PET and CSF biomarkers of amyloid-β are considered interchangeable in defining ‘amyloid positivity’. However, Leuzy et al. report discordance between these measures in a multicentre memory clinic population. This suggests that in a minority of individuals these metrics may not be interchangeable, and may instead reflect distinct but interrelated processes.
Keywords: Alzheimer’s disease, CSF Aβ42, CSF Aβ42/40, PiB PET, Centiloids
PET and CSF biomarkers of amyloid-β are considered interchangeable in defining ‘amyloid positivity’. However, Leuzy et al. report discordance between these measures in a multicentre memory clinic population. This suggests that in a minority of individuals these metrics may not be interchangeable, and may instead reflect distinct but interrelated processes.
Abstract
The aim of this study was to assess the agreement between data on cerebral amyloidosis, derived using Pittsburgh compound B positron emission tomography and (i) multi-laboratory INNOTEST enzyme linked immunosorbent assay derived cerebrospinal fluid concentrations of amyloid-β42; (ii) centrally measured cerebrospinal fluid amyloid-β42 using a Meso Scale Discovery enzyme linked immunosorbent assay; and (iii) cerebrospinal fluid amyloid-β42 centrally measured using an antibody-independent mass spectrometry-based reference method. Moreover, we examined the hypothesis that discordance between amyloid biomarker measurements may be due to interindividual differences in total amyloid-β production, by using the ratio of amyloid-β42 to amyloid-β40. Our study population consisted of 243 subjects from seven centres belonging to the Biomarkers for Alzheimer’s and Parkinson’s Disease Initiative, and included subjects with normal cognition and patients with mild cognitive impairment, Alzheimer’s disease dementia, frontotemporal dementia, and vascular dementia. All had Pittsburgh compound B positron emission tomography data, cerebrospinal fluid INNOTEST amyloid-β42 values, and cerebrospinal fluid samples available for reanalysis. Cerebrospinal fluid samples were reanalysed (amyloid-β42 and amyloid-β40) using Meso Scale Discovery electrochemiluminescence enzyme linked immunosorbent assay technology, and a novel, antibody-independent, mass spectrometry reference method. Pittsburgh compound B standardized uptake value ratio results were scaled using the Centiloid method. Concordance between Meso Scale Discovery/mass spectrometry reference measurement procedure findings and Pittsburgh compound B was high in subjects with mild cognitive impairment and Alzheimer’s disease, while more variable results were observed for cognitively normal and non-Alzheimer’s disease groups. Agreement between Pittsburgh compound B classification and Meso Scale Discovery/mass spectrometry reference measurement procedure findings was further improved when using amyloid-β42/40. Agreement between Pittsburgh compound B visual ratings and Centiloids was near complete. Despite improved agreement between Pittsburgh compound B and centrally analysed cerebrospinal fluid, a minority of subjects showed discordant findings. While future studies are needed, our results suggest that amyloid biomarker results may not be interchangeable in some individuals.
Introduction
Current thinking ascribes the pathogenesis of Alzheimer’s disease to the aggregation of amyloid-β in the brain. Specifically, the accumulation of various species of aggregated amyloid-β is believed to set in motion tau pathology and neurodegeneration, leading to cognitive impairment and, ultimately, dementia (Jack et al., 2013). Brain amyloid-β accumulation can be identified in vivo using CSF levels of the 42-amino acid form of amyloid-β (amyloid-β42), and PET with fibrillar amyloid-β specific radiotracers, such as the carbon-11 labelled thioflavin-T derivative, Pittsburgh compound B (PiB) (Blennow et al., 2015).
While brain retention of amyloid tracers such as PiB is elevated in Alzheimer’s disease, CSF levels of amyloid-β42 are decreased, an observation hypothesized to reflect the sequestration of brain soluble amyloid-β into insoluble plaques, with a resultant reduction in the amount of amyloid-β42 available for clearance into the CSF (Kawarabayashi et al., 2001; DeMattos et al., 2002). This inverse relationship has been confirmed by many groups across cognitively normal, mild cognitive impairment (MCI) and Alzheimer’s disease subjects, leading to the view that these biomarkers are interchangeable in defining ‘amyloid-positivity’ (Fagan, 2015). Though this inverse relationship has generally been observed between both measures, a subset of cases shows discordant results, with either abnormal CSF amyloid-β42 but normal amyloid PET, or normal CSF amyloid-β42 but abnormal amyloid PET (Forsberg et al., 2008; Jagust et al., 2009; Degerman Gunnarsson et al., 2010; Landau et al., 2013; Palmqvist et al., 2014; Zwan et al., 2014; Mattsson et al., 2015). While discordance in most cases is due to abnormal CSF amyloid-β42 in subjects with normal amyloid PET, isolated PET positivity has been reported in both MCI and Alzheimer’s disease (Koivunen et al., 2008; Forsberg et al., 2010; Landau et al., 2013; Zwan et al., 2014; Leuzy et al., 2015; Mattsson et al., 2015; Palmqvist et al., 2016).
With the exception of a recent European multicentre study (Zwan et al., 2016), however, large-scale studies addressing agreement between amyloid PET and CSF amyloid-β42 have to date been conducted using cohorts evaluated according to standardized clinical and biomarker assessment protocols (Landau et al., 2013; Mattsson et al., 2015; Palmqvist et al., 2016). In addition, there are no studies comparing the agreement between amyloid PET and CSF amyloid-β42 concentrations obtained by the novel antibody-free mass spectrometry (MS)-based reference measurement procedure (RMP) (Leinenbach et al., 2014). Moreover, there have as yet been no studies implementing the Centiloid method, a recently proposed standardization approach that aims to facilitate cross-centre comparison/combination of amyloid PET outcome data using a scaling procedure (Klunk et al., 2015). The aim of the present study was thus to assess agreement between CSF amyloid-β42 and PiB PET in a mixed memory clinic sample drawn from different academic European research centres, with patients assessed according to local clinical routines and imaged using differing acquisition protocols. Given the established between-centre variability in enzyme-linked immunosorbent assay (ELISA)-derived CSF amyloid-β42 values (Wiltfang et al., 2007; Bjerke et al., 2010; Andreasson et al., 2012; Mattsson et al., 2013), we likewise aimed to determine whether concordance rates would be affected by centralized reanalysis of CSF using Meso Scale Discovery ELISA technology (MSD) and an MS-based candidate RMP (Leinenbach et al., 2014). Finally, we sought to examine the hypothesis that discordance between amyloid biomarker results may be due to interindividual differences in total amyloid-β production (Wiltfang et al., 2007; Lewczuk et al., 2015), by adjusting MSD and MS-RMP determined amyloid-β42 levels, for those of amyloid-β40.
Materials and methods
Study population
Our study population consisted of 243 subjects for whom PiB PET data and CSF data and samples were available, collected across seven European academic centres belonging to the Biomarkers for Alzheimer’s and Parkinson’s Disease (BIOMARKAPD) initiative. Participating sites included the Hospital de Sant Pau, Barcelona, Spain (n = 24); Coimbra University Hospital, Coimbra, Portugal (n = 22); Danish Dementia Research Centre, Copenhagen, Denmark (n = 31); University of Lisbon, Lisbon, Portugal (n = 23); Karolinska Institutet, Stockholm, Sweden (n = 32); Turku University Hospital, Turku, Finland (n = 87); and the Ulm University Hospital, Ulm, Germany (n = 24). Patients had been referred for cognitive complaints and assessed according to standard local clinical routines. All diagnoses were made in a multidisciplinary team setting using a consensus-based approach.
Patients with Alzheimer’s disease (n = 122) fulfilled the 1984 National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable Alzheimer’s disease dementia (McKhann et al., 2011), with MCI (n = 81) diagnosed according to the Petersen criteria (Petersen et al., 1999). Other diagnoses were made according to the Neary criteria for frontotemporal dementia (FTD; n = 20), including behavioural, semantic, and progressive non-fluent variants (Neary et al., 1998), and the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia (VaD; n = 7) (Román et al., 1993).
In addition, 13 with normal cognition were recruited from relatives and carers of patients. Inclusion criteria were: the absence of memory or other cognitive complaints; independence in basic and instrumental activities of daily living; and no active neurological or psychiatric disease. All subjects provided written informed consent to participate in the investigation, which was conducted according to the Declaration of Helsinki and subsequent revisions. Ethical approval was obtained from local regional ethics committees.
Apolipoprotein E genotype
Apolipoprotein E (APOE) genotyping was performed in a subset of subjects (n = 106; four cognitively normal, 44 MCI, 52 Alzheimer’s disease, six FTD) via polymerase chain reaction (PCR) of genomic DNA extracted from EDTA-anticoagulated blood. Subjects were classified as ϵ4 allele carriers or non-carriers.
Local CSF amyloid-β42 and tau measurements
All centres used a similar protocol for CSF collection and processing. CSF samples were collected by lumbar puncture, between 8 am and 3 pm. A total of 10 ml was drawn, and stored in polypropylene tubes. After removal of the first 0.5 ml, samples were centrifuged at 1500g (3000–4000 rpm) for 10 min at +4°C. Samples were then stored at −80°C in 1 ml portions pending biochemical analysis, without being thawed or refrozen. Amyloid-β42, total tau, and phosphorylated tau were measured using commercially available sandwich ELISAs (INNOTEST, Fujirebio-Europe), according to kit inserts.
Centralized CSF reanalysis for amyloid-β
All CSF samples were those remaining from the clinical routine at participating centres. Samples were stored on dry ice and sent via express mail to the Clinical Neurochemistry Laboratory, Gothenburg University, Mölndal, Sweden. Given the low volume of CSF available for reanalysis per centre (∼500 μl), MSD was selected over the INNOTEST platform, given its lower sample volume requirement. MSD electrochemiluminescence analyses (amyloid-β42 and amyloid-β40) were performed according to the manufacturer’s protocol. For calibration and sample preparation for MS-RMP, native (unlabelled) and 15N uniformly labelled amyloid-β40 and amyloid-β42 and 13C uniformly labelled amyloid-β42 (rPeptide) were dissolved in 20% acetonitrile and 1% ammonium hydroxide (NH4OH) to a concentration of 50 µg/ml. Aliquots were stored at −80°C. Artificial CSF was prepared as described elsewhere (Dillen et al., 2011). Calibration samples for amyloid-β42 were prepared in human CSF as previously described (Leinenbach et al., 2014). For amyloid-β40, artificial CSF was spiked to a final concentration of 1.5, 5, 10, 20, 30 and 40 ng/ml native amyloid-β40 and a constant concentration of 15N-amyloid-β40 at 1600 pg/ml as internal standard. Unknown samples (180 µl) were spiked with 20 µl internal standard to a final concentration of 1600 pg/ml 13C-amyloid-β42 and 15N-amyloid-β40. Solid phase extraction (SPE), liquid chromatography, MS analysis and data processing were conducted as previously described (Leinenbach et al., 2014).
Determination of CSF amyloid-β cut-offs
Local amyloid-β42 values (pooled) were classified as positive (abnormal) or negative (normal) using an optimized cut-off of 557 pg/ml, established in a recent study using a large cohort of BIOMARKAPD subjects (Zwan et al., 2016). Unbiased cut-offs for MSD- and MS-RMP-derived amyloid-β42 and amyloid-β42/40 ratios were determined by mixture modelling (Benaglia et al., 2009), implemented in R (v.3.2.3, R Foundation for Statistical Computing, Vienna, Austria, 2015).
Pittsburgh compound B-PET imaging
PiB was synthesized using a previously described method at the individual centres according to good manufacturing practice requirements (Mathis et al., 2003; Klunk et al., 2004). PiB-PET acquisition protocols varied across sites, with late summation images created according to local clinical practice: Barcelona, four 5-min frames, 50- to 70-min post-injection (50- to 70-min summation); Coimbra, Lisbon, Stockholm, 60-min dynamic acquisition (40- to 60-min summation); Copenhagen, 30-min dynamic acquisition, 40-min post-injection (40- to 70-min summation); Turku, 30-min dynamic acquisition 60 min post-injection (60- to 90-min summation), and Ulm, four 5-min frames, 40- to 60-min post-injection (40- to 60-min summation). PiB summation images were rated locally by nuclear medicine physicians as either positive (binding in more than one cortical region; abnormal) or negative (predominantly white matter binding; normal), blinded to CSF results.
Pittsburgh compound B-PET image analysis
PiB summation images (40–60, 40–70, 50–70, and 60–90 min) were first non-linearly spatially normalized to a population-based PiB template (Nordberg et al., 2013), using the normalize function in SPM8 (Functional Imaging Laboratory, Wellcome Department of Imaging Neuroscience, UCL, London, UK). Spatially normalized images were then resampled using a 23-region grey matter atlas, created in parallel to the PiB template (Nordberg et al., 2013). Standardized uptake value ratio (SUVr) images were then calculated by normalizing PiB uptake within frontal, temporal, parietal, occipital, parahippocampal, anterior and posterior cingulate regions to mean cerebellar grey matter uptake.
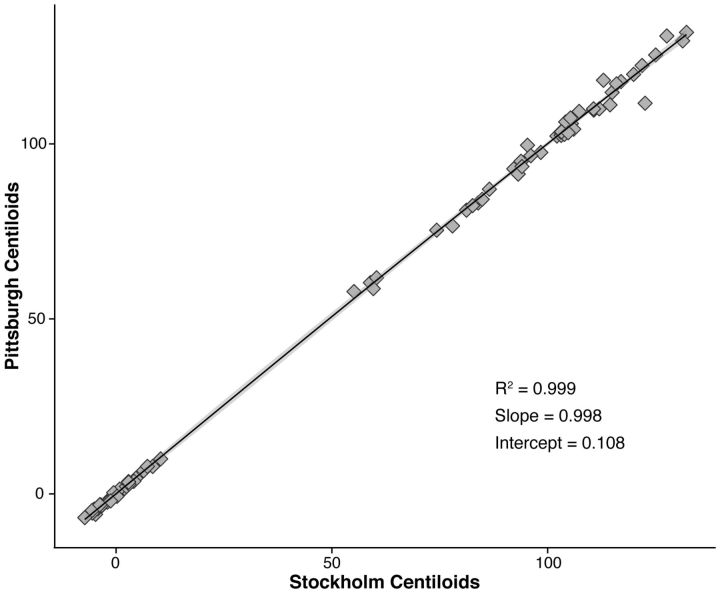
Owing to between-centre variability in PiB scanning windows, SUVr data were standardized using a recent method that allows for linear scaling of amyloid PET outcome data to a 100-point scale (Klunk et al., 2015), the units of which have been termed ‘Centiloids’. After downloading de-identified PiB and MRI data from the Global Alzheimer’s Association Information Network website (GAAIN; http://www.gaain.org) for 34 amyloid-negative young controls (YC-0) and 45 amyloid-positive Alzheimer’s disease subjects (AD-100), we confirmed the validity of our analysis pipeline via replication of the Level 1 analysis (linear correlation between downloaded/recalculated PiB Centiloid values: slope of 0.99, intercept of 1.03, and R2 of 0.99; Fig. 1) (Klunk et al., 2015). As a result of having scanning intervals up to 90 min, we were restricted to the subset of subjects with PET data over this time interval (0–90 min; 16 YC-0 and 40 AD-100) in the Level 2 calibration of our non-standard approach. Following exclusion of scans where there had been difficulty with subject positioning in the scan field, the final sample used to derive slope and intercept parameters for calculation of ‘PiB calculated’ SUVr values (PiB-CalcSUVr), were 11 for YC-0 and 35 for AD-100. After comparing SUVr values obtained using both standard (50–70 min, global cortical target region and whole cerebellum reference volumes of interest) and non-standard approaches via linear regression (PiBUNITIND: R2 between 0.96 and 0.97), PiB-CalcSUVr data for each of the four time windows was converted into Centiloid values (PiBCentiloidstd YC-0: SD between 6.62 and 25.4, relative variance between 0.26 and 5.17; PiBCentiloidstd AD-100: SD between 21.31 and 22.95, relative variance between 1 and 1.08), using the mean SUVr of the 34 YC-0 and 45 AD-100 subjects.
Figure 1.
Linear correlation plot showing the relationship between original/recalculated PiB Centiloids. Original (Pittsburgh) and recalculated (Stockholm) PiB Centiloids are shown on the ordinate and abscissa, respectively (YC-0, n = 34; AD-100, n = 45).
To set a threshold for PiB positivity using Centiloids, we adopted a composite cortical SUVr cut-off of 1.41, representing the upper 95% confidence limit from a previously characterized population of normally distributed healthy controls for whom PiB data have been acquired 40–60 min after injection (Nordberg et al., 2013). Using 40–60-min slope and intercept parameters, this cut-off was converted to Centiloid units, giving a cut-off of 34. This value was then used to subdivide subjects into PiB+ (>34) and PiB− (≤34).
Statistical analysis
Statistical analyses were performed with R, v.3.2.3. Between-group comparisons were done using Kruskal-Wallis ANOVA for continuous values and chi-square (sex, education) or Fisher’s exact tests (APOE) for categorical values. Post hoc Mann Whitney U-tests were performed where appropriate. These analyses were corrected for multiple comparisons using false discovery rate (FDR). The ratio of amyloid-β42 to amyloid-β40 (MSD and MS-RMP) was calculated according to a previously published formula: [(amyloid-β42) / (amyloid-β40) × 10] (Hansson et al., 2007). Linear regression analyses were performed to assess the relationship between values from the different CSF analytical platforms, as well as between published Centiloid values and those calculated at our site.
Concordance between PiB PET (visual, Centiloid) and CSF (amyloid-β42, amyloid-β42/40) was defined as the proportion of subjects positive or negative for both biomarkers (i.e. concordant positive, PiB+/CSF+, or concordant negative, PiB−/CSF−). Discordance between PiB and CSF was defined as the proportion of individuals with only one abnormal biomarker (i.e. discordant with PiB positivity, PiB+/CSF−, or discordant with CSF positivity, PiB−/CSF+). Agreement between visual and Centiloid based classification was assessed using percentage agreement and Fleiss k.
Results
Participant characteristics and CSF mixture modelling cut-offs
Subject characteristics according to diagnostic group are presented in Table 1. Groups did not differ in terms of age, sex, education, or in the period between CSF and PiB PET assessments. As expected, MMSE scores were lower in patient groups, relative to cognitively normal subjects (MCI, Alzheimer’s disease, FTD, P < 0.001; VaD, P = 0.01), and differed between patient groups (MCI > Alzheimer’s disease and FTD, P < 0.001). No significant differences in the prevalence of the APOE ϵ4 allele were found between groups. Levels of total tau were higher in Alzheimer’s disease with respect to cognitively normal subjects (P < 0.001), FTD and VaD (P < 0.01). Compared to cognitively normal subjects, phosphorylated tau levels were higher in MCI and Alzheimer’s disease (P < 0.01 and 0.001, respectively) and, relative to MCI, higher in Alzheimer’s disease (P < 0.001). Phosphorylated tau was also found to be higher in Alzheimer’s disease, compared to FTD and VaD (P < 0.001). The unbiased cut points to identify an abnormal concentration of amyloid-β in CSF were <515 pg/ml and 0.72 (MSD amyloid-β42 and amyloid-β42/40, respectively) and <896 pg/ml and 0.76 (MS-RMP amyloid-β42 and amyloid-β42/40, respectively).
Table 1.
Demographic, clinical and biomarker characteristics according to diagnostic group
| CN | MCI | AD | FTD | VaD | |
|---|---|---|---|---|---|
| (n = 13) | (n = 81) | (n = 122) | (n = 20) | (n = 7) | |
| Age, years | 67 (69, 60) | 64 (70, 58) | 65 (72, 59) | 64 (70, 60) | 61 (74, 57) |
| Sex, M: F (% F) | 6: 7 (54%) | 37: 44 (54%) | 50: 72 (59%) | 9: 11 (55%) | 3: 4 (57%) |
| Education, 1–4 | 3 (4, 2.8) | 3 (4, 2) | 3 (3, 2) | 2 (3, 2) | 2 (3, 1.5) |
| MMSE, points | 29 (30, 28) | 27 (28, 26) | 23 (26, 20) | 23 (27, 20) | 26 (27.5, 22.5) |
| APOE ε4, ≥ 1 allele† | 4 (0%) | 25 (57%) | 29 (56%) | 2 (33%) | N/A |
| INNOTEST Aβ42, pg/ml | 843 (900, 732) | 535 (698, 409) | 413 (530, 308) | 641 (726, 433) | 491 (675.5, 386.5) |
| INNOTEST Aβ42 positive | 1 (8%) | 46 (57%) | 96 (79%) | 10 (50%) | 5 (71%) |
| INNOTEST T-tau, pg/mla | 252 (318, 204) | 313 (520, 210) | 488 (772, 326) | 307 (409, 193) | 261 (306, 219) |
| INNOTEST P-tau, pg/mlb | 43 (58, 33) | 59 (77, 43) | 74 (107, 57) | 43 (64, 31) | 41 (48, 28) |
| CSF-PiB, months | 2.63 (7.05, 1.55) | 3.93 (8.30, 1.77) | 2.43 (5.14, 0.74) | 2.03 (3.87, 1.09) | 3.47 (5.20, 2.93) |
| PiB positive (Visual) | 1 (8%) | 50 (62%) | 114 (93%) | 3 (15%) | 0 (0%) |
| PiB, SUVr | 1.26 (1.32, 1.21) | 1.54 (1.87, 1.24) | 1.83 (2.05, 1.67) | 1.18 (1.25, 1.09) | 1.25 (1.27, 1.11) |
| PiB, Centiloid | 15.1 (20.8, 8.3) | 47.5 (87.6, 10.9) | 84.1 (110.8, 63.1) | 4.6 (13.8, −5.9) | 12.8 (14.7, -3.9) |
| PiB positive (Centiloid) | 1 (8%) | 47 (58%) | 112 (92%) | 3 (15%) | 0 (0%) |
Values are reported as median (quartile3, quartile 1), or as n (%). PiB SUVr and Centiloid refer to global cortical composite.
Aβ42 = amyloid-β42; AD = Alzheimer’s disease; APOE ε4 = ε4 allele of apolipoprotein E; CN = cognitively normal older individuals; MMSE = Mini-Mental State Examination; N/A = not applicable; P-tau = tau hyperphosphorylated at threonine 181: T-tau = total tau.
Owing to between country differences, a 4-point scale was used for educational level: 1 = basic schooling, 2 = professional training, 3 = college education, 4 = university degree.
aData missing for nine cognitively normal, 37 MCI, 70 Alzheimer’s disease, 14 FTD, and seven VaD subjects.
bData missing for three MCI, three Alzheimer’s disease, one FTD, and one VaD subject.
cData missing for eight MCI, 16 Alzheimer’s disease, and three FTD subjects.
Pittsburgh compound B PET findings
The proportion of PiB scans visually rated as positive was greatest in Alzheimer’s disease (93%), followed by MCI (62%), FTD (15%), cognitively normal (8%), and VaD (0%). Global Centiloid was higher in patient groups in comparison to cognitively normal (MCI, Alzheimer’s disease, P < 0.001), in Alzheimer’s disease, FTD, and VaD, in comparison to MCI (P < 0.001, 0.001, 0.01, respectively) and in Alzheimer’s disease, relative to FTD and VaD (P < 0.001). No difference was found between FTD and VaD.
CSF amyloid-β findings
Locally measured INNOTEST and reanalysed CSF levels (Table 2) were lower in patients, relative to controls (P < 0.05), with levels in Alzheimer’s disease lower than those in MCI (P < 0.001). Using MSD and MS-RMP, amyloid-β42 concentrations were lower in MCI (P < 0.05) and Alzheimer’s disease (P < 0.001), with respect to cognitively normal subjects. Further, amyloid-β42 values were lower in Alzheimer’s disease CSF, compared to MCI (P < 0.001), FTD, and VaD (P < 0.01). Findings using the ratio of amyloid-β42 to amyloid-β40 were in agreement with those for amyloid-β42: cognitively normal versus MCI (P < 0.001), MCI versus Alzheimer’s disease (P < 0.001), Alzheimer’s disease versus FTD (P < 0.001) and VaD (P < 0.01). No between-group differences were found for amyloid-β40.
Table 2.
Results for INNOTEST and reanalysed CSF according to diagnostic group
| CN | MCI | AD | FTD | VaD | |
|---|---|---|---|---|---|
| (n = 13) | (n = 81) | (n = 122) | (n = 20) | (n = 7) | |
| INNOTEST Aβ42, pg/ml | 843 (900, 732) | 535 (698, 409) | 413 (530, 309) | 641 (726, 433) | 491 (675.5, 386.5) |
| Aβ42 positive | 1 (8%) | 46 (57%) | 96 (79%) | 10 (50%) | 5 (71%) |
| MSD Aβ40, pg/ml | 5363 (7369, 4425) | 5607 (7188, 4536) | 5476 (6556, 4333) | 5213 (6149, 4225) | 5187 (6180, 4499) |
| Aβ42, pg/ml | 524 (719, 428) | 352 (510, 249) | 258 (374, 193) | 448 (556, 300) | 529 (630, 369) |
| Aβ42, positive | 6 (46%) | 60 (74%) | 115 (94%) | 12 (60%) | 3 (43%) |
| Aβ42/Aβ40 | 0.97 (1.17, 0.85) | 0.60 (0.89, 0.48) | 0.51 (0.57, 0.42) | 0.93 (1.05, 0.78) | 1.03 (1.05, 0.79) |
| Aβ42/Aβ40 positive | 3 (23%) | 52 (64%) | 113 (93%) | 7 (35%) | 2 (29%) |
| MS-RMP Aβ40, pg/ml | 9305 (12647, 7301) | 8619 (11493, 6324) | 8160 (10903, 6353) | 7788 (9715, 6590) | 7510 (9982, 5762) |
| Aβ42, pg/ml | 956 (1286, 654) | 568 (863, 396) | 441 (617, 318) | 704 (968, 484) | 760 (1060, 498) |
| Aβ42 positive | 6 (46%) | 63 (78%) | 115 (94%) | 14 (70%) | 5 (71%) |
| Aβ42/Aβ40 | 0.96 (1.01, 0.76) | 0.61 (0.92,0.47) | 0.5 (0.6, 0.38) | 0.91 (1.06, 0.38) | 1.02 (1.10, 0.81) |
| Aβ42/Aβ40 positive | 3 (23%) | 58 (72%) | 117 (96%) | 10 (50%) | 2 (29%) |
Values are reported as median (quartile3, quartile 1), or as n (%).
Aβ = amyloid-β; AD = Alzheimer’s disease; CN = cognitively normal older individuals; MMSE = Mini-Mental State Examination.
Cut-offs used to determine positivity were as follows: INNOTEST amyloid-β42, <557 pg/ml; MSD amyloid-β42, <515 pg/ml; MSD amyloid-β42/40, <0.72; MS amyloid-β42, <896 pg/ml; MS amyloid-β42/40, < 0.76.
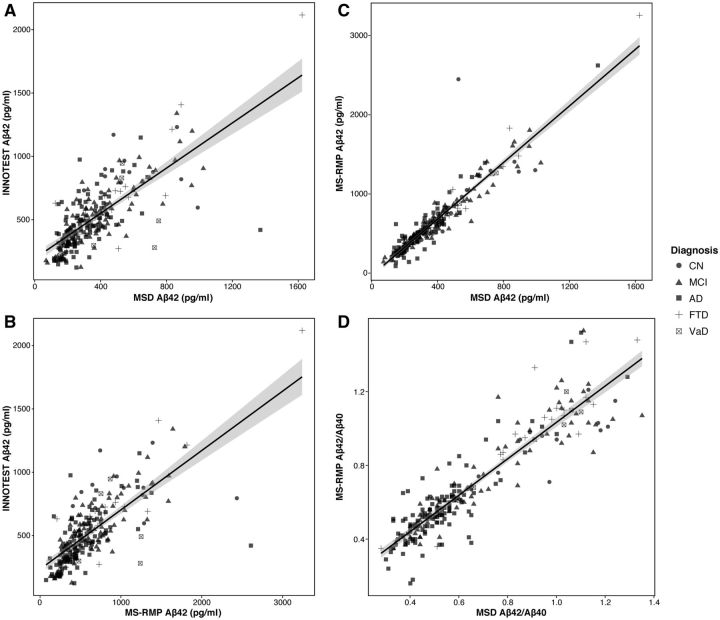
Relative to INNOTEST, amyloid-β42 concentrations were lower using MSD, and higher using MS-RMP; this pattern held across all groups, save for VaD subjects, where the inverse was found. For all subjects, however, MS-RMP amyloid-β40 levels were higher than those for MSD. Only minor differences were seen between platforms when using the ratio of amyloid-β42 to amyloid-β40. The correlation between local and centralized CSF measurements was moderate (INNOTEST amyloid-β42 and amyloid-β42 from MSD and MS-RMP: Spearman’s ρ = 0.74, P < 0.001; Spearman’s ρ = 0.74, P < 0.001, Fig. 2A and B, respectively), but high between the new, centralized measurements (MSD and MS-RMP): (amyloid-β42, Spearman’s ρ = 0.93, P < 0.001, Fig. 2C; amyloid-β42/40, Spearman’s ρ = 0.91, P < 0.001, Fig. 2D).
Figure 2.
Linear correlation plots showing the relationship between locally and centrally measured CSF amyloid-β. (A) INNOTEST and MSD amyloid-β42; (B) INNOTEST and MS-RMP amyloid-β42; (C) MSD and MS-RMP amyloid-β42; and (D) MSD and MS-RMP amyloid-β42/40.
Agreement between visual and Centiloid-based Pittsburgh compound B classification
Across groups, agreement between visual and Centiloid based PiB ratings was 97% (235 of 243; Cohen k = 0.92). Of the eight instances of disagreement, six (75%) were rated as visually positive, Centiloid negative (four MCI, two Alzheimer’s disease), and two as visually negative, Centiloid positive (one cognitively normal, one MCI). While discordance rates in FTD and VaD between visual assessment and Centiloid did not differ, rates in MCI were consistently higher when using Centiloid, with mixed findings in cognitively normal and Alzheimer’s disease subjects (Table 3).
Table 3.
Discordance findings between PiB and CSF across the various platforms investigated and using both visual and Centiloid
| CN | MCI | AD | FTD | VaD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 13) |
(n = 81) |
(n = 122) |
(n = 20) |
(n = 7) |
||||||
| Visual | Centiloid | Visual | Centiloid | Visual | Centiloid | Visual | Centiloid | Visual | Centiloid | |
| INNOTEST Aβ42, PiB | 2 (15%) | 3 (23%) | 18 (22%) | 20 (25%) | 29 (24%) | 30 (25%) | 9 (45%) | 9 (45%) | 5 (71%) | 5 (71%) |
| PiB+/CSF− | 1 | 2 | 11 | 10 | 23 | 23 | 2 | 2 | 0 | 0 |
| PiB−/CSF+ | 1 | 1 | 7 | 10 | 6 | 7 | 7 | 7 | 5 | 5 |
| MSD Aβ42, PiB | 7 (54%) | 6 (46%) | 18 (22%) | 21 (26%) | 12 (10%) | 13 (11%) | 9 (45%) | 9 (45%) | 3 (43%) | 3 (43%) |
| PiB+/CSF− | 1 | 1 | 4 | 4 | 5 | 5 | 0 | 0 | 0 | 0 |
| PiB−/CSF+ | 6 | 5 | 14 | 17 | 7 | 8 | 9 | 9 | 3 | 3 |
| Aβ42/Aβ40, PiB | 2 (15%) | 3 (23%) | 6 (7%) | 12 (15%) | 6 (5%) | 6 (5%) | 4 (20%) | 4 (20%) | 2 (29%) | 2 (29%) |
| PiB+/CSF− | 0 | 1 | 2 | 3 | 3 | 3 | 0 | 0 | 0 | 0 |
| PiB−/CSF+ | 2 | 2 | 4 | 9 | 3 | 3 | 4 | 4 | 2 | 2 |
| MS-RMP Aβ42, PiB | 7 (54%) | 6 (46%) | 21 (26%) | 25 (31%) | 12 (10%) | 13 (11%) | 11 (55%) | 11 (55%) | 5 (71%) | 5 (71%) |
| PiB+/CSF− | 1 | 1 | 4 | 4 | 5 | 5 | 0 | 0 | 0 | 0 |
| PiB−/CSF+ | 6 | 5 | 17 | 21 | 7 | 8 | 11 | 11 | 5 | 5 |
| Aβ42/Aβ40, PiB | 3 (23%) | 4 (31%) | 10 (12%) | 14 (17%) | 8 (7%) | 9 (7%) | 7 (35%) | 7 (35%) | 2 (29%) | 2 (29%) |
| PiB+/CSF− | 0 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 |
| PiB−/CSF+ | 3 | 3 | 9 | 13 | 6 | 7 | 7 | 7 | 2 | 2 |
Values are reported as n or n (%).
AD = Alzheimer’s dementia; Aβ42 = amyloid-β42; Aβ42/40 = amyloid-β42/40; CN = cognitively normal older individuals; MMSE = Mini-Mental State Examination.
Concordance between PiB Centiloid and CSF amyloid-β
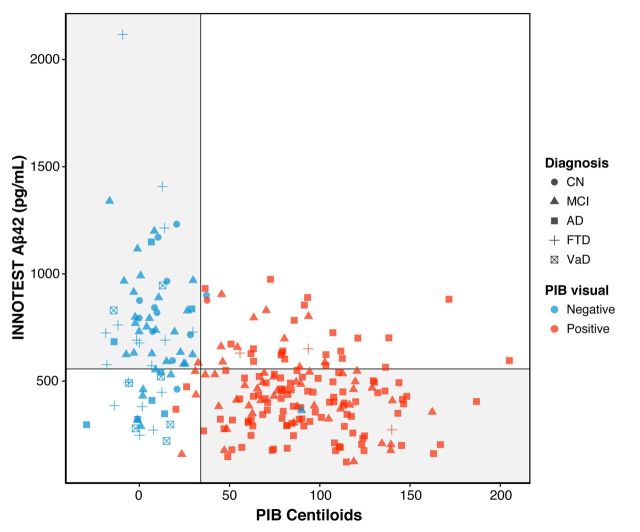
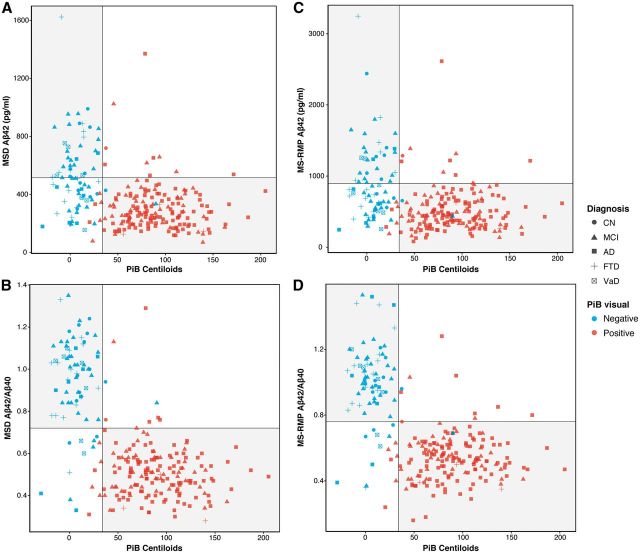
Using the total sample set, the concordance between PiB and CSF amyloid-β42 concentrations measured by INNOTEST, MSD and MS-RMP were 73%, 77% and 76%, respectively (Figs 3, 4A and B). Using the ratio of amyloid-β42 to amyloid-β40 further improved the concordance (MSD, 90%; MS-RMP 88%, see Fig. 4C and D).
Figure 3.
Scatterplot showing concordance between INNOTEST amyloid-β42 and PiB Centiloids. Circles indicate cognitively normal subjects, triangles MCI, squares Alzheimer’s disease, crosses FTD, and crossed squares VaD. The vertical line reflects the Centiloid cut-off of 34; the horizontal line the cut-off of 557 pg/ml for INNOTEST amyloid-β42. Blue indicates PiB scans were visually rated as negative, red as positive. The grey quadrants indicate concordance between amyloid-β biomarkers (top left, concordant negative: PiB−/CSF−; bottom right, concordant positive: PiB+/CSF+). The white quadrants indicate discordance between amyloid-β biomarkers (bottom left, discordant with isolated CSF positivity: PiB−/CSF+; top right, discordant with isolated PiB positivity: a PiB+/CSF−).
Figure 4.
Scatterplots reflecting concordance between PiB Centiloids and reanalysed CSF. (A) MSD amyloid-β42 (cut-off < 515 pg/ml). (B) MSD amyloid-β42/40 (cut-off < 0.72); (C) MS-RMP amyloid-β42 (cut-off < 896 pg/ml), and (D) MS-RMP amyloid-β42/amyloid-β40 (cut-off < 0.76). Grey circles indicate cognitively normal healthy control subjects, triangles indicate MCI, squares indicate Alzheimer’s disease, crosses FTD, and crossed squares VaD. The vertical lines reflects the Centiloid cut-off of 34; the horizontal lines the cut-offs of < 515 pg/ml, <0.72, <896 pg/ml, and <0.76 for MSD (amyloid-β42, amyloid-β42/40) and MS-RMP (amyloid-β42, amyloid-β42/40), respectively. Blue indicates PiB scans visually rated as negative, red as positive. The grey quadrants indicate concordance between amyloid-β biomarkers (top left, concordant negative: PiB−/CSF−; bottom right, concordant positive: PiB+/CSF+). The white quadrants indicate discordance between amyloid-β biomarkers (bottom left, discordant with isolated CSF positivity: PiB−/CSF+; top right, discordant with isolated PiB positivity: a PiB+/CSF−). Aβ = amyloid-β.
Using reanalysed amyloid-β42, concordance was highest in Alzheimer’s disease and MCI, with findings for cognitively normal, FTD, and VaD varying between MSD and MS-RMP techniques. In VaD subjects, discordance was the same as INNOTEST using MS-RMP, but lower using MSD; while across FTD, MCI, and cognitively normal subjects, discordance was higher using MSD and MS-RMP. Across all groups, however, the ratio of amyloid-β42 to amyloid-β40 was better than when using amyloid-β42 alone, with the greatest change seen in the VaD group. When looking at discordance with local amyloid-β42, PET was overall more often abnormal than CSF, with isolated CSF positivity predominant when using the reanalysed data (Fig. 5).
Figure 5.

Frequency plots showing different agreement profiles between PiB PET and CSF. Values of <557 pg/ml (INNOTEST amyloid-β42), <515 pg/ml (MSD amyloid-β42), <0.72 (MSD amyloid-β42/40), <896 pg/ml (MS-RMP amyloid-β42), <0.76 (MS-RMP amyloid-β42/40), and global Centiloid value > 34 were used to classify subjects as concordant positive (PiB+/CSF+), concordant negative (PiB−/CSF−), discordant with CSF positivity (PiB−/CSF+), and discordant with PiB positivity (PiB+/CSF−). Aβ = amyloid-β.
Comparison of concordant and discordant subjects
Comparison of subjects showing concordant and discordant amyloid biomarker results are shown in Supplementary Tables 1–5. Using INNOTEST amyloid-β42, total and phosphorylated tau were found to be higher in concordant positive subjects, relative to those concordant negative and those discordant with abnormal CSF. Tau levels were likewise found to higher the discordant PET positive group, relative to both discordant with isolated abnormal CSF and discordant negative subgroups (Supplementary Table 1). When using reanalysed CSF values, the prevalence of the APOE ϵ4 allele was found to be higher in those concordant positive, relative to those concordant negative. Age differences were noted between groups defined using MSD and MS-RMP amyloid-β42, though findings were not consistent. Similarly, a greater percentage of females was found in the concordant positive group for MSD amyloid-β42/40, relative to PiB−/CSF−, though for MS-RMP amyloid-β42, a greater percentage of females was noted in the PiB−/CSF+ group, relative to those concordant positive. Mini-Mental State Examination scores were lower in those with concordant positive findings, relative to those concordant negative; findings, however, varied across platforms in comparison to the other biomarker pairings. With respect to the interval between CSF and PET, only for MS-RMP amyloid-β42 was a difference found (PiB+/CSF− > PiB+/CSF+ and PiB−/CSF+, P < 0.05). Supplementary Fig. 1 shows the relationship between concordance (INNOTEST amyloid-β42 and PiB Centiloids) and CSF tau findings.
Discussion
Here, we report concordance levels between PiB PET and CSF amyloid-β (amyloid-β42 and amyloid-β42/40) using subjects from the European BIOMARKAPD initiative. Despite good agreement between these measures, discordance was observed in a subset of patients across all groups, using both local and centrally analysed measurements. Given the well-described intra- and intercentre variability in CSF amyloid-β42 concentrations using INNOTEST ELISA (Mattsson et al., 2011), it was expected that concordance with PiB would be increased using reanalysed measurements. Comparison of discordance using local and reanalysed CSF, however, yielded variable results, with improved agreement with PiB classification seen only in the Alzheimer’s disease patients using reanalysed amyloid-β42—and VaD, when using MSD amyloid-β42—and across patient groups when using amyloid-β42/40. This pattern may suggest that the reanalysed results are a more faithful approximation of amyloid-β42 levels across groups, the centralized analysis component having removed the variance imposed by differences in INNOTEST measurements between laboratories. While it is thus tempting to speculate that MSD and MS-RMP providing more accurate estimates of amyloid-β42 concentration levels, this explanation seems unlikely since these analytical techniques have been shown to correlate tightly—both with one another, and with INNOTEST—when samples are analysed in a single run under standardized conditions (Bjerke et al., 2016).
As expected, concordance rates matched closely across groups using MSD and MS-RMP. Relative to MSD, MS-RMP classified an additional four patients (one in both the MCI and FTD groups, two in VaD group) as CSF positive using amyloid-β42, and one cognitively normal subject using amyloid-β42/40. Concordance findings using the ratio of amyloid-β42 to amyloid-β40—a measure which adjusts for ‘high’ and ‘low’ amyloid-β production levels, and thus for false negative (just above the cut-off) and false positive amyloid-β42 results, respectively (Wiltfang et al., 2007; Lewczuk et al., 2015)—were, as expected, greater than when using amyloid-β42 alone. This effect was greatest in the FTD and VaD groups. Though based on a relatively small sample size, this finding ties into recent work showing that subcortical changes non-specific to Alzheimer’s disease may alter global levels of amyloid-β isoforms (Janelidze et al., 2016; van Westen et al., 2016). This raises the possibility that the amyloid-β42/40 ratio may allow for differentiating conditions in which amyloid-β40 and amyloid-β42 can be reduced in parallel, from true amyloid-positive cases. Future studies, however, will be needed to better understand the relationship between Alzheimer’s disease pathology and vascular changes, as well as matrix effects on amyloid-β42 measurements.
PiB-PET scans in the present study ranged from 40- to 90-min post-injection, with 20- or 30-min time windows used to normalize global cortical uptake to that within the cerebellar grey matter. Given that the range of PiB SUVr values using this reference tissue have been shown to vary dynamically over time (Lopresti et al., 2005; McNamee et al., 2009), the Centiloid approach was implemented to ensure appropriate comparability of imaging results. In line with previous studies using visual and quantitative-based reads (Rabinovici et al., 2011; Mountz et al., 2015), high agreement was observed between both classification methods, with all cases rated as visually positive, Centiloid negative, exhibiting values close to the cut-off. Though few in number, these cases highlight that subjects with borderline positive values can prove visually challenging and contribute to discrepant interpretations across assessment approaches. In the two subjects classified as amyloid-positive using Centiloids and negative using visual assessment, the pattern of cortical uptake was unclear owing to atrophy, high white matter signal, and poor image quality. Though the clinical use of amyloid-PET in routine clinical practice will likely depend on visual assessment alone, our findings suggest that Centiloid ratings can be used independently, or as a valuable adjunct to visual reads in multicentre studies.
The global composite PiB SUVr cut-off of 1.41 was selected for use in the present study owing to it having been previously established using our processing pipeline, and due to the fact that we were restricted to the use of a PiB-PET template owing to limited availability of structural imaging in our study population. Though somewhat more conservative than the only other Centiloid cut-off of 27.78 available in the literature (Ayakta et al., 2016), our higher cut-off reflects differences in populations, method for cut-off selection, and processing pipelines, including the choice of scanning window and volumes of interest. For instance, our approach used slope and intercept parameters from a 40–60 min post-injection interval to determine Centiloid equivalence to our SUVr cut-off (in contrast to 50–70 min), itself established using the 95th percentile approach in healthy older subjects [versus receiver operating characteristic (ROC) curve in a mixed patient population, with CERAD score as standard of truth]. Indeed, back calculation of the 27.78 Centiloid cut-off to SUVr using our pipeline yielded an SUVr close to ours (1.38), highlighting the effects of varying analysis methods, the resulting limited comparability of SUVr data across centres, and the attendant importance of Centiloid standardization.
Classification mismatch using PiB and amyloid-β42 may be due a number of factors. Most mismatched subjects in our study had isolated low CSF amyloid-β. In some individuals, this was due PiB retention being only somewhat elevated, and thus falling below the cut point for positivity. In addition, since PiB binds only weakly to amorphous amyloid-β plaques (Bacskai et al., 2007; Ikonomovic et al., 2008), PiB prove unable to detect variants of Alzheimer’s disease characterized by the predominance of diffuse (non-fibrillar) plaques (Cairns et al., 2009; Schöll et al., 2012). Of course low amyloid-β42 in CSF is also known to occur in isolation in non-Alzheimer’s disease conditions (Ewers et al., 2015; Skillbäck et al., 2015) and may also be related to entrapment in interstitial drainage pathways (Weller et al., 2001), epitope masking due to binding of amyloid-β42 to carrier proteins (Otto et al., 2000; Wiltfang et al., 2003), or the presence of amyloid as a secondary pathology. In cognitively normal subjects, abnormal CSF amyloid-β42 can also be seen, and is thought to possibly represent preclinical Alzheimer’s disease (Fagan et al., 2009a; Sperling et al., 2011; Palmqvist et al., 2016). Isolated increased PiB uptake using INNOTEST data likely reflected between-centre variability in INNOTEST results; using reanalysed data, however, this pattern was infrequent and was seen mainly in MCI and Alzheimer’s disease. This finding may in some cases be due to cut-off levels but may also reflect the possibility that fibrillar amyloid-β can be detected first in some individuals, as has been reported previously (Koivunen et al., 2008; Forsberg et al., 2010; Landau et al., 2013).
Among the studies that have thus far examined the association between amyloid PET and CSF amyloid-β42 (Fagan et al., 2006, 2007, 2009b; Forsberg et al., 2008, 2010; Jagust et al., 2009; Tolboom et al., 2009; Degerman Gunnarsson et al., 2010; Weigand et al., 2011; Landau et al., 2013; Zwan et al., 2014, 2016; Palmqvist et al., 2014, 2016; Mattsson et al., 2015), few have directly compared tau levels in those with concordant and discordant biomarker results. In the studies that have, tau was found to be higher in PET+/CSF+ subjects, relative to those PET−/CSF+ (Palmqvist et al., 2016; Zwan et al., 2016), in PET+/CSF+ subjects, relative to those PET−/CSF+ (Mattsson et al., 2015; Zwan et al., 2016), and in subjects discordant with PET positivity, relative to both those concordant negative and discordant with abnormal CSF (Zwan et al., 2016). Using INNOTEST amyloid-β42, our findings match those reported up to now. Comparison of tau levels between biomarker subgroups defined using reanalysed CSF, however, was not performed, due to differences in CSF methodology (INNOTEST versus xMAP Luminex multiplexing). Though group differences were noted for gender and APOE in the present study, in the aggregate with findings from other studies (Mattsson et al., 2015; Toledo et al., 2015), the precise modulatory role exerted by these variables is not yet clear. Lastly, concordance findings here reported are unlikely to have been much altered by inclusion of CSF tau data, with the majority of abnormal tau values clustered in the concordant positive quadrant. Future studies incorporating new approaches for CSF tau quantification and multivariate modelling are required to further clarify differences between biomarker subgroups.
Though discordance between CSF and amyloid-β PET has thus far been discussed primarily in the context of the increasing use of these two techniques in the diagnosis of cognitively impaired individuals, an additional area of importance is that of future clinical trials. In one of the largest studies conducted so far on the relationship between amyloid-β biomarkers, it was shown that concordance increased significantly as a function of disease stage (Mattsson et al., 2015), with the highest levels of discordance noted in cognitively normal and subjective memory complaint subjects. In a related study focusing on non-demented subjects diagnosed as cognitively healthy controls or MCI, evidence was shown in support of the hypothesis that CSF may detect amyloid-β accumulation in preclinical Alzheimer’s disease earlier than amyloid PET (Palmqvist et al., 2016). There is thus increasing evidence to suggest that discussions surrounding the interchangeability of amyloid-β biomarkers may be less applicable with respect to the detection of cerebral amyloidosis in the preclinical phase of Alzheimer’s disease. Specifically, isolated CSF positivity may represent a profile suitable for inclusion in clinical trials of amyloidocentric agents (Hardy et al., 2014), or related interventions targeting modifiable risk factors (Ngandu et al., 2015). Given unresolved differences between current classification schemes (Sperling et al., 2011; Dubois et al., 2014, 2016), further follow-up studies to verify progression to amyloid PET positivity, neurodegenerative changes and cognitive decline, are required.
A possible limitation of this study was the lack of gold standard autopsy confirmation. In addition to confirming or refuting clinical diagnoses for included subjects, post-mortem data would possibly have allowed for an improved understanding of PiB negative discordant cases, including the potential effects of coincident pathologies. Autopsy data would, moreover, have helped clarify the seven cases showing discrepant classifications using visual and Centiloid based methods; at least five of the six visually positive cases, however, would likely have crossed the SUVr/Centiloid threshold for positivity had partial volume correction (PVC) been applied. Though the lack of PVC stands as a potential caveat, its application would likely only have resulted in the relocation of a subset of isolated CSF+ cases to the concordant positive quadrant. Future studies on amyloid biomarkers, however, should examine the effect of this type of correction. As a further caveat, the INNOTEST amyloid-β42 ELISA would ideally have been used instead of MSD, though the effect of this is likely to have been minimal (Bjerke et al., 2016). Other possible limitations include the relatively low number of cognitively normal subjects and patients with FTD and VaD, as well as the fact that APOE genotype data was not available for all subjects. Lastly, CSF classification cut-offs were established with mixture modelling; while a robust approach for establishing unbiased thresholds, and used in several studies (Buchhave et al., 2012; Palmqvist et al., 2014), these cut-offs cannot be viewed as generalizable beyond the present work.
Overall, the agreement levels between amyloid PET and CSF amyloid-β42 here reported are in line with previous studies, and support the strong agreement of these two metrics in MCI and Alzheimer’s disease, with mainly isolated low CSF amyloid-β42 in FTD, VaD and cognitively normal subjects. While technical factors cannot be excluded outright as contributing to measured amyloid-β42 and amyloid-β40 levels in CSF assays, even when run in the same time and place, continued discordance using centrally reanalysed samples suggests that biological factors are also at play. While further studies are required, in particular longitudinal studies on amyloid biomarker trajectories, with a focus on intraindividual change, our findings suggest that, at least in a minority of subjects, these measures may not be interchangeable, reflecting instead distinct but interrelated processes. Future work using standardized amyloid PET and CSF amyloid-β42, as well as post-mortem pathology data, will be critical to gaining an improved understanding of amyloid biomarker discordance.
Supplementary Material
Acknowledgements
The authors would like to thank William E. Klunk, PhD (University of Pittsburgh) and Victor L. Villemagne, MD (University of Melbourne) for valuable discussions pertaining to the Centiloid method.
Glossary
Abbreviations
- AD-100
amyloid-β-positive Alzheimer’s disease subjects used as part of Centiloid standardization
- FTD
frontotemporal dementia
- MCI
mild cognitive impairment
- MSD
Meso Scale Discovery ELISA
- MS-RMP
mass spectrometry-based candidate reference measurement procedure
- PiB
Pittsburgh compound B
- SUVr
standardized uptake value ratio
- VaD
vascular dementia
- YC-0
amyloid-β-negative young controls used as part of Centiloid standardization
Funding
This study was part of BIOMARKAPD, EU Joint Programme–Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organizations under the aegis of JPND (www.jpnd.eu): Stockholm (A.L., K.C. and A.N.), the Swedish Research Council (projects 529-2012-14 and 05817), the Karolinska Institutet Strategic Neuroscience program, the Stockholm Country Council-Karolinska Institutet regional agreement on medical training and clinical research (ALF grant), Swedish Brain Power, the Swedish Brain Foundation, Swedish Alzheimer Foundation, Gun and Bertil Stohnes foundation, Demensfonden, the Alzheimer Foundation in Sweden, the Foundation for Old Servants, the Swedish Foundation for Strategic Research (SSF), and the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° HEALTH-F2-2011-278850 (INMiND); Gothenburg (E.P., J.P., H.Z., and K.B.) the Swedish Research Council (project 529-2012-14), the Gamla Tjänarinnor foundation, ERC (681712), the Wolfson Foundation, the Knut and Alice Wallenberg Foundation, Frimurarestiftelsen and the Alzheimer Foundation, the Torsten Söderberg foundation, Hjärnfonden and the Swedish Alzheimer Foundation; Barcelona (A.Leó, R.B. and J.F.), Instituto de Salud Carlos III (PI11/03035-BIOMARKAPD, PI11/02425 and PI14/01126, PI13/01532, PI14/01561), jointly funded by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, ‘Una manera de hacer Europa’ and ‘Marató TV3’ grant 20142610; Turku (JOR and SKH), Academy of Finland (decision no. 263193), Sigrid Juselius Foundation, Turku University Hospital Clinical Grants; Ulm (M.O., S.A.S., C.A.F.V.A., and A.B.), BMBF (Ministry of Science and Technology): Competence net neurodegenerative dementias (project: FTLDc), the JPND networks for standardisation of biomarkers (SOPHIA) and the JPND project, PreFronals, the, foundation of the state of Baden-Wuerttemberg and The Thierry Latran Foundation and BIU (Boehringer Ingelheim Ulm University BioCentre).
Conflict of interest
K.B. reports personal fees from IBL International, Roche Diagnostics and Eli Lilly. C.A.F.V.A. reports personal fees from Desitin Arzneimittel GmbH, Dr Willmar Schwabe GmbH &Co, personal fees and non-financial support from Nutricia GmbH, Lilly Deutschland GmbH, and grants from Roche Diagnostics GmbH, Biologische Heilmittel Heel GmbH, and ViaMed GmbH. J.O.R. serves as a consultant neurologist for Clinical Research Services Turku (CRST) Ltd. A.N. has received grants from GE Healthcare and Bayer Healthcare, served on the scientific advisory boards of GE Healthcare, Avid, and Eli Lilly, and received speaker honorarium from GE Healthcare, Piramal, Novartis, and Bayer Healthcare.
Supplementary material
Supplementary material is available at Brain online.
References
- Andreasson U, Vanmechelen E, Shaw LM, Zetterberg H, Vanderstichele H. Analytical aspects of molecular Alzheimer's disease biomarkers. Biomark Med 2012; 6: 377–89. [DOI] [PubMed] [Google Scholar]
- Ayakta N, Lockhart S, O'Neill J, Ossenkoppele R, Reed B, Olichney J, et al. Centiloid thresholds for amyloid positivity derived from autopsy-proven cases. In: Human Amyloid Imaging Conference Book of Abstracts, ID PP89, 29–30, Miami, FL, Jan 13–15, 2016, World Events Forum, Inc. [Google Scholar]
- Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, et al. Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch Neurol 2007; 64: 431–4. [DOI] [PubMed] [Google Scholar]
- Benaglia T, Chauvreau D, Hunter DR, Young DS. mixtools: an R package for analyzing finite mixture models. J Stat Softw 2009; 32: 1–29. [Google Scholar]
- Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, et al. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010; 2010: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke M, Andreasson U, Kuhlmann J, Portelius E, Pannee J, Lewczuk P, et al. Assessing the commutability of reference material formats for the harmonization of amyloid beta measurements. Clin Chem Lab Med 2016; 54: 1177–91. [DOI] [PubMed] [Google Scholar]
- Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer's disease. Trends Pharmacol Sci 2015; 36: 297–309. [DOI] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 2012; 69: 98–106. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Ikonomovic MD, Benzinger T, Storandt M, Fagan AM, Shah AR, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol 2009; 66: 1557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degerman Gunnarsson M, Lindau M, Wall A, Blennow K, Darreh-Shori T, Basu S, et al. Pittsburgh compound-B and Alzheimer's disease biomarkers in CSF, plasma and urine: an exploratory study. Dement Geriatr Cogn Disord 2010; 29: 204–12. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, et al. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J Neurochem 2002; 81: 229–36. [DOI] [PubMed] [Google Scholar]
- Dillen L, Cools W, Vereyken L, Timmerman P. A screening UHPLC-MS/MS method for the analysis of amyloid peptides in cerebrospinal fluid of preclinical species. Bioanalysis 2011; 3: 45–55. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–29. [DOI] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016; 12: 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Mattsson N, Minthon L, Molinuevo JL, Antonell A, Popp J, et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease: A large-scale international multicenter study. Alzheimers Dement 2015; 11: 1306–15. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006; 59: 512–9. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007; 64: 343–9. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009a; 65: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med 2009b; 1: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM. What does it mean to be ‘amyloid-positive'? Brain 2015; 138 (Pt 3): 514–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 2008; 29: 1456–65. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res 2010; 7: 56–66. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, et al. Prediction of Alzheimer's disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord 2007; 23: 316–20. [DOI] [PubMed] [Google Scholar]
- Hardy J Bogdanovic N Winblad B Portelius E Andreasen N Cedazo-Minguez A, et al. Pathways to Alzheimer's disease. J Intern Med 2014; 275: 296–303. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008; 131 (Pt 6): 1630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS Jagust WJ Petersen RC Weiner MW Aisen PS et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology 2009; 73: 1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S Zetterberg H Mattsson N Palmqvist S Vanderstichele H Lindberg O, et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016; 3: 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 2001; 21: 372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306–19. [DOI] [PubMed] [Google Scholar]
- Klunk WE Koeppe RA Price JC Benzinger TL Devous MD Sr, Jagust WJ et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 2015; 11: 1–15.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen J, Pirttila T, Kemppainen N, Aalto S, Herukka SK, Jauhianen AM, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid beta-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord 2008; 26: 378–83. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013; 74: 826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinenbach A, Pannee J, Dulffer T, Huber A, Bittner T, Andreasson U, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem 2014; 60: 987–94. [DOI] [PubMed] [Google Scholar]
- Leuzy A, Carter SF, Chiotis K, Almkvist O, Wall A, Nordberg A. Concordance and diagnostic accuracy of [11C]PIB PET and cerebrospinal fluid biomarkers in a sample of patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 2015; 45: 1077–88. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer's disease: validation of two novel assays. J Alzheimers Dis 2015; 43: 183–91. [DOI] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005; 46: 1959–72. [PubMed] [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003; 46: 2740–54. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, et al. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement 2011; 7: 386–95.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer's Association quality control program. Alzheimers Dement 2013; 9: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer's disease. Brain 2015; 138 (Pt 3): 772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM Knopman DS Chertkow H Hyman BT Jack CR Jr, Kawas CH et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee RL, Yee SH, Price JC, Klunk WE, Rosario B, Weissfeld L, et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med 2009; 50: 348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountz JM, Laymon CM, Cohen AD, Zhang Z, Price JC, Boudhar S, et al. Comparison of qualitative and quantitative imaging characteristics of [(11)C]PiB and [(18)F]flutemetamol in normal control and Alzheimer's subjects. Neuroimage Clin 2015; 9: 592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–63. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer's disease. Eur J Nucl Med Mol Imaging 2013; 40: 104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M, Esselmann H, Schulz-Shaeffer W, Neumann M, Schroter A, Ratzka P, et al. Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology 2000; 54: 1099–102. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 2014; 71: 1282–9. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Mattsson N, Hansson O, Alzheimer's Disease Neuroimaging I. Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain 2016; 139 (Pt 4): 1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56: 303–8. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Rosen HJ, Alkalay A, Kornak J, Furst AJ, Agarwal N, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011; 77: 2034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993; 43: 250–60. [DOI] [PubMed] [Google Scholar]
- Schöll M, Wall A, Thordardottir S, Ferreira D, Bogdanovic N, Langstrom B, et al. Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology 2012; 79: 229–36. [DOI] [PubMed] [Google Scholar]
- Skillbäck T, Farahmand BY, Rosen C, Mattsson N, Nagga K, Kilander L, et al. Cerebrospinal fluid tau and amyloid-beta1-42 in patients with dementia. Brain 2015; 138 (Pt 9): 2716–31. [DOI] [PubMed] [Google Scholar]
- Weller RO. How well does the CSF inform upon pathology in the brain in Creutzfeldt-Jakob and Alzheimer's diseases? J Pathol 2001; 194: 1–3. [DOI] [PubMed] [Google Scholar]
- Sperling RA Aisen PS Beckett LA Bennett DA Craft S Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 2009; 50: 1464–70. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Bjerke M, Da X, Landau SM, Foster NL, Jagust W, et al. Nonlinear association between cerebrospinal fluid and florbetapir F-18 beta-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurol 2015; 72: 571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand SD, Vemuri P, Wiste HJ, Senjem ML, Pankratz VS, Aisen PS, et al. Transforming cerebrospinal fluid Abeta42 measures into calculated Pittsburgh Compound B units of brain Abeta amyloid. Alzheimers Dement 2011; 7: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westen D, Lindqvist D, Blennow K, Minthon L, Nagga K, Stomrud E, et al. Cerebral white matter lesions - associations with Abeta isoforms and amyloid PET. Sci Rep 2016; 6: 20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltfang J, Esselmann H, Bibl M, Hull M, Hampel H, Kessler H, et al. Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-Tau in patients with low- and high-CSF A beta 40 load. J Neurochem 2007; 101: 1053–9. [DOI] [PubMed] [Google Scholar]
- Wiltfang J, Esselmann H, Smirnov A, Bibl M, Cepek L, Steinacker P, et al. Beta-amyloid peptides in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Ann Neurol 2003; 54: 263–7. [DOI] [PubMed] [Google Scholar]
- Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. J Alzheimers Dis 2014; 41: 801–7. [DOI] [PubMed] [Google Scholar]
- Zwan MD, Rinne JO, Hasselbalch SG, Nordberg A, Lleo A, Herukka SK et al. Use of amyloid-PET to determine cutpoints for CSF markers: a multicenter study. Neurology 2016; 86: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.