Abstract
Protein G, a bacterial cell-wall protein with high affinity for the constant region of IgG (IgGFc) antibodies, contains homologous repeats responsible for the interaction with IgGFc. A synthetic peptide corresponding to an 11-amino acid-long sequence in the COOH-terminal region of the repeats was found to bind to IgGFc and block the interaction with protein G. Moreover, two other IgGFc-binding bacterial proteins (proteins A and H), which do not contain any sequences homologous to the peptide, were also inhibited in their interactions with IgGFc by the peptide. Finally, a decapeptide based on a sequence in IgGFc blocked the binding of all three proteins to IgGFc. This unusually clear example of convergent evolution emphasizes the complexity of protein-protein interactions and suggests that bacterial surface-protein interaction with host protein adds selective advantages to the microorganism.
Full text
PDF

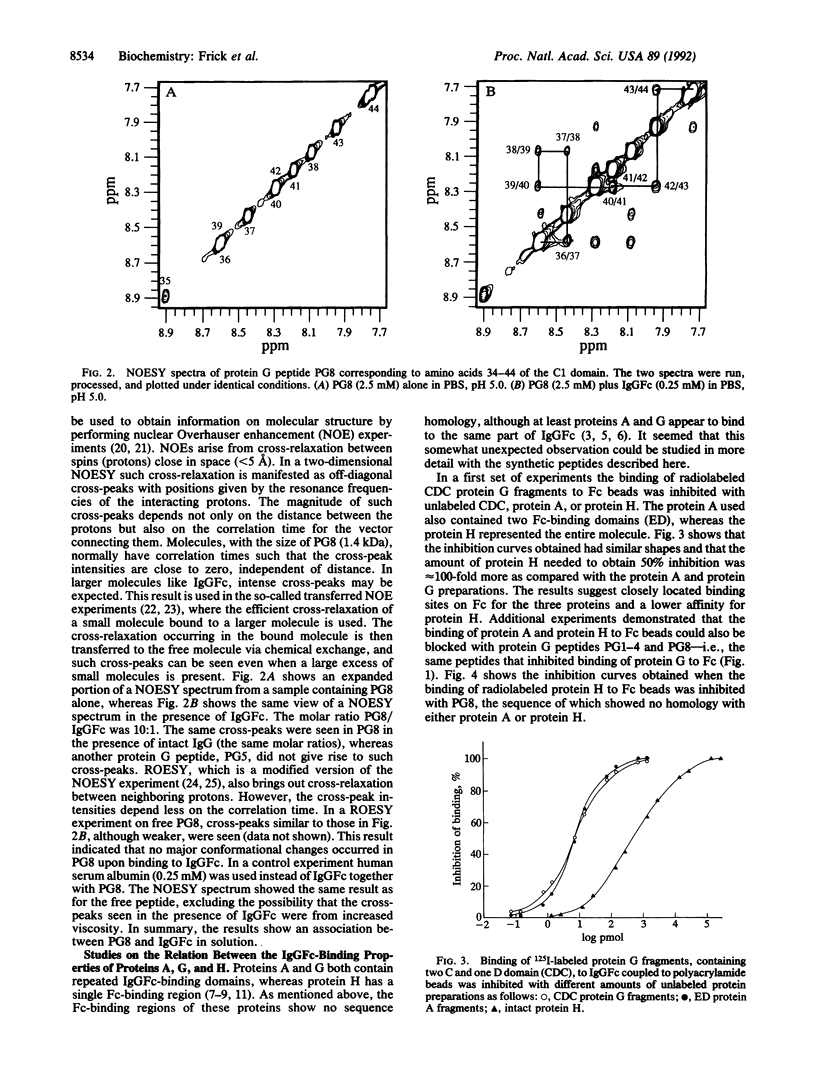

Images in this article
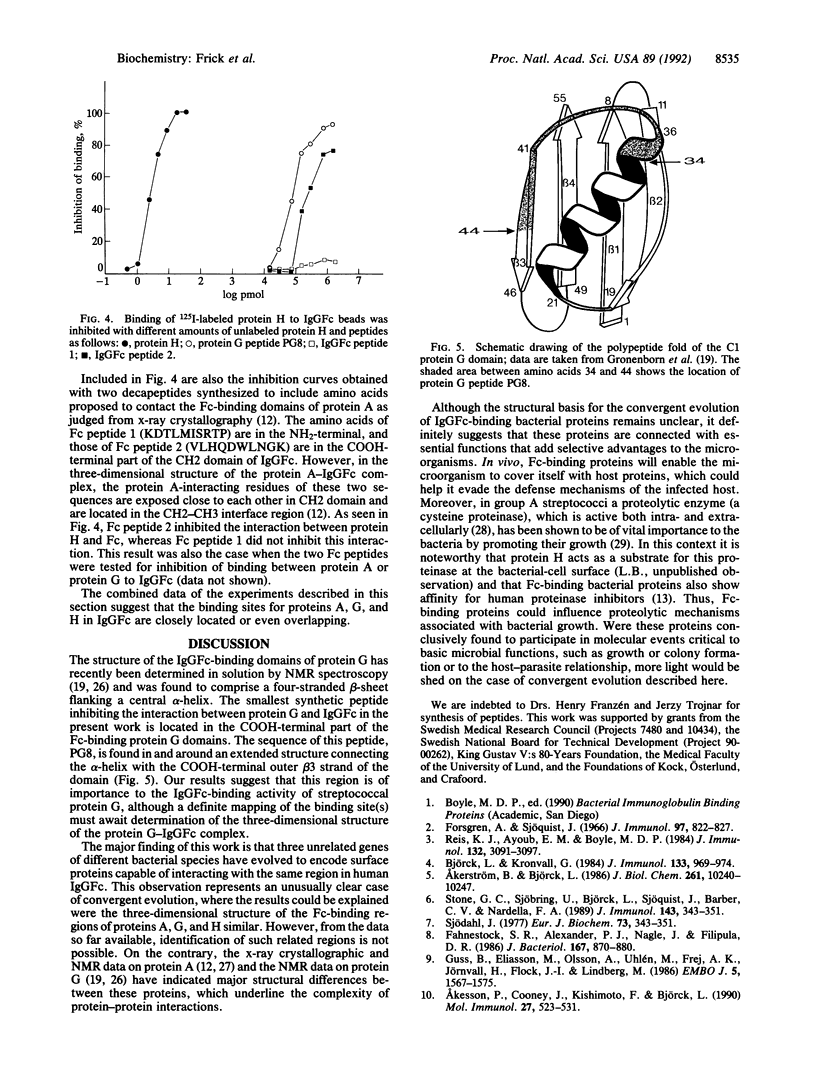
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986 Aug 5;261(22):10240–10247. [PubMed] [Google Scholar]
- Akerström B., Björck L. Protein L: an immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J Biol Chem. 1989 Nov 25;264(33):19740–19746. [PubMed] [Google Scholar]
- Akesson P., Cooney J., Kishimoto F., Björck L. Protein H--a novel IgG binding bacterial protein. Mol Immunol. 1990 Jun;27(6):523–531. doi: 10.1016/0161-5890(90)90071-7. [DOI] [PubMed] [Google Scholar]
- Björck L., Akesson P., Bohus M., Trojnar J., Abrahamson M., Olafsson I., Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989 Jan 26;337(6205):385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- Björck L., Kronvall G. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J Immunol. 1984 Aug;133(2):969–974. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981 Apr 28;20(9):2361–2370. [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Gomi H., Hozumi T., Hattori S., Tagawa C., Kishimoto F., Björck L. The gene sequence and some properties of protein H. A novel IgG-binding protein. J Immunol. 1990 May 15;144(10):4046–4052. [PubMed] [Google Scholar]
- Gronenborn A. M., Filpula D. R., Essig N. Z., Achari A., Whitlow M., Wingfield P. T., Clore G. M. A novel, highly stable fold of the immunoglobulin binding domain of streptococcal protein G. Science. 1991 Aug 9;253(5020):657–661. doi: 10.1126/science.1871600. [DOI] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L. Y., Yang J. C., Derrick J. P., Sutcliffe M. J., Roberts G. C., Murphy J. P., Goward C. R., Atkinson T. Sequential 1H NMR assignments and secondary structure of an IgG-binding domain from protein G. Biochemistry. 1991 Jun 4;30(22):5335–5340. doi: 10.1021/bi00236a002. [DOI] [PubMed] [Google Scholar]
- Lo S. S., Liang S. M., Liu T. Y. Intracellular form of streptococcal proteinase: a clue to a novel mechanism of secretion. Anal Biochem. 1984 Jan;136(1):89–92. doi: 10.1016/0003-2697(84)90309-9. [DOI] [PubMed] [Google Scholar]
- Nilson B. H., Solomon A., Björck L., Akerström B. Protein L from Peptostreptococcus magnus binds to the kappa light chain variable domain. J Biol Chem. 1992 Feb 5;267(4):2234–2239. [PubMed] [Google Scholar]
- Olsson A., Eliasson M., Guss B., Nilsson B., Hellman U., Lindberg M., Uhlén M. Structure and evolution of the repetitive gene encoding streptococcal protein G. Eur J Biochem. 1987 Oct 15;168(2):319–324. doi: 10.1111/j.1432-1033.1987.tb13423.x. [DOI] [PubMed] [Google Scholar]
- Reis K. J., Ayoub E. M., Boyle M. D. Streptococcal Fc receptors. I. Isolation and partial characterization of the receptor from a group C streptococcus. J Immunol. 1984 Jun;132(6):3091–3097. [PubMed] [Google Scholar]
- Sjodahl J. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur J Biochem. 1977 Mar 1;73(2):343–351. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- Sjöbring U., Trojnar J., Grubb A., Akerström B., Björck L. Ig-binding bacterial proteins also bind proteinase inhibitors. J Immunol. 1989 Nov 1;143(9):2948–2954. [PubMed] [Google Scholar]
- Torigoe H., Shimada I., Saito A., Sato M., Arata Y. Sequential 1H NMR assignments and secondary structure of the B domain of staphylococcal protein A: structural changes between the free B domain in solution and the Fc-bound B domain in crystal. Biochemistry. 1990 Sep 18;29(37):8787–8793. doi: 10.1021/bi00489a040. [DOI] [PubMed] [Google Scholar]