Abstract
Increased concentrations of dissolved organic carbon (DOC), often labelled “browning”, is a current trend in northern, particularly boreal, freshwaters. The browning has been attributed to the recent reduction in sulphate (S) deposition during the last 2 to 3 decades. Over the last century, climate and land use change have also caused an increasing trend in vegetation cover (“greening”), and this terrestrially fixed carbon represents another potential source for export of organic carbon to lakes and rivers. The impact of this greening on the observed browning of lakes and rivers on decadal time scales remains poorly investigated, however. Here, we explore time-series both on water chemistry and catchment vegetation cover (using NDVI as proxy) from 70 Norwegian lakes and catchments over a 30-year period. We show that the increase in terrestrial vegetation as well as temperature and runoff significantly adds to the reduced SO4-deposition as a driver of freshwater DOC concentration. Over extended periods (centuries), climate mediated changes in vegetation cover may cause major browning of northern surface waters, with severe impact on ecosystem productivity and functioning.
The observed increased concentrations of dissolved organic carbon (DOC) of northern boreal lakes during the past two to three decades, often termed “browning”1,2 has attracted considerable attention due to the role of DOC in the global carbon cycle3, as well as its multitude of impacts on ecosystem processes and services4,5,6.
The browning has been attributed to various ambient drivers related to sulphate (SO42−) deposition, climate, hydrology and land-use1,7,8. Reduced sulphate deposition affects the export of DOC from terrestrial pools into freshwaters by altering the solubility of organic matter1,7,8. The underlying mechanisms have been suggested to involve a combination of increased charge density of humic substances and reduced ionic strength, both contributing to increasing organic matter solubility7,8. Also increased temperature and altered hydrology, enhancing soil carbon decomposition rates and the amount of water flow through soil as well as retention time, respectively, have been suggested to be important drivers of changes in DOC-fluxes9,10. The main source of coloured, allochthonous DOC in boreal surface water is terrestrially fixed organic carbon3 with origin from forests and peatlands11,12, and increases in soil organic matter pools, mediated by increases in terrestrial productivity, have been documented to promote DOC in surface waters on long (century) time scales13.
Currently, there is a prominent “greening” trend in many northern and alpine areas, i.e. increased vegetation, biomass carbon pools and/or forest cover. This trend has been attributed to climate warming, nitrogen deposition, reduced grazing activities and reduced forestry harvesting intensity14,15. Forest inventories and remote sensing studies have revealed a large increase in standing forest mass of Northern temperate areas over the past decades16. Also, a pronounced increase in growing season has been recorded over northern latitudes17. In a survey of 1000 Norwegian lakes and catchments, vegetation cover represented by the remote sensing derived Normalized Difference Vegetation Index (NDVI) was found to be the key spatial predictor of DOC, followed by bogs18. Linked to space-for-time predictions of changes in NDVI under a 2 °C climate scenario, this approach was used to predict a profound future browning of northern waters19. A large portion of riverine DOC export is constituted by “modern” (decade-old) carbon20,21 suggesting that an increase in terrestrial vegetation biomass and productivity in the course of decades may contribute to increases in DOC. However, empirical support for terrestrial carbon fixation promoting browning in boreal surface waters on a decadal timescale has yet not been presented.
In the present study, we investigate to what extent the observed greening of terrestrial vegetation contributes to changes in surface water DOC. We used time-series (1986–2013) of measured DOC from annual water samples in 70 lakes, and extracted annual composites of catchment vegetation proxies along with along with annual composites of the other suggested main drivers of the observed increase in DOC: catchment sulphur (S) deposition, temperature and runoff. The study sites were all from Norway and cover primarily pristine boreal headwater catchments along a large climatic gradient, as well as a gradient of sulphate deposition. All lakes in this region have experienced a substantial decline in S deposition since the 1980s2 resulting in considerable post-acidification chemical recovery.
Results
There was an increasing trend of DOC in the majority of the lakes during the study period (1986–2013) (Fig. 1) which corresponded to an average decadal increase of 1.7 mg l−1. The strongest increase in DOC was observed in the most acidified areas in south-eastern Norway which also have seen the strongest decreases in S-deposition. This is consistent with previously reported increase of organic carbon in many European and North American freshwaters2,22, and supports the strong link between decreased S deposition and increased DOC in previously acidified areas1.
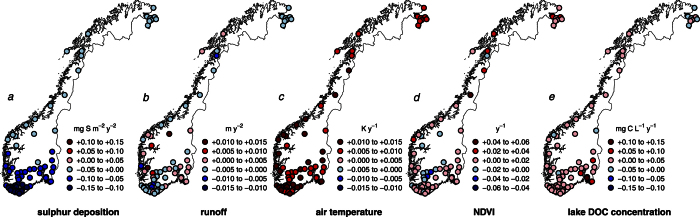
Figure 1. Lake specific trends in total atmospheric S deposition, runoff, surface air temperature, NDVI and lake DOC concentration.
Estimated Theil-Sen’s slope values (y−1) based on site-specific Regional Kendall Tests for (a) catchment total atmospheric S deposition, (b) runoff, (c) surface air temperature, (d) NDVI and (e) lake DOC concentration during the study period (1986–2011). Positive temporal trends are depicted using shades of red, whereas negative temporal trends are depicted using shades of blue. A stronger shade in either trend direction denotes a faster trend when compared regionally. The categorization levels were determined using R’s pretty function on absolute slope values73. All S deposition trends where negative, and all temperature trends where positive, 85% of the DOC trends and 76% of the NDVI trends were positive, and 65% of the catchments displayed negative runoff trends. Overall, the mean regional trends for the whole study area for all five variables were significant (positive for NDVI, temperature and DOC, negative for S deposition and runoff, Regional Kendall Tests with lakeID as block, n = 70, all with p < 0.017). Figure created in R v. 3.2.1 (URL http://www.R-project.org/)61 using the libraries raster60 and sp74.
Catchment vegetation (NDVI) and temperature also showed increasing trends (Fig. 1) 76% of the catchments had a positive NDVI trend, with a mean decadal increase of 5.2%. This supports previous findings of an increase in vegetation coverage or density in many northern and boreal areas14,15,23. Some areas had no response, and high altitude sites (above ca. 1000 m) in southern Norway showed a decreased trend for NDVI. The negative NDVI-trends in truly alpine areas possibly reflects increased precipitation as snowfall, hence later snowmelt and decreased growing season17,24, but could also be attributed to vegetation damage due to extreme weather events, herbivory and pests25. Runoff in the majority of the catchments tended to decline, but with no clear regional pattern (Fig. 1). We additionally regressed the trend slopes of DOC against the trend slopes of the investigated potential driver variables (Tables S10 and S11) using a multiple linear regression. Comparison of standarized model coefficients showed that the strongest contribution to the observed increase in DOC came from the reduction in S deposition (−0.24), followed by runoff (−0.19) and temperature (0.15) and NDVI (0.08). For a more direct visualization of trends we plotted pairwise smoothed time series curves for the relationship between lake DOC and S deposition, runoff, temperature and NDVI (Fig. 2).
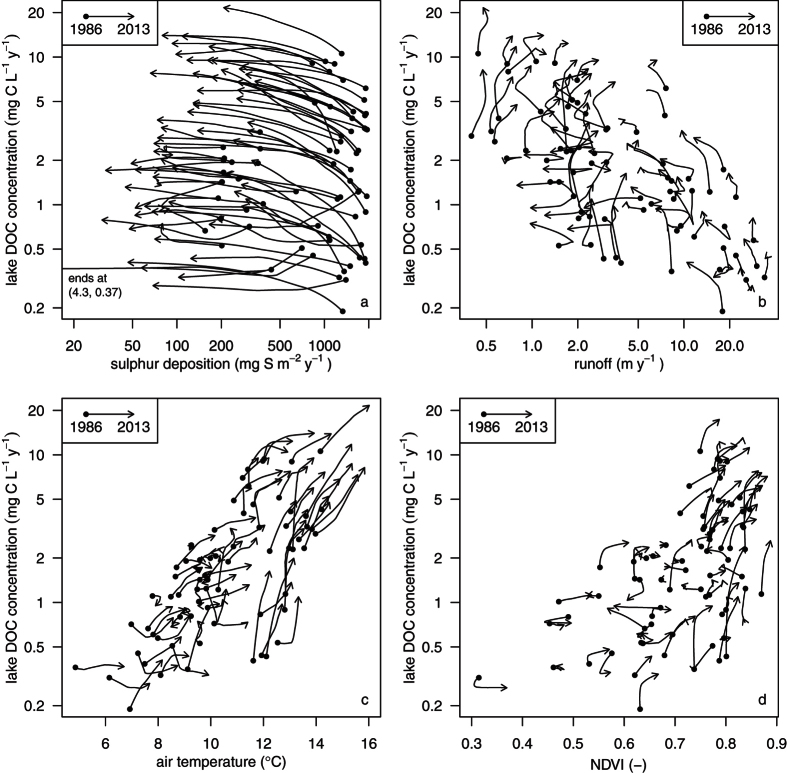
Figure 2. Pairwise time-trend relations between individual driver variables and DOC.
Two-dimensional cross smoothed time series curves starting from 1986 (dots) to 2013 (arrow heads) for individual lakes, where DOC concentration is plotted against (a) sulphur deposition, (b) runoff, (c) surface air temperature and (d) NDVI. Each curve represents a time series for a lake. Coordinates were obtained by smoothing lake-specific time series using lowess61 with a smooth span of 0.8. Only those years with a complete pair of observations are used in this figure. Lake DOC concentration, total sulphur deposition and runoff are scaled logarithmically.
Further, we used DOC and annual means for NDVI, S-deposition, runoff and temperature in a mixed effect multiple regression model with lake as a random factor, and a temporal trend (year) effect nested within lake as a controlling variable to account for time trends that cannot be represented by the explanatory variables in our models. There was strong support for the effect of S deposition, catchment vegetation (NDVI), but also runoff (Table 1). Models with different time lags gave similar relative importance rankings for the explanatory variables. Despite the robustness of the general model predictions we were unable to produce one single best model. Accordingly, model averaging was used for summarizing coefficients (Table 2). Judged from the standardized coefficients and the relative influence of the selected variables (RI), the most important explanatory variables were S deposition (1), followed by runoff (0.96) and NDVI (0.93). There was a smaller, but still significant, effect of temperature (0.26) (Table 2). The pronounced contribution from changes in catchment vegetation suggests that increased terrestrial C-fixation promotes export of allochthonous C to surface waters on a decadal timescale. This finding complements previous studies that demonstrated a strong spatial effect of NDVI on DOC18, and which was used to predict an increase in DOC as a result of a climate-induced landscape greening19.
Table 1. Model selection tables for the mixed effect model of mean yearly total organic carbon (DOC) against normalized difference vegetation index (NDVI), runoff (Q), Sulphur deposition (S-dep), temperature (T), as well as year.
| Intercept | NDVI | Q | S-dep | T | year | n | logLik | AIC | ∆i | Wi |
|---|---|---|---|---|---|---|---|---|---|---|
| −0.150 | 0.046 | −0.056 | −0.098 | 0.148 | 9 | −189.69 | 397.4 | 0.00 | 0.63 | |
| −0.151 | 0.046 | −0.058 | −0.098 | −0.008 | 0.150 | 10 | −189.56 | 399.1 | 1.72 | 0.27 |
| −0.147 | −0.068 | −0.103 | 0.143 | 8 | −192.94 | 401.9 | 4.50 | 0.07 | ||
| −0.153 | 0.057 | −0.101 | 0.150 | 8 | −193.39 | 402.8 | 5.38 | 0.04 |
The tables show parameter estimates for model terms included in the models, number of model parameters (n), log likelihood (LogLik), AIC, AIC difference from best model (∆i), and Akaike weights (Wi). Only models from the top 95% confidence model set shown (cumulative AIC weight of models ≥ 0.95).
Table 2. Summary result for model averaging of fixed effects in the 95% confidence model set (cumulative Wi ≥ 0.95) on total organic carbon (DOC) against normalized difference vegetation index (NDVI), runoff (Q), Sulphur deposition (S-dep), temperature (T), as well as year.
| Estimate | SE | 95% CI | Relative importance | |
|---|---|---|---|---|
| Intercept | −0.151 | 0.105 | (−0.356, 0.055) | |
| NDVI | 0.047 | 0.018 | (0.010, −0.082) | 0.93 |
| Q | −0.057 | 0.021 | (−0.098, −0.016) | 0.96 |
| S-dep | −0.098 | 0.023 | (−0.144, −0.052) | 1 |
| year | 0.147 | 0.038 | (0.073, 0.223) | 1 |
| T | −0.008 | 0.015 | (−0.038, 0.022) | 0.26 |
Parameter estimates (on standardized scale) are interpretable as effect size (i.e. describes changes in unites of standard deviation of the original variable), unconditional SE, 95% Confidence intervals and relative importance.
Discussion
Our analysis agrees with the previously documented effect of reduced S deposition (or lake S-concentration) on DOC1, and also supports the role of NDVI as a proxy of terrestrial vegetation promoting aquatic DOC19. This is, however, the first study to suggest that terrestrial carbon fixation indeed promotes browning in boreal surface waters on a decadal timescale. The role of coniferous forests along with peatlands as a major source of DOC has been confirmed for boreal catchments in general11,12,26. Thus, it seems reasonable to assume that increased terrestrial primary production or forest cover will induce an increase in DOC export. Since S-deposition is now approaching pre-industrial levels and thus is unlikely to decrease much further, and because terrestrial greening may continue for centuries to come, it can be assumed that the relative impact of vegetation on aquatic DOC will increase.
Climate change has been shown to increase vegetation cover and plant biomass (greening) of northern latitude landmasses during the past few decades17. For example, in Norway, tree-lines have moved upwards by more than 40 meters or advanced northwards by 15 km, likely as a combined response of climate change, reduced grazing by domestic animals and elevated nitrogen deposition27,28,29. Also, forest biomass has increased with 27% since 1990 15, comparable with biomass changes in Sweden30,31 and Finland30. The increase in biomass has in part been attributed to reduced harvesting intensity in managed European forest but climate warming and N deposition32 are also promoting forest growth. The tree-line advancement and greening of Arctic and boreal regions may not be a uniform response, however, as there may be both spatial and temporal differences29,33,34. In fact there may be regions also experiencing temporal set-backs of terrestrial vegetation. More than 5% of the vegetation in the boreal region and 4% of the Arctic region have experienced periods of “browning” in the period 1981–2011 17. Recent observations shows that the northern part of Fennoscandia have experienced winter warming events that caused detrimental damage to vegetation25,35 and caterpillar attacks on mountain birch forests36. The short and long terms impacts of such browning events of catchment export of DOC are not settled, however.
Feedbacks from declining snow-cover extent leading to longer growing seasons are promoting vegetation compositional/structural changes. Enhanced nitrogen mineralization in warmer soils insulated by increased shrub cover17,37 may also contribute to the elevated DOC. For taiga and tundra areas, increased permafrost thaw and changing vegetation cover over extended timescales also promote elevated DOC-levels of surface waters38,39,40. The catchments included in our study do not have permafrost, with potential exception for the northernmost areas where DOC changes are relatively small. For catchments at higher elevation is has been suggested, however, that permafrost declines during the last decades may have locally increased the export of organic carbon, as well as sulphur and nutrients39,41,42.
Changes in redox conditions mediated by combinations of hydrology and iron (Fe) reduction and oxidation cycles have been proposed as drivers of elevated DOC43,44. Fe may play a twofold role in this context, both as a mobilizer of DOC and SO4 itself44, and also by its chromophoric properties, making the DOC more “coloured”43, thus attenuating more light45. Also, we may expect increases in DOC of surface waters due to changing temperature and precipitation patterns, as well as associated changes in bogs and wetland.
The impacts of freshwater browning are manifold, e.g. reduced drinking water quality, changes in food web structure and productivity5,46, as well as increased anoxia47,48. Increased levels of coloured, allochthonous DOC will affect aquatic productivity at various trophic levels in numerous ways. Organic carbon may stimulate heterotrophic productivity from bacteria49 to fish50, or indirectly promote autotroph production via elevated levels of CO2 51, increased attenuation of detrimental UV-radiation52 or increase net heterotrophy through enhanced microbial oxygen consumption of bioavailable DOC47. Also nutrients associated with dissolved organic material may stimulate autotroph productivity in nutrient-poor areas5. However, the overall net effect of increased DOC on aquatic productivity is likely negative. This is primarily attributed to its shading effect, with increased light attenuation causing reduced primary production45,53, as well as promoting benthic anoxia48,54. This may cause negative impacts up the trophic ladder to fish which will suffer both from bottom-up cascade effects as well as reduced search field for prey due to reduced visibility5,55. Downstream effects of increased terrestrial export of DOC and sediments have been observed in recipient coastal areas56, with corresponding negative impacts on marine primary and secondary production, and potentially a shift from visual predators (fish) to non-visuals (gelatinous plankton).
The wide range of potential impacts on productivity and community composition thus calls for more knowledge of short- and long-term (i.e., inter-annual and decadal to centuries) drivers of browning, which again can serve as basis for future predictions of impacts on aquatic systems.
Methods
Lake chemistry data
The data in this analysis are obtained from a Norwegian lake monitoring program where 70 lakes covering the Norway mainland have been sampled annually from 1986 to 2013 (Fig. 1). These lakes represent acid sensitive, headwater lakes on granitic or gneissic bedrock with negligible local pollution sources. Water samples were collected at the outlet after the autumn circulation period and analysed at the Norwegian Institute of Water Research57. Due to that the analytical protocol measures organic carbon on unfiltered water, we use total organic C (TOC) as a proxy for DOC. In these boreal, low-productivity systems TOC are for this purpose substitutable with dissolved organic carbon (DOC) since DOC typically constitutes >90% of TOC58.
Environmental drivers
Lake catchments where delineated from a 10 m digital terrain model (obtained from The Norwegian Mapping Authority)59. Time series of annual mean catchment vegetation biomass/productivity (NDVI), runoff, temperature and sulphur deposition were then extracted using the raster library60 in R v. 3.2.161.
We used NDVI (Normalized Difference Vegetation Index; GIMMS NDVI3g) as proxy for catchment vegetation biomass/productivity62. Although care should be taken before interpreting NDVI changes directly as net changes in primary production, the index has previously proven a good surrogate of photosynthetic activity and for capturing long-term changes in vegetation63. The NDVI3g data set has a pixel resolution of 8 × 8 km and is recorded as bimonthly maximum NDVI. Here, we used the mean NDVI3g record for the main snow free period (June–August) averaged annually from each catchment area. Other composites, such as maximum yearly NDVI3g and June–August maxima gave similar results in terms of temporal dynamics, and were therefore not included in further analyses.
Time series of temperature and runoff for individual catchment were extracted using gridded data from 1980 to 2013 (1 km2 resolution from the Norwegian Meteorological Institute and the Norwegian Water Resources and Energy Directorate64. The original data with a daily resolution were aggregated to June to August means for individual catchments, as for NDVI time series.
Since S concentration in lake water may originate from various sources and also be confounded by hydrology and temperature, we here use time series of S deposition at the catchment level which were interpolated from EMEP deposition raster temporal composite (1980, 1985, 1990, and annually from 1995 to 2011)65 using recalculations if available. Total S deposition was used in the present study, comprising both dry and wet deposition. Each type of deposition was modelled using regression kriging using EMEP precipitation as a linear regression model predictor, with residuals fitted using a linear variogram model with no nugget. Using the variogram model and linear model, deposition flux over the catchment area was predicted using block kriging with the target geometry being the catchment polygon. Missing values from 1985–1990 and 1990–1995 where estimated within each catchment using loess regression61. For comparison we did also run the model with SO4 concentrations measured as part of the monitoring program, which yielded the same basic predictions as for deposition data (see Table S8 and S9).
Data standardization and software
All pre-processing of data and analyses was done in R v. 3.2.161. Prior to the analyses, DOC were log transformed, and all variables standardized by subtracting mean and dividing on standard deviation.
Analyses
We first extracted temporal trends (Theil-Sen’s slopes)66 for lake DOC and catchment specific NDVI, run-off, atmospheric S deposition using the rkt library67. We tested for overall trends in DOC and each of the catchment drivers using a regional Kendall trend test from the same library, using the individual lake as block66. Additionally, we illustrated the concordance between the temporal trends by using the Theil-Sen slopes for DOC as dependent variable and corresponding slopes for catchment specific NDVI, run-off, and atmospheric S deposition as explanatory variables in a generalized least-squares regression model (gls). We compared models with and without spatial autocorrelation structure (fitted by REML) using Akaike’s information criterion (AIC) (Zuur et al. 2009). However, no support for inclusion of a spatial autocorrelation was apparent (∆AIC > 4.00 in support of model without spatial autocorrelation). Although such analyses have some issues in terms of using Theil-Sen slopes as input variables by for example disregarding the inherent between-year variation in vegetation measures, they nevertheless provide a useful illustration. See supplementary material (section D) for further description.
We then included effects of S deposition, catchment NDVI, runoff, and air temperature as driver variables in a linear mixed effect model with DOC as dependent variable (nlme library)68. Year was initially included in the full model as a controlling variable69 in order to control for potential temporal trends in DOC not accounted for by our explanatory variables. Model selection and parameter estimation re-runs with and without year as controlling variable did not indicate that this had any major effects on the main conclusions (see supplementary material Table S4 and S5). Selection of random structure where done on a full fixed model structure (fitted by REML) by model comparison70. This resulted in a full model including lake-ID as random intercept and year nested within lake-ID as random slope (∆AIC > −76.65 in favor of the selected random structure). By introducing lake ID as random factor, we model between-lake variation in DOC caused by catchment variables not considered in the current study (static land cover properties that not are expected to change on the relevant time span such as bogs and wetlands, catchment size, lake size, etc.). Temporal autocorrelation (AR1) was initially also tried, by entering year nested within lake-ID, but proved redundant in the initial selection of the random structure. There was no signs of multi-collinearity (variance inflation factors70, all VIF < 1.96, and maximum correlation between predictors where 0.61, see Fig. S2).
Catchment vegetation may be expected to influence lake DOC with certain time lags. However, in order to avoid data-dredging issues, we selected a-priori a one year’s time lag for NDVI and runoff as the input for our analyses, but subsequently performed sensitivity analyses on the effect of the time lag chosen (see Figure S4). The main predictions were insensitive to choice of time lag.
Model selection and multi-model inference
Model selection and model averaging on fixed effect structure where done by model comparison using the MuMIn library71. All possible combinations of fixed factors where compared and candidate models ranked according to the Akaike information criterion (AIC). Since there was no clear top-ranked candidate model, we applied Akaike weight-based averaging over the 95% confidence model set (cumulative AIC weights of models ≥0.95) to estimating coefficients for the candidate models as well as their 95% confidence intervals. The relative influence (RI) of each variable was given as the summation of wi across all models including that variable in the 95% confidence model set72.
Residuals of the final selected models were visually inspected for deviations from normality, heteroscedasticity, and spatial or temporal autocorrelation without finding evidence for violation of model assumptions (see Supplementary material).
Additional Information
How to cite this article: Finstad, A. G. et al. From greening to browning: Catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Sci. Rep. 6, 31944; doi: 10.1038/srep31944 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the Research Council of Norwegian (RCN 221410 and 224779). We acknowledge the NASA GIMMS group for sharing the NDVI3g data through the Arctic Biomass project (RCN 227064).
Footnotes
The authors declare no competing financial interests.
Author Contributions A.G.F., S.L., D.O.H. and T.A. conceived the original idea and designed the study; Data extraction and preparation where performed by K.T., H.T. and S.B.; The analysis was performed by A.G.F and K.T.; Ideas, writing and discussions with contributions from A.G.F., T.A., S.L., K.T., S.B., H.A.d.W., H.T and D.O.H.
References
- Monteith D. T. et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450, 537–U9 (2007). [DOI] [PubMed] [Google Scholar]
- Garmo Ø. et al. Trends in Surface Water Chemistry in Acidified Areas in Europe and North America from 1990 to 2008. Water. Air. Soil Pollut. 225, 1–14 (2014). [Google Scholar]
- Cole J. J. et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 10, 172–185 (2007). [Google Scholar]
- Ask J. et al. Terrestrial organic matter and light penetration: Effects on bacterial and primary production in lakes. Limnol. Oceanogr. 54, 2034–2040 (2009). [Google Scholar]
- Karlsson J. et al. Light limitation of nutrient-poor lake ecosystems. Nature 460, 506–U80 (2009). [DOI] [PubMed] [Google Scholar]
- Matilainen A., Vepsäläinen M. & Sillanpää M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 159, 189–197 (2010). [DOI] [PubMed] [Google Scholar]
- de Wit H. A., Mulder J., Hindar A. & Hole L. Long-Term Increase in Dissolved Organic Carbon in Streamwaters in Norway Is Response to Reduced Acid Deposition. Environ. Sci. Technol. 41, 7706–7713 (2007). [DOI] [PubMed] [Google Scholar]
- Oulehle F. et al. Soil–solution partitioning of DOC in acid organic soils: results from a UK field acidification and alkalization experiment. Eur. J. Soil Sci. 64, 787–796 (2013). [Google Scholar]
- Hongve D., Riise G. & Kristiansen J. Increased colour and organic acid concentrations in Norwegian forest lakes and drinking water – a result of increased precipitation? Aquat. Sci. 66, 231–238 (2004). [Google Scholar]
- Tranvik L. & Jansson M. Climate change (Communication arising): Terrestrial export of organic carbon. Nature 415, 861–862 (2002). [Google Scholar]
- Xenopoulos M. A. et al. Regional comparisons of watershed determinants of dissolved organic carbon in temperate lakes from the Upper Great Lakes region and selected regions globally. Limnol. Oceanogr. 48, 2321–2334 (2003). [Google Scholar]
- Dillon P. J. & Molot L. A. Long-term trends in catchment export and lake retention of dissolved organic carbon, dissolved organic nitrogen, total iron, and total phosphorus: The Dorset, Ontario, study, 1978–1998. J. Geophys. Res. Biogeosciences 110, G01002, doi: 10.1029/2004JG000003 (2005). [DOI] [Google Scholar]
- Meyer-Jacob C., Tolu J., Bigler C., Yang H. & Bindler R. Early land use and centennial scale changes in lake-water organic carbon prior to contemporary monitoring. Proc. Natl. Acad. Sci. 112, 6579–6584 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay K. C. et al. Vegetation productivity patterns at high northern latitudes: a multi-sensor satellite data assessment. Glob. Change Biol. 20, 3147–3158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H., Austnes K., Hylen G. & Dalsgaard L. A carbon balance of Norway: terrestrial and aquatic carbon fluxes. Biogeochemistry 123, 147–173 (2015). [Google Scholar]
- Myneni R. B. et al. A large carbon sink in the woody biomass of Northern forests. Proc. Natl. Acad. Sci. 98, 14784–14789 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. et al. Temperature and vegetation seasonality diminishment over northern lands. Nat. Clim Change 3, 581–586 (2013). [Google Scholar]
- Larsen S., Andersen T. & Hessen D. O. Predicting organic carbon in lakes from climate drivers and catchment properties. Glob. Biogeochem. Cycles 25, GB3007 (2011). [Google Scholar]
- Larsen S., Andersen T. O. M. & Hessen D. O. Climate change predicted to cause severe increase of organic carbon in lakes. Glob. Change Biol. 17, 1186–1192 (2011). [Google Scholar]
- Butman D., Raymond P. A., Butler K. & Aiken G. Relationships between Δ14C and the molecular quality of dissolved organic carbon in rivers draining to the coast from the conterminous United States. Glob. Biogeochem. Cycles 26, doi: 10.1029/2012GB004361 (2012). [DOI] [Google Scholar]
- Marwick T. R. et al. The age of river-transported carbon: A global perspective. Glob. Biogeochem. Cycles 29, 122–137 (2015). [Google Scholar]
- Weyhenmeyer G. A. & Karlsson J. Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperatures. Limnol. Oceanogr. 54, 2513–2519 (2009). [Google Scholar]
- Elmendorf S. C. et al. Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457 (2012). [Google Scholar]
- Borgstrøm R. Relationship between Spring Snow Depth and Growth of Brown Trout, Salmo trutta, in an Alpine Lake: Predicting Consequences of Climate Change. Arct. Antarct. Alp. Res. 33, 476–480 (2001). [Google Scholar]
- Bjerke J. W. et al. Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environ. Res. Lett. 9, 084006, doi: 10.1088/1748-9326/9/8/084006 (2014). [DOI] [Google Scholar]
- Kortelainen P. Content of Total Organic Carbon in Finnish Lakes and Its Relationship to Catchment Characteristics. Can. J. Fish. Aquat. Sci. 50, 1477–1483 (1993). [Google Scholar]
- Karlsson P. S. & Nordell K. O. Effects of soil temperature on the nitrogen economy and growth of mountain birch seedlings near its presumed low temperature distribution limit. Écoscience 3, 183–189 (1996). [Google Scholar]
- Bryn A. Recent forest limit changes in south-east Norway: Effects of climate change or regrowth after abandoned utilisation? Nor. Geogr. Tidsskr. - Nor. J. Geogr. 62, 251–270 (2008). [Google Scholar]
- Hofgaard A., Tømmervik H., Rees G. & Hansen F. Latitudinal forest advance in northernmost Norway since the early 20th century. J. Biogeogr. 40, 938–949 (2013). [Google Scholar]
- Jari Liski et al. Carbon accumulation in Finland’s forests 1922–2004 – an estimate obtained by combination of forest inventory data with modelling of biomass, litter and soil. Ann Sci. 63, 687–697 (2006). [Google Scholar]
- Agren A., Buffam I., Jansson M. & Laudon H. Importance of seasonality and small streams for the landscape regulation of dissolved organic carbon export. J. Geophys. Res. Biogeosciences 112, G03003, doi: 10.1029/2006JG000381 (2007). [DOI] [Google Scholar]
- Quinn Thomas R., Canham C. D., Weathers K. C. & Goodale C. L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci 3, 13–17 (2010). [Google Scholar]
- Dalen L. & Hofgaard A. Differential regional treeline dynamics in the Scandes Mountains. Arct. Antarct. Alp. Res. 37, 284–296 (2005). [Google Scholar]
- Schuur E. et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Change 119, 359–374 (2013). [Google Scholar]
- Bokhorst S. et al. Extreme winter warming events more negatively impact small rather than large soil fauna: shift in community composition explained by traits not taxa. Glob. Change Biol. 18, 1152–1162 (2012). [Google Scholar]
- Jepsen J. et al. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sens. Environ 113, 1939–1947 (2009). [Google Scholar]
- Chapin F. S. et al. Role of land-surface changes in Arctic summer warming. Science 310, 657–660 (2005). [DOI] [PubMed] [Google Scholar]
- Abbott B. W. et al. Biomass offsets little or none of permafrost carbon release from soils, streams, and wildfire: an expert assessment. Environ. Res. Lett. 11, 034014 (2016). [Google Scholar]
- Abbott B. W., Jones J. B., Godsey S. E., Larouche J. R. & Bowden W. B. Patterns and persistence of hydrologic carbon and nutrient export from collapsing upland permafrost. Biogeosciences 12, 3725–3740 (2015). [Google Scholar]
- Macias-Fauria M., Forbes B. C., Zetterberg P. & Kumpula T. Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nat. Clim Change 2, 613–618 (2012). [Google Scholar]
- Kokelj S., Jenkins R., Milburn D., Burn C. & Snow N. The influence of thermokarst disturbance on the water quality of small upland lakes, Mackenzie Delta region, Northwest Territories, Canada. Permafr. Periglac. Process 16, 343–353 (2005). [Google Scholar]
- Borge A. F., Westermann S., Solheim I. & Etzelmüller B. Strong degradation of palsas and peat plateaus in northern Norway during the last 60 years. Cryosphere Discuss 2016, 1–31 (2016). [Google Scholar]
- Kritzberg E. S. & Ekström S. M. Increasing iron concentrations in surface waters – a factor behind brownification? Biogeosciences 9, 1465–1478 (2012). [Google Scholar]
- Knorr K.-H. DOC-dynamics in a small headwater catchment as driven by redox fluctuations and hydrological flow paths - are DOC exports mediated by iron reduction/oxidation cycles? Biogeosciences 10, 891–904 (2013). [Google Scholar]
- Thrane J.-E., Hessen D. & Andersen T. The Absorption of Light in Lakes: Negative Impact of Dissolved Organic Carbon on Primary Productivity. Ecosystems 17, 1040–1052 (2014). [Google Scholar]
- Williamson C. E. et al. Ecological consequences of long-term browning in lakes. Sci. Rep. 5, 18666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture R., DeWit H., Tominaga K., Kiuru P. & Markelov I. Oxygen dynamics in a boreal lake responds to long-term changes in climate, ice phenology and DOC inputs. J. Geophys. Res. Biogeosci. 120, 2441–24456 (2015) [Google Scholar]
- Craig N., Jones S. E., Weidel B. C. & Solomon C. T. Habitat, not resource availability, limits consumer production in lake ecosystems. Limnol. Oceanogr. 60, 2079–2089 doi: 10.1002/lno.10153 (2015). [DOI] [Google Scholar]
- Hessen D. O., Andersen T. & Lyche A. Carbon metabolism in a humic lake - pool sizes and cycling through zooplankton. Limnol. Oceanogr. 35, 84–99 (1990). [Google Scholar]
- Tanentzap A. J. et al. Forests fuel fish growth in freshwater deltas. Nat Commun 5, 4077, doi: 10.1038/ncomms5077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson M., Karlsson J. & Jonsson A. Carbon dioxide supersaturation promotes primary production in lakes. Ecol. Lett. 15, 527–532 (2012). [DOI] [PubMed] [Google Scholar]
- Williamson C. E., Stemberger R. S., Morris D. P., Frost T. M. & Paulsen S. G. Ultraviolet radiation in North American lakes: Attenuation estimates from DOC measurements and implications for plankton communities. Limnol. Oceanogr. 41, 1024–1034 (1996). [Google Scholar]
- Kankaala P., Huotari J., Tulonen T. & Ojala A. Lake-size dependent physical forcing drives carbon dioxide and methane effluxes from lakes in a boreal landscape. Limnol. Oceanogr. 58, 1915–1930 (2013). [Google Scholar]
- Couture R.-M. et al. Modelling phosphorus loading and algal blooms in a Nordic agricultural catchment-lake system under changing land-use and climate. Environ. Sci. Process. Impacts 16, 1588–1599 (2014). [DOI] [PubMed] [Google Scholar]
- Finstad A. G., Helland I. P., Ugedal O., Hesthagen T. & Hessen D. O. Unimodal response of fish yield to dissolved organic carbon. Ecol. Lett. 17, 36–43 (2014). [DOI] [PubMed] [Google Scholar]
- Dupont N. & Aksnes D. L. Centennial changes in water clarity of the Baltic Sea and the North Sea. Estuar. Coast. Shelf Sci. 131, 282–289 (2013). [Google Scholar]
- Skjelkvåle B. L., Mannio J., Wilander A. & Andersen T. Recovery from acidification of lakes in Finland, Norway and Sweden 1990–1999. Hydrol Earth Syst Sci 5, 327–338 (2001). [Google Scholar]
- Wetzel R. G. Limnology: lake and river ecosystems (Academic Press, 2001). [Google Scholar]
- Finstad A. G. et al. Competitive exclusion along climate gradients: energy efficiency influences the distribution of two salmonid fishes. Glob. Change Biol. 17, 1703–1711 (2011). [Google Scholar]
- Hijmans R. J. raster: Geographic Data Analysis and Modeling. http://CRAN.R-project.org/package=raster (2015).
- R. Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/ (2015).
- Pinzon J. E. & Tucker C. J. A Non-Stationary 1981–2012 AVHRR NDVI3g Time Series. Remote Sens. 6, 6929–6960 (2014). [Google Scholar]
- Pettorelli N. et al. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 20, 503–510 (2005). [DOI] [PubMed] [Google Scholar]
- Mohr M. New Routines for Gridding of Temperature and Precipitation Observations for ‘seNorge.no’. MET report No. 08/2008 (2008).
- Schulz M. et al. Transboundary acidification, eutrophication and ground level ozone in Europe in 2011. (Norwegian Meteorological Institute, 2013). [Google Scholar]
- Helsel D. R. & Frans L. M. Regional Kendall Test for Trend. Environ. Sci. Technol. 40, 4066–4073 (2006). [DOI] [PubMed] [Google Scholar]
- Marchetto A. rkt: Mann-Kendall Test, Seasonal and Regional Kendall Tests. URL http://CRAN.R-project.org/package=rkt (2015).
- Pinheiro J., Bates D., DebRoy S., Sarkar D. & Team R. D. C. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3 1–104 (2012). [Google Scholar]
- Freckleton R. P. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. J. Anim. Ecol. 71, 542–545 (2002). [Google Scholar]
- Zuur A. F., Ieno E. N., Walker N. J., Savaeliev A. A. & Smith G. M. Mixed effects models and extensions in ecology with R. (Springer, 2009). [Google Scholar]
- Grueber C. E., Nakagawa S., Laws R. J. & Jamison I. G. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711 (2011). [DOI] [PubMed] [Google Scholar]
- Johnson J. B. & Omland K. S. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 (2004). [DOI] [PubMed] [Google Scholar]
- Becker R. A., Chambers J. M. & Wilks A. R. The new S language: a programming environment for data analysis and graphics. (Wadsworth and Brooks/Cole, Pacific Grove, 1988). [Google Scholar]
- Bivand R., Pebesma E. & Gomez-Rubio V. Applied spatial data analyses with R. (Springer, NY, 2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.