increase in I sc, which was 9.94 ± 3.38 μEq·h−1·cm−2 in its absence (n = 6) and 7.74 ± 1.75 μEq·h−1·cm−2 in the presence of tetrodotoxin (n = 6, difference not significant).
Submucosal neurons are excited by butyrylcholine
In order to prove the stimulatory action of atypical choline esters on submucosal neurons suggested by Ussing chamber experiments with tetrodotoxin (Figure 5), submucosal ganglionic cells were loaded with the Ca2 +‐sensitive dye, fura‐2, and changes in the cytosolic Ca2 + concentration were measured. For these experiments butyrylcholine was selected as agonist as its responses were more sensitive to the effects of TTX in the Ussing chamber experiments (Figure 5). When tested on 42 neurons (finally verified as neurons by the fura‐2 ratio increase evoked by 30 mM KCl depolarization), 26 neurons (i.e. 60%) responded to butyrylcholine (1 mM) with a significant increase in the fura‐2 ratio signal. This increase amounted to 0.74 ± 0.09 (n = 26). Acetylcholine (10 mM), subsequently administered after washout of butyrylcholine, evoked an increase in the fura‐2 ratio signal of 0.58 ± 0.07 (n = 24, Figure 6).
Figure 6.
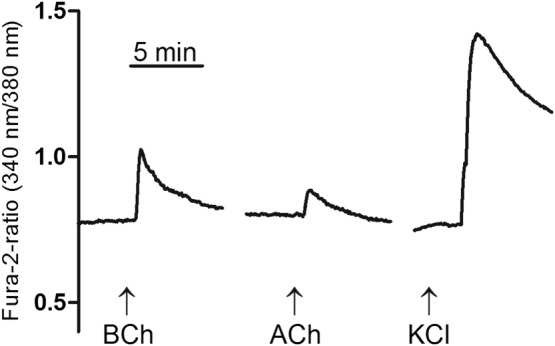
Increase in the cytosolic Ca2 + concentration of submucosal neurons measured by the change in the fura‐2 ratio signal evoked by butyrylcholine (1 mM) and acetylcholine (1 mM). At the end of experiment, neurons were depolarized by KCl (30 mM) in order to evoke a Ca2 + influx via voltage‐dependent Ca2 + channels. Typical tracing from 26 (out of 42 tested) cells; for statistics, see text. BCh, butyrylcholine.
Sensitivity of the Isc response evoked by atypical choline esters to acetylcholine receptor antagonists
Both nicotinic (Bader and Diener, 2015) and muscarinic (Lindqvist et al., 1998, Haberberger et al., 2006, Bader and Diener, 2015) acetylcholine receptors are expressed in the colonic epithelium. Therefore, the sensitivity of the I sc induced by both atypical choline esters against muscarinic and nicotinic receptor blockers was tested in mucosal–submucosal and mucosal preparations, that is, preparations with and without an intact submucosal plexus.
In mucosal–submucosal preparations, blockade of nicotinic acetylcholine receptors with hexamethonium (0.1 mM at the serosal side) or mecamylamine (10 μM at the serosal side) did not affect the increase in I sc induced by propionylcholine (Table 3). In mucosa preparations, however, the secretory response to this agonist tended to be reduced by about one third by hexamethonium and inhibition reached statistical significance in the case of mecamylamine, suggesting that propionylcholine stimulates in part epithelial (but not neuronal) nicotinic receptors.
Table 3.
Effects of several acetylcholine receptor antagonists on Isc induced by propionylcholine across mucosal–submucosal as well as mucosal preparations of rat distal colon
| Blocker | PCh ( − blocker) | PCh (+ blocker)h | n | PCh ( − blocker) | PCh (+ blocker) | n |
|---|---|---|---|---|---|---|
| ΔI sc (μEq·h−1·cm−2) | ||||||
| Mucosa–submucosa | Mucosa | |||||
| ACh receptors | ||||||
| Hexamethonium and atropine | 4.43 ± 0.66 | 0.64 ± 0.22a | 8/7 | 10.30 ± 1.11 | 0.15 ± 0.02a | 6/7 |
| Nicotinic acetylcholine receptors | ||||||
| Hexamethonium | 2.61 ± 0.83 | 5.72 ± 1.19 | 6 | 9.49 ± 1.75 | 6.34 ± 1.38 | 6 |
| Mecamylamine | 5.02 ± 0.45 | 5.39 ± 1.16 | 6 | 9.63 ± 1.27 | 5.22 ± 0.78a | 6 |
| Muscarinic acetylcholine receptors | ||||||
| Atropine | 9.97 ± 1.88 | 3.10 ± 0.66a | 6 | 10.82 ± 1.37 | 1.60 ± 0.44a | 6/7 |
| M1 acetylcholine receptors | ||||||
| Telenzepine | 4.55 ± 1.01 | 2.78 ± 0.35 | 6 | 12.16 ± 1.62 | 3.79 ± 0.87a | 6 |
| Pirenzepine | 4.28 ± 0.90 | 1.69 ± 0.21a | 7 | 9.68 ± 1.45 | 4.31 ± 0.52a | 6/7 |
| M3 acetylcholine receptors | ||||||
| Darifenacine | 6.84 ± 1.68 | 1.41 ± 0.41a | 6 | 11.21 ± 1.46 | 2.45 ± 0.44a | 6 |
| J104129 | 4.30 ± 0.49 | 0.39 ± 0.10a | 6 | 8.85 ± 1.82 | 0.47 ± 0.18a | 6 |
The response to propionylcholine (500 μM at the serosal side) was either tested under control conditions, that is, in the absence of any inhibitor (− blocker), or in the presence of different inhibitors of acetylcholine receptors (+ blocker). Inhibitors used were as follows: hexamethonium (100 μM ), mecamylamine (10 μM ), atropine (1 μM), telenzepine (0.1 μM), pirenezepine (1 μM), darifenacine (10 μM) and J104129 (5 μM). All inhibitors were administered to the serosal side. Values are given as increase in I sc (ΔI sc) above baseline just before administration of propionylcholine and are means ± SEM.
P < 0.05 versus response evoked by propionylcholine in the respective control series. PCh, propionylcholine.
Unselective blockade of muscarinic acetylcholine receptors with atropine (1 μM at the serosal side) significantly reduced the propionylcholine response in both preparations. Inhibition was mimicked by selective M1 receptor blockers (for references to the inhibitors, e.g. Hammer and Giachetti, 1980, Galvan et al., 1989) such as telenzepine (0.1 μM at the serosal side) or pirenzepine (1 μM at the serosal side) and M3 receptor blockers (for references to the inhibitors, e.g. Mitsuya et al., 1999, Zinner, 2007) such as darifenacine (1 μM at the serosal side) or J104129 (5 μM at the serosal side). With the exception of the effect of telenzepine in mucosal–submucosal preparations, inhibition of the propionylcholine‐induced increase in I sc was statistically significant in all cases (P < 0.05, Table 3).
A different pattern of inhibition was observed when using butyrylcholine (500 μM ) as agonist. In this case, both nicotinic receptor blockers inhibited a significant part of the epithelial response (measured in mucosa preparations) and – at least in the case of mecamylamine – also strongly inhibited the effect of this agonist in mucosal–submucosal preparations, where a major part of the secretory response is mediated by the excitation of secretomotor submucosal neurons (Figures 5 and 6). Also, muscarinic receptor blockade with atropine, pirenzepine or the two M3 receptor blockers, darifenacine and J104129, inhibited significantly the increase in I sc evoked by butyrylcholine, whereas telenzepine was ineffective at the concentration used (Table 4). In both preparations, the increase in I sc evoked by propionyl‐ or butyrylcholine was nearly abolished, when both nicotinic and muscarinic receptors were blocked by a combination of hexamethonium and atropine (Tables 3 and 4), suggesting that their effect is solely mediated by ACh receptors.
Table 4.
Effects of acetylcholine receptor antagonists on Isc induced by butyrylcholine across mucosal–submucosal or mucosal preparations of rat distal colon
| Blocker | BCh ( − blocker) | BCh (+ blocker) | n | BCh (+ blocker) | BCh ( − blocker) | n |
|---|---|---|---|---|---|---|
| ΔIsc ( μEq·h−1·cm−2) | ||||||
| Mucosa–submucosa | Mucosa | |||||
| ACh receptors | ||||||
| Hexamethonium and atropine | 5.11 ± 1.10 | 1.17 ± 0.48a | 6 | 7.50 ± 1.22 | 0.26 ± 0.05 | 6 |
| Nicotinic acetylcholine receptors | ||||||
| Hexamethonium | 3.67 ± 0.58 | 2.39 ± 0.71 | 8 | 5.10 ± 1.13 | 1.51 ± 0.19a | 5/6 |
| Mecamylamine | 5.18 ± 0.81 | 0.53 ± 0.13a | 6 | 6.93 ± 1.65 | 1.30 ± 0.24a | 5/6 |
| Muscarinic acetylcholine receptors | ||||||
| Atropine | 8.63 ± 2.30 | 2.96 ± 0.74a | 8/7 | 10.24 ± 1.87 | 3.44 ± 0.37a | 6 |
| M1 acetylcholine receptors | ||||||
| Telenzepine | 3.99 ± 0.71 | 4.00 ± 1.22 | 7 | 8.59 ± 1.67 | 6.61 ± 1.13 | 6/7 |
| Pirenzepine | 5.92 ± 0.80 | 3.17 ± 0.55a | 7 | 9.74 ± 1.08 | 6.21 ± 0.90a | 6 |
| M3 acetylcholine receptors | ||||||
| Darifenacine | 5.22 ± 0.46 | 2.76 ± 0.75a | 7 | 9.50 ± 1.59 | 3.07 ± 0.53a | 6 |
| J104129 | 3.28 ± 0.83 | 0.43 ± 0.16a | 7 | 6.43 ± 1.51 | 0.40 ± 0.06a | 6/7 |
The response to butyrylcholine (500 μM at the serosal side) was either tested under control conditions, that is, in the absence of any inhibitor (− blocker), or in the presence of different inhibitors of acetylcholine receptors (+ blocker). Inhibitors used were: hexamethonium (100 μM), mecamylamine (10 μM ), atropine (1 μM), telenzepine (0.1 μM), pirenezepine (1 μM), darifenacine (1 μM) and J104129 (5 μM). All inhibitors were administered to the serosal side. Values are given as increase in I sc (ΔIsc) above baseline just before administration of butyrylcholine and are means ± SEM.
P < 0.05 versus response evoked by butyrylcholine in the respective control series. BCh, butyrylcholine.
Taken together, these inhibitor experiments suggest a stronger effect of butyrylcholine on nicotinic receptors in comparison with propionylcholine. Furthermore, close inspection of the data presented in Tables 3 and 4 reveal an over‐additive inhibitory action of atropine and hexamethonium, as both drugs inhibit, for example, the response to butyrylcholine by more than two thirds in mucosal preparations (Discussion).
Ionic nature of the Isc response to propionylcholine and butyrylcholine
In order to characterize the ionic nature of the increase in I sc evoked by the two atypical choline esters, experiments were performed in colonic mucosal preparations. To find out if the I sc induced by propionylcholine is due to Cl− secretion, anion substitution experiments were performed. When administered under control conditions, that is, with Cl− ions present on both sides of the epithelium, propionylcholine (500 μM at the serosal side) evoked an increase in I sc of 10.0 ± 2.35 μEq·h−1·cm−2 (n = 6, Figure 7A). However, after substitution of Cl− on both sides of the tissue with the impermeable anion gluconate, the increase in I sc was significantly reduced and only amounted to 1.35 ± 0.36 μEq·h−1·cm−2 (P < 0.05 vs. control response in the presence of Cl−, n = 6, Figure 7A). The same was observed when the tissue was pre‐incubated with bumetanide (100 μM at the serosal side), an inhibitor of the Na+‐K+‐2Cl−‐cotransporter (Kaplan et al., 1996), which is responsible for the basolateral uptake of Cl− during Cl− secretion (Barrett and Keely, 2000). The blockade of this transporter resulted in a significant inhibition of the propionylcholine‐induced increase in I sc from 9.65 ± 1.59 μEq·h−1·cm−2 (n = 5) to 3.14 ± 0.40 μEq·h−1·cm−2 (n = 6, P < 0.05, Figure 7A). As a consequence, these series of experiments indicate that the I sc evoked by propionylcholine is due to Cl− secretion. Similarly, also the increase in I sc evoked by butyrylcholine (500 μM at the serosal side) was significantly reduced by both manoeuvres (Figure 7B) indicating that both agonists – like native acetylcholine – evoke the secretion of Cl− across the colonic epithelium.
Figure 7.
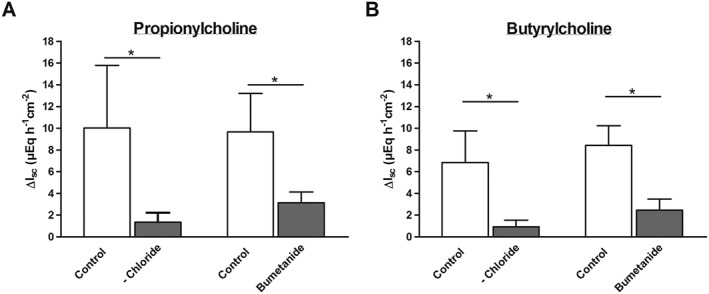
Chloride‐dependence of the I sc response evoked by propionylcholine (500 μM at the serosal side, A) or butyrylcholine (500 μM at the serosal side, B) across colonic mucosal preparations. For each experimental series the response to propionylcholine or butyrylcholine was tested after chloride substitution by gluconate (on the mucosal and serosal side; ‐Chloride) or in the presence of bumetanide (100 μM at the serosal side), respectively, and compared with the response under control conditions (open bars). Values are given as increase in I sc (ΔI sc) above baseline just before administration of the respective choline ester and are means ± SEM; n = 6 for all series except the control response to the bumetanide series for propionylcholine (n = 5) or the series with butyrylcholine in the presence of bumetanide (n = 7). * P < 0.05 versus response evoked by propionylcholine or butyrylcholine in the respective control series.
Discussion
Acetylcholine is not only a neurotransmitter, but is also released as a paracrine mediator from the so‐called non‐neuronal cholinergic system. This system includes mainly barrier‐forming epithelial cells, for example, in skin, airways or gastrointestinal tract and cells with a defence function such as macrophages or granulocytes, which express and release acetylcholine (Wessler et al., 2003). Both the classical acetylcholine synthesizing enzyme, choline acetyltransferase, as well as the mitochondrial enzyme carnitine acetyltransferase have been suggested to be involved in the synthesis of non‐neuronal acetylcholine in urothelium (Lips et al., 2007). In the rat colon, experiments with inhibitors of both enzymes indicate that only choline acetyltransferase, expressed in the surface epithelium of the large intestine of different species (Klapproth et al., 1997), but not the carnitine acetyltransferase (Bader et al., 2014), is responsible for the epithelial production of this signalling molecule.
Choline acetyltransferase, however, is known to be quite unselective concerning the fatty acid residue used for esterification of choline and does not distinguish between acetylCoA, propionylCoA or butrylCoA as a substrate (Rossier, 1977). Furthermore, colonic bacteria permanently produce high amounts of short‐chain fatty acids like acetate, propionate and butryrate during the fermentation of carbohydrates such as cellulose which cannot be digested by mammalian enzymes during their passage through the small intestine (Bugaut, 1987). As short‐chain fatty acids are rapidly absorbed via non‐ionic diffusion or via anion transporters (e.g. Hadjiagapiou et al., 2000), it seemed of interest in the present study to investigate whether propionylcholine or butyrylcholine are produced within the colonic epithelium. Indeed, scanning the mucosa with DESI‐MS clearly revealed – besides acetylcholine – the presence of propionyl‐ and butyrylcholine in the analytes released from the mucosa (Figure 1). It seems reasonable to assume that the electrospray applied under high pressure and high voltage destroys the apical membranes so that the intracellular content of the esters is measured. This is further supported by the observation that all three esters were also detected, when a DESI‐MS analysis was performed in mucosal homogenates (data not shown).
Despite their relatively low physiological expression (about 2–3% in comparison with native acetylcholine), the ability of these atypical esters to induce changes in ion transport across the colonic epithelium was tested in two preparations from rat distal colon, mucosal–submucosal and mucosal preparations. The functional difference between both is determined by the presence or absence of the submucosal plexus, which contains the main secretomotor neurons innervating the colonic epithelium (Andres et al., 1985). In both preparations, the two atypical esters evoked an increase in I sc (Figure 2). Anion substitution experiments and sensitivity to bumetanide (Figure 7) indicate that the increase in I sc evoked by atypical choline esters was due to chloride secretion. In the presence of the submucosal plexus, the effect of both esters was significantly reduced, when the tissue was pretreated with tetrodotoxin (Figure 5), suggesting a stimulation of submucosal secretomotor neurons, which was confirmed by Ca2 +‐imaging experiments at submucosal ganglia (Figure 6). Nevertheless, in general, the maximal increase in I sc evoked by these esters tended to be smaller in mucosal–submucosal preparations in comparison with mucosal preparations with the ablated submucosa (c.f. the control responses in Tables 2, 3, 4). This might suggest that a part of these esters might be degraded, for example, by cellular metabolism during diffusion across the submucosa, preventing these esters from reaching their epithelial action sites.
Due to the lower production rate (Figure 1) and the lower affinity of the atypical esters for ACh receptors (Figure 2), it seems unlikely that they play a physiological role as agonists in regulating epithelial transport, although the concentration‐response experiments show that they act as full agonists at their receptors (Figure 2). However, they might well function as modulators of cholinergic signalling due to desensitization of the receptors against the dominant signalling molecule, acetylcholine, released from enteric neurons or from colonic epithelium. This hypothesis is indicated by the fact that prior desensitization against propionylcholine significantly inhibited the action of a subsequent administration of acetylcholine (Figure 4). This is in analogy to the effect of choline, which is a low affinity agonist at ACh receptors eliciting biological responses due to binding at the receptors only at very high, unphysiological concentrations. Nevertheless, choline is thought to modulate cholinergic signalling in the brain via desensitization of α7 nicotinic receptors by choline (Alkondon et al., 1997); similarly in the rat colon, where it desensitizes muscarinic receptors (Bader and Diener, 2015). So despite their low production rate (Figure 1), these esters might well modulate cholinergic signalling under physiological conditions. However, it must be noted that for the experiment depicted in Figure 4, a quite high concentration of propionylcholine (500 μM at the serosal side) was selected in order to produce a maximal desensitization, that is, the molar ratio of propionylcholine to acetylcholine in this experiment was far from that observed in physiological conditions, where the local production of acetylcholine is much larger than that of propionylcholine. Nevertheless, under in vivo conditions, a continuous release of choline esters is expected as the epithelium is in permanent contact with the colonic content, in which short‐chain fatty acids represent the predominant anions (Bugaut, 1987). It remains to be investigated whether manoeuvres modifying the pattern of short‐chain fatty acid production within the colonic lumen (e.g. dietary modulation of fibre content or treatment with antibiotics) might change the molar ratios of choline ester production within the epithelium and the functional response evoked by stimulation of their release after stimulation of the epithelial receptors for short‐chain fatty acids (Yajima et al., 2011b).
Both esters have a distinct profile at ACh receptors. Muscarinic receptors are found in colonic epithelial cells. However, they also express different subunits (i.e. α2, α4, α5, α6, α7, α10 and β4) of nicotinic receptors (Bader and Diener, 2015) previously thought to be only characteristic of excitable cells. The butyrylcholine‐induced increase in I sc was strongly inhibited by mecamylamine, a nonselective blocker of nicotinic receptors (Table 4). This inhibition was particularly marked in the presence of the submucosa. In the mucosal–submucosal preparation, mecamylamine reduced the I sc response to this ester by about 90% suggesting that butyrylcholine is able to stimulate nicotinic receptors on enteric neurons as well as epithelial nicotinic receptors. In contrast, in the case of propionylcholine, mecamylamine was ineffective in mucosal–submucosal preparations and reduced the response to this ester by only about 45% in the mucosal preparations, suggesting that this ester might only stimulate nicotinic receptors expressed by epithelial cells. Experiments with the non‐selective muscarinic receptor blocker atropine supported by data obtained with darifenacin and J104129, clearly indicate that a part of the action of both atypical esters is mediated by muscarinic receptors (Tables 3 and 4), especially those of the M3 subtype, which is the predominant muscarinic receptor involved in cholinergic induction of epithelial anion secretion (Haberberger et al., 2006). Surprisingly, in some experimental series, the ‘selective’ subtype blockers exerted a stronger inhibitory action than the nonselective muscarinic blocker atropine (e.g. the inhibitory effect of J104129, Tables 3 and 4). This might either indicate an additional effect of J104129 on other muscarinic receptors or might be caused by an insufficient concentration of atropine.
Thus, in principle, like acetylcholine both atypical choline esters are able to act on both types of ACh receptors albeit with differences in their relative efficacy. Interestingly, butyrylcholine is considered in most studies as a nicotinic receptor agonist (e.g. Andreev et al., 1984), whereas actions on muscarinic receptors – apart from a study in the first half of the last century (Hunt and Renshaw, 1934) – seem to have been neglected.
Close inspection of the data presented in Tables 3 and 4 reveal that the effect of muscarinic and nicotinic receptor blockade seems to be over additive. For example, blockade of nicotinic receptors by mecamylamine reduces the I sc response evoked by propionylcholine by 45%, and blockade of muscarinic receptors with atropine inhibits about 85% of the current increase. This observation might indicate some kind of potentiation between muscarinic and nicotinic signalling at the level of the epithelium, which is equipped with both types of ACh receptors, leading to a synergistic stimulation of anion secretion, when both receptor types are stimulated simultaneously.
We know from studies with carbachol that the main anion channel mediating Cl− secretion induced by this ACh receptor agonist is the CFTR. It is not activated itself by the increase in the cytosolic Ca2 + concentration, but instead the driving force for Cl− movement is increased by hyperpolarization of the membrane via activation of Ca2 +‐dependent K+ channels (Strabel and Diener, 1995). This is supported by the transient activation of Ca2 +‐dependent apical Cl− channels (Hennig et al., 2008). Although it was behind the scope of the present study to confirm this for propionyl‐ and butyrylcholine, the clear mediation of the response to both esters by ACh receptors (Tables 3 and 4) gives reason to assume that the same channels underlie the increase in I sc evoked by these esters.
Taken together, the current experiments suggest the possibility of a new form of communication between the intestinal microbiome and the colonic epithelium via the delivary of different short‐chain fatty acids for the synthesis of choline esters by the epithelial choline acetyltransferase. Depending on the relative concentrations of acetate, propionate or butyrate within the colonic lumen and the resulting pattern of choline esters synthesized by the epithelium, the sensitivity of ACh receptors might be altered by production of lower‐affinity choline esters, such as, propionyl‐ or butyrylcholine, which reduce the sensitivity of ACh receptors to the high affinity agonist, acetylcholine, due to desensitization. Modulation of colonic fermentation with pre‐ or probiotics is often used therapeutically to treat colonic disorders, thus this pathway might well have clinical relevance for humans or implications for animal experiments with regard to a sufficient fibre content of the diet. In another part of the gastrointestinal tract, the forestomach of ruminants, it is well known that the composition of the food strongly affects the luminal production pattern of short‐chain fatty acids (Church, 1988). Therefore, we are currently investigating whether different feeding conditions, which should modify colonic production of short‐chain fatty acids due to a different content of plant fibres, has an impact on non‐neuronal cholinergic signalling in the colon of rodents.
Author contributions
S.M., S.G. and S.B. designed the study, performed experiments and interpreted data and drafted the manuscript. S.S., B.S. and M.D. designed the study, interpreted data and drafted the manuscript. All authors approved the version to be submitted.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The diligent technical contributions of Mrs. B. Brück, E. Haas, B. Schmidt and A. Stockinger are gratefully acknowledged. Supported by the LOEWE research focus “Non‐neuronal cholinergic systems” and a Just'us grant (to S.B.) of the University Giessen.
Moreno, S. , Gerbig, S. , Schulz, S. , Spengler, B. , Diener, M. , and Bader, S. (2016) Epithelial propionyl‐ and butyrylcholine as novel regulators of colonic ion transport. British Journal of Pharmacology, 173: 2766–2779. doi: 10.1111/bph.13555.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX (1997). Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9: 2734–2742. [DOI] [PubMed] [Google Scholar]
- Andreev AA, Veprintsev BN, Vulfius CA (1984). Two‐component desensitization of nicotinic receptors induced by acetylcholine agonists in Lymnaea stagnalis neurones. J Physiol 353: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres H, Bock R, Bridges RJ, Rummel W, Schreiner J (1985). Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol 364: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S, Diener M (2015). Novel aspects of cholinergic regulation of colonic ion transport. Pharmacol Res Perspect 3: e00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S, Klein J, Diener M (2014). Choline acetyltransferase and organic cation transporters are responsible for synthesis and propionate‐induced release of acetylcholine in colon epithelium. Eur J Pharmacol 733: 23–33. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Keely SJ (2000). Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572. [DOI] [PubMed] [Google Scholar]
- Bell A, Althaus M, Diener M (2015). Communication between mast cells and rat submucosal neurons. Pflügers Arch Eur J Physiol 467: 1809–1823. [DOI] [PubMed] [Google Scholar]
- Bugaut M (1987). Occurence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol 86B: 439–472. [DOI] [PubMed] [Google Scholar]
- Catterall WA (1980). Neurotoxins that act on voltage‐sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol 20: 15–43. [DOI] [PubMed] [Google Scholar]
- Church DC (1988). The ruminant animal In: Digestive physiology and nutrition, 2nd edn. Prentice Hall: London. [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Brit J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener M, Knobloch SF, Bridges RJ, Keilmann T, Rummel W (1989). Cholinergic‐mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol 168: 219–229. [DOI] [PubMed] [Google Scholar]
- Galvan M, Boer R, Schudt C (1989). Interaction of telenzepine with muscarinic receptors in mammalian sympathetic ganglia. Eur J Pharmacol 167: 1–10. [DOI] [PubMed] [Google Scholar]
- Haberberger R, Schultheiss G, Diener M (2006). Epithelial muscarinic M1 receptors contribute to carbachol‐induced ion secretion in mouse colon. Eur J Pharmacol 530: 229–233. [DOI] [PubMed] [Google Scholar]
- Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K (2000). Mechanism(s) of butyrate transport in Caco‐2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780. [DOI] [PubMed] [Google Scholar]
- Hammer R, Giachetti A (1980). Muscarinic receptor subtypes M1 and M2. Biochemical and functional classification. Life Sci 31: 2991–2998. [DOI] [PubMed] [Google Scholar]
- Hennig B, Schultheiss G, Kunzelmann K, Diener M (2008). Ca2 +‐induced Cl− efflux at rat distal colonic epithelium. J Membrane Biol 221: 61–72. [DOI] [PubMed] [Google Scholar]
- Hunt R, Renshaw RR (1934). Further studies of the methyl cholines and analogous compounds. J Pharmacol Exp Ther 51: 237–262. [Google Scholar]
- Kaplan MR, Mount DB, Delpire E (1996). Molecular mechanisms of NaCl cotransport. Annu Rev Physiol 58: 649–668. [DOI] [PubMed] [Google Scholar]
- Karasov WH, Douglas AE (2013). Comparative digestige physiology. Comprehen Physiol 3: 741–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Münch M, Bittinger F, Kirkpatrick CJ et al. (1997). Non‐neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 355: 515–523. [DOI] [PubMed] [Google Scholar]
- Lindqvist SM, Sharp P, Johnson IT, Satoh Y, Williams MR (1998). Acetylcholine‐induced calcium signaling along the rat colonic crypt axis. Gastroenterology 115: 1131–1143. [DOI] [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W et al. (2007). Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51: 1042–1053. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Brit J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya M, Mase T, Tsuchiya Y, Kawakami K, Hattori H, Kobayashi K et al. (1999). J‐104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem 7: 2555–2567. [DOI] [PubMed] [Google Scholar]
- Rossier J (1977). Acetyl‐coenzyme A and coenzyme A analogues. Their effects in rat brain choline acetyltransferase. Biochem J 165: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strabel D, Diener M (1995). Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol 274: 181–191. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ (2003). The non‐neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci 72: 2055–2061. [DOI] [PubMed] [Google Scholar]
- Yajima T, Inoue R, Matsumoto M, Yajima M (2011a). Non‐neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol 589: 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Inoue R, Yajima M, Tsuruta T, Karaki S, Hira T et al. (2011b). The G‐protein on cholesterol‐rich membrane microdomains mediates mucosal sensing of short‐ chain fatty acid and secretory response in rat colon. Acta Physiol 203: 381–389. [DOI] [PubMed] [Google Scholar]
- Zinner N (2007). Darifenacin: a muscarinic M3‐selective receptor antagonist for the treatment of overactive bladder. Exp Opin Pharmacother 8: 511–523. [DOI] [PubMed] [Google Scholar]


