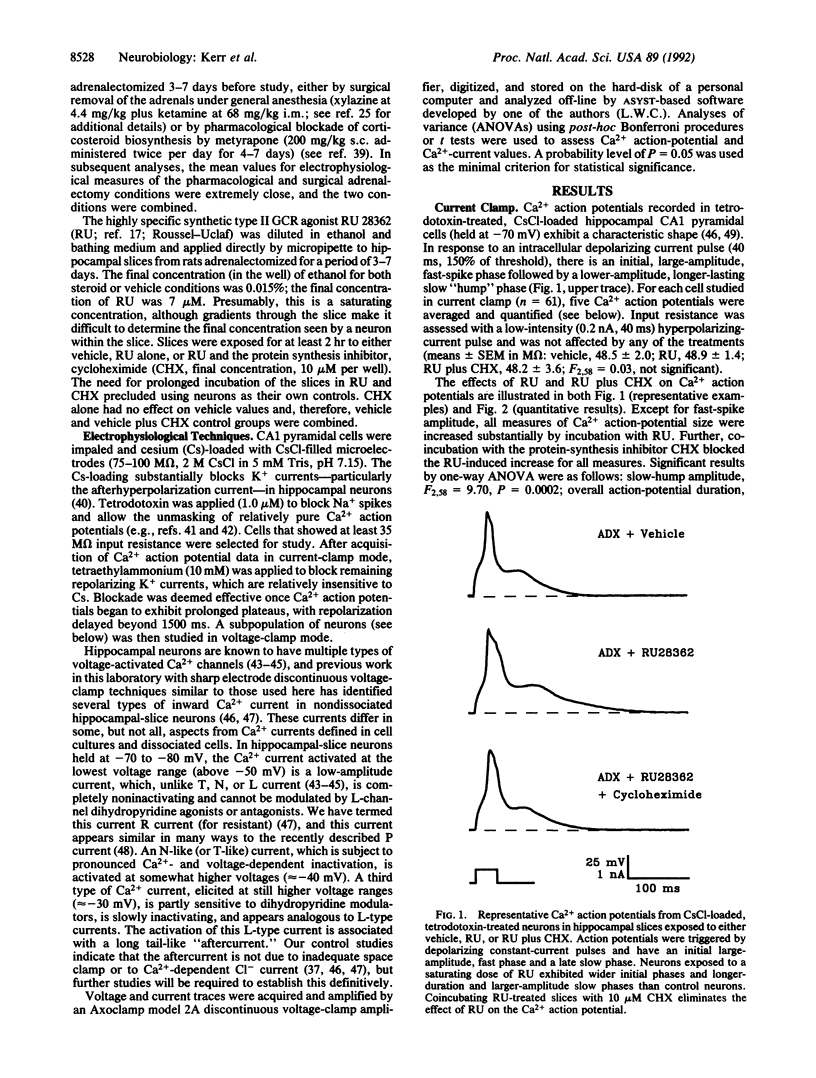
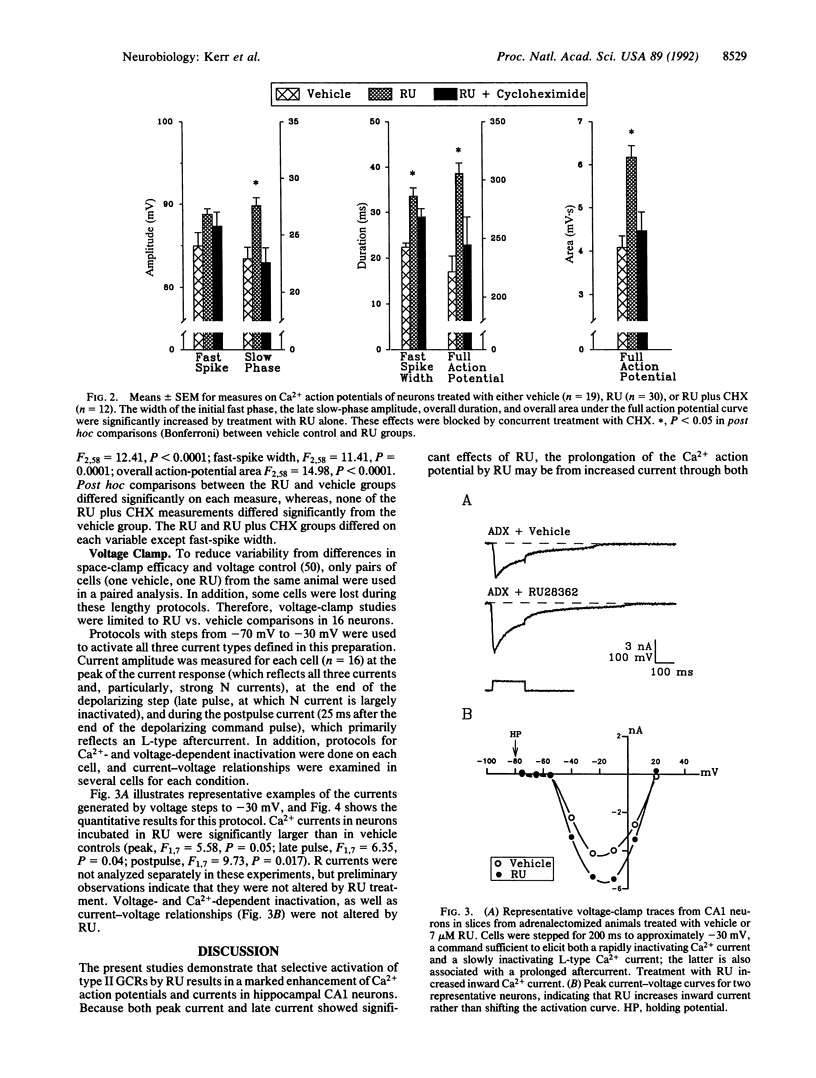
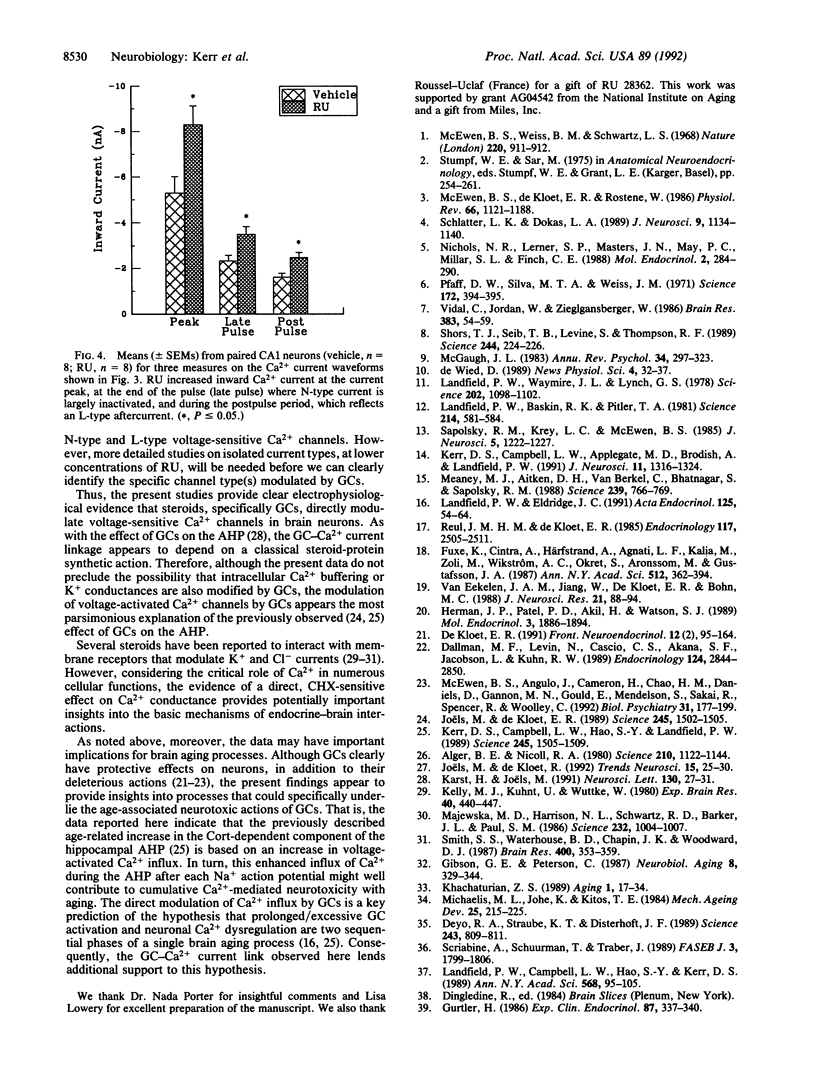
Abstract
Glucocorticoids (GCs) activate several biochemical/molecular processes in the hippocampus through two receptor types. In addition, GCs influence cognitive behaviors and hippocampal neural activity and can also increase the rate of aging-dependent cell loss in the hippocampus. However, the ionic mechanisms through which GCs modulate hippocampal neuronal function are not well understood. We report here direct evidence that activation of cytosolic steroid receptors, specifically of the type II GC receptor, can enhance voltage-dependent Ca2+ conductances in brain neurons. Ca2+ current was assessed by current-clamp measures of Ca2+ action potentials and by sharp electrode voltage-clamp analyses of voltage-sensitive currents in cesium-, tetrodotoxin-, and tetraethylammonium-treated CA1 neurons in hippocampal slices. Both Ca2+ action potentials and voltage-activated Ca2+ currents (N- and L-like) were increased by 2-hr exposure to the synthetic GC receptor agonist, RU 28362. This effect of RU 28362 was blocked by coincubation with cycloheximide, indicating that the GC receptor-Ca2+ channel interaction depends on de novo protein synthesis. Dysregulated calcium homeostasis is also viewed as a candidate mechanism in brain aging. Thus, present results are consistent with the hypothesis that excessive GC-receptor activation and resultant increased Ca2+ influx may be two sequential phases of a brain-aging process that results initially in impairment of function and eventually in neuronal loss.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Levin N., Cascio C. S., Akana S. F., Jacobson L., Kuhn R. W. Pharmacological evidence that the inhibition of diurnal adrenocorticotropin secretion by corticosteroids is mediated via type I corticosterone-preferring receptors. Endocrinology. 1989 Jun;124(6):2844–2850. doi: 10.1210/endo-124-6-2844. [DOI] [PubMed] [Google Scholar]
- Deyo R. A., Straube K. T., Disterhoft J. F. Nimodipine facilitates associative learning in aging rabbits. Science. 1989 Feb 10;243(4892):809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Cintra A., Härfstrand A., Agnati L. F., Kalia M., Zoli M., Wikström A. C., Okret S., Aronsson M., Gustafsson J. A. Central glucocorticoid receptor immunoreactive neurons: new insights into the endocrine regulation of the brain. Ann N Y Acad Sci. 1987;512:362–393. doi: 10.1111/j.1749-6632.1987.tb24974.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987 Jul-Aug;8(4):329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Gürtler H. Effects of metyrapone and a combined alpha- and beta-adrenergic blockade on plasma glucose recovery from insulin-induced hypoglycaemia in pigs. Exp Clin Endocrinol. 1986 Aug;87(3):337–340. doi: 10.1055/s-0029-1210564. [DOI] [PubMed] [Google Scholar]
- Herman J. P., Patel P. D., Akil H., Watson S. J. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989 Nov;3(11):1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992 Jan;15(1):25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989 Sep 29;245(4925):1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Karst H., Joëls M. The induction of corticosteroid actions on membrane properties of hippocampal CA1 neurons requires protein synthesis. Neurosci Lett. 1991 Sep 2;130(1):27–31. doi: 10.1016/0304-3940(91)90219-j. [DOI] [PubMed] [Google Scholar]
- Kelly M. J., Kuhnt U., Wuttke W. Hyperpolarization of hypothalamic parvocellular neurons by 17 beta-estradiol and their identification through intracellular staining with procion yellow. Exp Brain Res. 1980;40(4):440–447. doi: 10.1007/BF00236152. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Campbell L. W., Applegate M. D., Brodish A., Landfield P. W. Chronic stress-induced acceleration of electrophysiologic and morphometric biomarkers of hippocampal aging. J Neurosci. 1991 May;11(5):1316–1324. doi: 10.1523/JNEUROSCI.11-05-01316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D. S., Campbell L. W., Hao S. Y., Landfield P. W. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989 Sep 29;245(4925):1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano) 1989 Sep;1(1):17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Baskin R. K., Pitler T. A. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981 Oct 30;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Campbell L. W., Hao S. Y., Kerr D. S. Aging-related increases in voltage-sensitive, inactivating calcium currents in rat hippocampus. Implications for mechanisms of brain aging and Alzheimer's disease. Ann N Y Acad Sci. 1989;568:95–105. doi: 10.1111/j.1749-6632.1989.tb12495.x. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Eldridge J. C. The glucocorticoid hypothesis of brain aging and neurodegeneration: recent modifications. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):54–64. [PubMed] [Google Scholar]
- Landfield P. W., Waymire J. C., Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978 Dec 8;202(4372):1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Llinás R. R., Sugimori M., Cherksey B. Voltage-dependent calcium conductances in mammalian neurons. The P channel. Ann N Y Acad Sci. 1989;560:103–111. doi: 10.1111/j.1749-6632.1989.tb24084.x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Angulo J., Cameron H., Chao H. M., Daniels D., Gannon M. N., Gould E., Mendelson S., Sakai R., Spencer R. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992 Jan 15;31(2):177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986 Oct;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Weiss J. M., Schwartz L. S. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968 Nov 30;220(5170):911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L. Hormonal influences on memory. Annu Rev Psychol. 1983;34:297–323. doi: 10.1146/annurev.ps.34.020183.001501. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Aitken D. H., van Berkel C., Bhatnagar S., Sapolsky R. M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988 Feb 12;239(4841 Pt 1):766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Michaelis M. L., Johe K., Kitos T. E. Age-dependent alterations in synaptic membrane systems for Ca2+ regulation. Mech Ageing Dev. 1984 Apr-May;25(1-2):215–225. doi: 10.1016/0047-6374(84)90142-8. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Nichols N. R., Lerner S. P., Masters J. N., May P. C., Millar S. L., Finch C. E. Rapid corticosterone-induced changes in gene expression in rat hippocampus display type II glucocorticoid receptor specificity. Mol Endocrinol. 1988 Mar;2(3):284–290. doi: 10.1210/mend-2-3-284. [DOI] [PubMed] [Google Scholar]
- Pfaff D. W., Silva M. T., Weiss J. M. Telemetered recording of hormone effects on hippocampal neurons. Science. 1971 Apr 23;172(3981):394–395. doi: 10.1126/science.172.3981.394. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Landfield P. W. Aging-related prolongation of calcium spike duration in rat hippocampal slice neurons. Brain Res. 1990 Jan 29;508(1):1–6. doi: 10.1016/0006-8993(90)91109-t. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Landfield P. W. Probable Ca2+-mediated inactivation of Ca2+ currents in mammalian brain neurons. Brain Res. 1987 Apr 28;410(1):147–153. doi: 10.1016/s0006-8993(87)80037-9. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985 May;5(5):1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter L. K., Dokas L. A. Receptor specificity of a glucocorticoid- and stress-induced hippocampal protein. J Neurosci. 1989 Apr;9(4):1134–1140. doi: 10.1523/JNEUROSCI.09-04-01134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriabine A., Schuurman T., Traber J. Pharmacological basis for the use of nimodipine in central nervous system disorders. FASEB J. 1989 May;3(7):1799–1806. [PubMed] [Google Scholar]
- Shors T. J., Seib T. B., Levine S., Thompson R. F. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989 Apr 14;244(4901):224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Waterhouse B. D., Chapin J. K., Woodward D. J. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987 Jan 6;400(2):353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Van Eekelen J. A., Jiang W., De Kloet E. R., Bohn M. C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988 Sep;21(1):88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Vidal C., Jordan W., Zieglgänsberger W. Corticosterone reduces the excitability of hippocampal pyramidal cells in vitro. Brain Res. 1986 Sep 24;383(1-2):54–59. doi: 10.1016/0006-8993(86)90007-7. [DOI] [PubMed] [Google Scholar]


