Abstract
Proanthocyanidin rich plant extracts derived from grape seed extract (GSE), hawthorn and cranberry are on markets for their preventive effects against cardiovascular diseases and uroinfections in woman. However, the importance of these health beneficial effects of these botanicals remains elusive due to incomplete understanding of uptake, metabolism and bioavailability of proanthocyanidins in vivo. In the present study rats were given GSE orally (300 mg/kg, twice a day) and blood and urine were collected over a 24 h period. Monomeric catechins and their methylated metabolites, and proanthocyanidins up to trimers were detected in blood samples treated with GSE using LC-MS/MS operating in the multiple reaction monitoring (MRM) mode. A new tetramethylated metabolite of dimeric proanthocyanidin (m/z 633) in GSE-treated urine was tentatively identified. Using LC-MS/MS, (+)-catechin and (−)-epicatechin were identified in the brain conclusively. These data suggested that GSE catechins cross the blood brain barrier and may be responsible for the neuroprotective effects of GSE.
Keywords: Grape seed proanthocyanidins, metabolism, LC-MS/MS, Brain
INTRODUCTION
Grape seeds, one of the richest sources of polyphenols, have potent antioxidant activity. Extracts prepared from the grape seeds contain a rich mixture of monomeric flavan-3-ols, phenolic acids and oligomeric proanthocyanidins. There has been a great surge in commercialization of botanical extracts containing proanthocyanidins. In Europe some standardized plant extracts (e.g. cranberry-or hawthorn-extracts) are on the market as conventional drugs which are used because of their content of oligomeric proanthocyanidins for the treatment of recurrent uroinfections in woman and cardiovascular diseases grade I and II respectively.
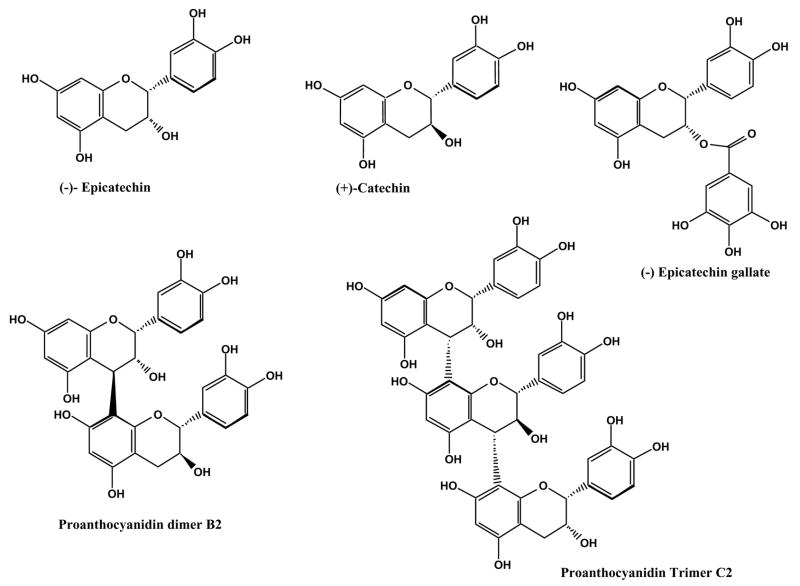
Proanthocyanidins are mixtures of dimers and higher oligomers of monomeric flavan-3-ol units linked mainly by a C4–C8 bond and, to a lesser extent, a C4–C6 bond (both are called B-type proanthocyanidins) (Fig. 1) (da Silva et al., 1991; Boukharta, 1988; Prieur et al., 1994). Emerging evidence has indicated that grape seed proanthocyanidins have cardioprotective effects against reperfusion-induced injury via their ability to decrease, directly or indirectly, free radicals in myocardium (Pataki et al., 2002). It has also been demonstrated that consumption of GSE decreases the incidence of cataracts in the eyes of hereditary cataractous (ICR/f) rats) (Yamakoshi et al., 2002). In addition, studies involving grape seed extract (GSE) found that chronic exposure to GSE modulates expression of specific mouse brain proteins that are either linked to Alzheimer’s Disease or neuron-degeneration (Deshane et al., 2004).
Figure 1.
Structures of flavan-3-ols and proanthocyanidin dimers and proanthocyanidin trimers.
Given the increasing significance of a potential health beneficial role of GSE proanthocyanidins in human health, there is a need for a fuller understanding of their absorption, metabolism and excretion. (Epi)catechins are absorbed from the intestines in humans and animals, appearing in plasma and urine primarily as glucuronidated, methylated and sulphated metabolites following the ingestion of diet containing monomeric catechins and proanthocyanidis such as chocolate, black and green tea, and red wine (Yang et al., 1998; Rein et al., 2000; Piskula et al., 1998; Donovan et al., 1999). However, the metabolic fate of proanthocyanidins is still elusive as there is conflicting evidence on the absorption and metabolism of the oligomeric and polymeric flavan-3-ols in humans and animals. For example, Koga et al. (1999) reported the presence of (+)-catechin and (−)-epicatechin and an absence of dimers in the plasma of rats following ingestion of a GSE. However, studies by Holt et al. (2002) demonstrated the presence of dimeric procyanidins in human plasma as early as 30 min after the consumption of a flavanol-rich food such as cocoa. There is evidence in support of absorption of monomeric catechins and proanthocyanidins through the human intestinal caco-2 epithelial cells (Deprez et al., 2001; Faria et al., 2006). Another study by Tsang et al. (2005) reported the absorption and metabolism of catechin and proanthocyanidins up to trimers in urine following the oral intake of GSE. However, this study was inconclusive in establishing the accessibility of catechin in the brain. It is, therefore, critical to understand the metabolic fate of proanthocyanidins in an experimental animal model. In this study, we describe identification of major metabolites of monomeric flavan-3-ols and proanthocyanidins after oral administration of GSE and their accessibility to the brain by LC-MS/MS methods.
EXPERIMENTALS
Chemicals and reagents
(+)-Catechin (98% purity), (−)-epicatechin (98% purity) and apigenin were purchased from Indofine chemical company Inc. (Hillsborough, NJ, USA). All other HPLC solvents and reagents were purchased from Fisher (Norcross, GA) and were of HPLC grade. A powdered GSE preparation was provided by Kikkoman Corp. (Chiba, Japan) and stored at 4°C in light-tight containers until used. The composition of GSE was previously described to consist of >95% flavanols, of which 86% were proanthocyanidins (Yamaguchi et al., 1999).
GSE administration and sample collection
Male Sprague-Dawley rats (SD, n = 8, body weight = 298–340 g, 12 weeks of age) were obtained from Harlan Sprague-Dawley Inc, Indianapolis, IN. They were kept in a controlled environment at 23°C and 55% relative humidity under a 12 h dark-light cycle, with free access to soy-free custom diet TD86369 (Harlan Teklad, Wisconsin, MD) and tap water for one week. All experimental procedures were conducted in accordance with Institutional Animal Care and Use Committee of the University of Alabama, Birmingham, and National Institutes of Health guidelines. After overnight fasting, GSE in aqueous solution was gavaged (300 mg/kg, twice a day) to the rats. Blood and brain were collected after 4 h of GSE administration on the 3rd day. Urine samples were collected using metabolic cages over 16 h after each GSE treatment. Rats were euthanized by cervical dislocation following isoflurane anesthesia and blood was collected via heart puncture using heparin as anticoagulant. Plasma was isolated by centrifugation of the blood at 3,000 × g for 5 min at stored at −20 °C. The brain was dissected after perfusion with ice-cold normal saline for 10 min and immediately frozen in liquid nitrogen. For the analysis of plasma and urinary metabolites, samples were hydrolyzed enzymatically with sulfatase extracted from Helix pomatia (Sigma, St, Louis, MO) to hydrolyze glucuronide and sulfate conjugates. In brief, phenolphthalein glucuronide, 4-methylumbelliferyl sulfate, and apigenin were added as internal standards to each plasma and urine sample before hydrolysis with 400 U of β-glucuronidase and aryl sulfatase in 150 mmol/L ammonium acetate buffer, pH 5, for 16 h at 37°C. The samples were extracted with ethyl acetate, then evaporated to dryness, and finally reconstituted in 80% aqueous methanol.
Two brain samples were combined into one (3.4–3.7 g), minced and added to methanol (6.5 mL) containing 0.5% acetic acid. The tissue was homogenized with a Tissue Tearor homogenizer (Biospace Product Inc., Racine, WI). The samples were vortex mixed and tumbled for 2 h at room temperature. The homogenate was centrifuged at 3,000 × g for 10 min and the supernatant was removed and dried under air and reconstituted in 80% aqueous methanol (200 μL). The reconstituted solutions were transferred to HPLC auto sampler vials and injected into the mass spectrometer to analyze the samples.
MALDI-TOF MS Analysis
Reflectron mode MALDI-TOF mass spectral data were acquired on an Applied Biosystems inc. (Foster City, CA) Voyager DE-Pro instrument equipped with delayed extraction and a nitrogen laser at 337 nm. The positive reflectron mode spectra collected using an accelerating voltage of 20 kV and a delay time of 100 ns; 300 laser shots were accumulated and averaged. The spectra were calibrated using an external calibration of a bradykinin peptide at m/z 1060.22. The laser intensity was set at 2500 with mass range of 500–3000 Da.
Liquid chromatography-mass spectrometry
LC-MS/MS analyses of rat urine, plasma and brain samples were performed using a system consisting of a model SIL-HT refrigerated Shimadzu auto sampler, an HPLC instrument (Shimadzu Scientific Instruments, Inc. Columbia, MD), and an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was carried out on a reversed-phase Phenomenex Fusion C18 column (150 × 2.0 mm i.d.) with the mobile phase consisted solvent A (0.1% formic acid) and solvent B (methanol containing 0.1% formic acid). A 10 min gradient was established running from 10 to 100% B over the first 5 min and the system was returned to the initial 10%B at 6 min.
The column effluent was introduced into the mass spectrometer using electrospray ionization (ESI) in the negative and positive ion modes. Nitrogen was used as nebulizer, and curtain gas. The nebulizer current and temperature were 5 A and 250 °C, respectively. The collision gas (N2) was set at high and collision energy was 30 eV with electron multiplier voltage (1900 V). The following mass transitions were used for MRM analysis of (epi)catechin m/z 291/139 (positive ion mode) and 289/137 (negative ion mode), methyl (epi)catechin m/z 305/139 and 305/137, proanthocyanidin dimers m/z 579/289 and 579/291, proanthocyanidin trimers m/z 867/579 in positive ion mode. The LC-MS-MS system was controlled by BioAnalyst 1.4.1 software.
Chromatographic separation of catechins and proanthocyanidins in the methanolic extract of grape seed was performed using a 250 × 2.0 mm i.d. (4 micron) Synergy Hydro-RP 80R (Phenomenex, Torrance, CA) with a 15 min gradient running from 10 to 60% B in the first 7 min and increased from 60 to 100% over the next 2 min and then returned to 10% from 9 to 10 min. LC- MS/MS conditions were similar as explained above. For quantification of (+)- catechin and (−)-epicatechin in GSE, a methanolic solution of GSE (164 μg/mL) was prepared in 80% methanol in water, filtered, and transferred to auto samplers for LC-MS/MS analysis. GSE solution, (+)-catechin and (−)-epicatechin standards were injected (10 μL) onto the LC-MS/MS system. The quantification of (+)-catechin and (−)-epicatechin in GSE was based on a calibration curve over the concentration range 0.01–10 μM. The assay method for quantification was demonstrated to be very sensitive and specific, with a correlation coefficient of 0.99.
RESULTS
Analysis of grape seed extract
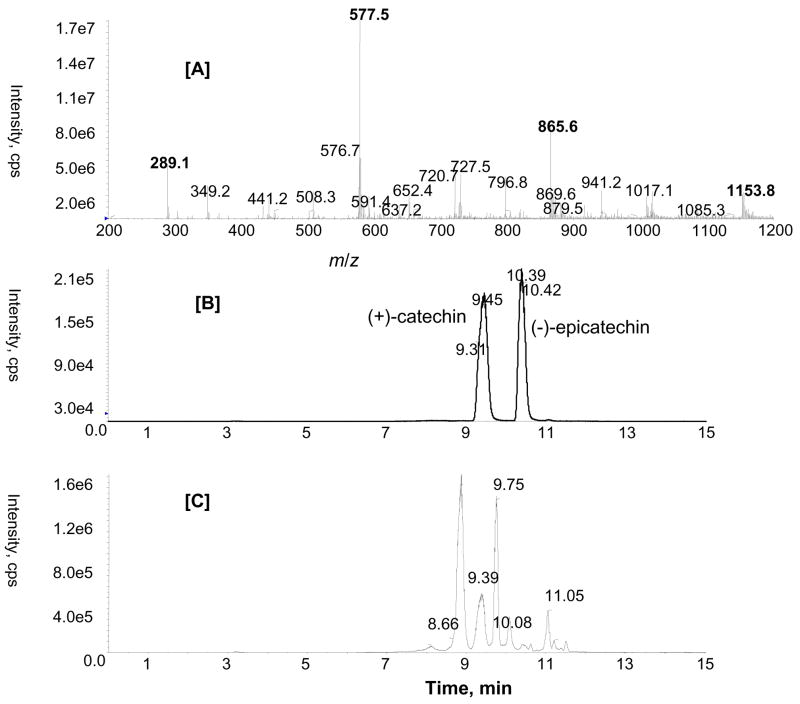
ESI-MS analysis of GSE in the negative ion mode indicated the extract was a complex mixture containing monomeric catechin and epicatechin (m/z 289.1), and proanthocyanidin dimers (m/z 577.5), trimers (m/z 865.5), tetramers (m/z 1153.8) and their gallate esters such as (epi)catechin gallate (m/z 441.2) and gallate of trimeric proanthocyanidins (m/z 1017.1) (Fig. 2A). A number of reversed-phase LC columns were screened in order to obtain the best separation of monomeric catechins and proanthocyanidis. A synergi Hydro-RP column (C18 with polar end capping) provided excellent chromatographic separation and peak shape of monomric catechins and proanthocyanidins. Fig. 2B illustrates typical chromatograms for GSE that demonstrate the base line separation of (+)- catechin (tR 9.45 min) and (−)-epicatechin (tR 10.39 min). The mean amounts of (+)-catechin and (−)-epicatechin in GSE were 65.95 and 27.40 μg/mg, respectively.
Figure 2.
ESI-MS spectrum of the methanolic extract of GSE showing monomeric catechins and proanthocyanidins [A]; LC-MS/MS chromatogram in negative MRM mode with mass transitions m/z 289/137 [B]; m/z 577/289 [C] obtained from the methanolic extract of GSE.
Since the proanthocyanidins are formed from the condensation of monomeric units of catechin or epicatechin, they differ in their position and configuration of their monomeric linkage. As can be seen in Fig. 2C, a MRM ion chromatogram of m/z 577/289 in negative ion mode showed at least five well resolved peaks corresponding to different dimers. They could be dimers B1–B5, as previously reported for GSE (Sun et al., 1999). In order to determine the composition of oligomeric and polymeric proanthocyanidins, analysis using MALDI-TOF/MS was also carried out.
MALDI-TOF MS Analysis
Matrix-assisted-laser-desorption-ionization-time-of-flight (MALDI-TOF) analysis is a facile way to determine the presence of molecules of intermediate molecular weight with high accuracy. Reflectron mode MALDI-TOF MS analysis was performed to identify oligomeric proanthocyanidins present in GSE. 2,4,6-trihydroxyacetophenone (THAP) matrix was used as the MALDI matrix. MALDI-TOF MS spectra of GSE contained peaks with m/z values corresponding to an oligomeric series of catechin/epicatechin/gallo(epi)catechin units up to nonamers in the reflectron mode (Table 1). Ions were detected in the positive ion mode as sodium adducts [M+Na]+. Galloylated proanthocyanidins were identified with the mass shifts [M + 152x + Na]+ where x represents the number of gallic acid esters (Table 1) (17).
Table 1.
Proanthocyanidins (PA) detected in GSE by MALDI-TOFMS in reflectron mode
| PA | Number of galloyl unit | Observed [M+Na]+ |
|---|---|---|
| Dimer | 0 | 601 |
| 1 | 753 | |
| Trimer | 0 | 889 |
| 1 | 1041 | |
| 2 | 1193 | |
| 3 | 1345 | |
| Tetramer | 0 | 1177 |
| 1 | 1329 | |
| 2 | 1481 | |
| Pentamer | 0 | 1465.5 (1466 calculated) |
| 1 | 1618 | |
| Hexamer | 0 | 1754 |
| 1 | 1907 | |
| Heptamer | 0 | 2041.7 (2042 calculated) |
| Octamer | 0 | 2330 |
| Nonamer | 0 | 2619 |
Identification of flavan-3-ol and proanthocyanidin metabolites
Urinary metabolites
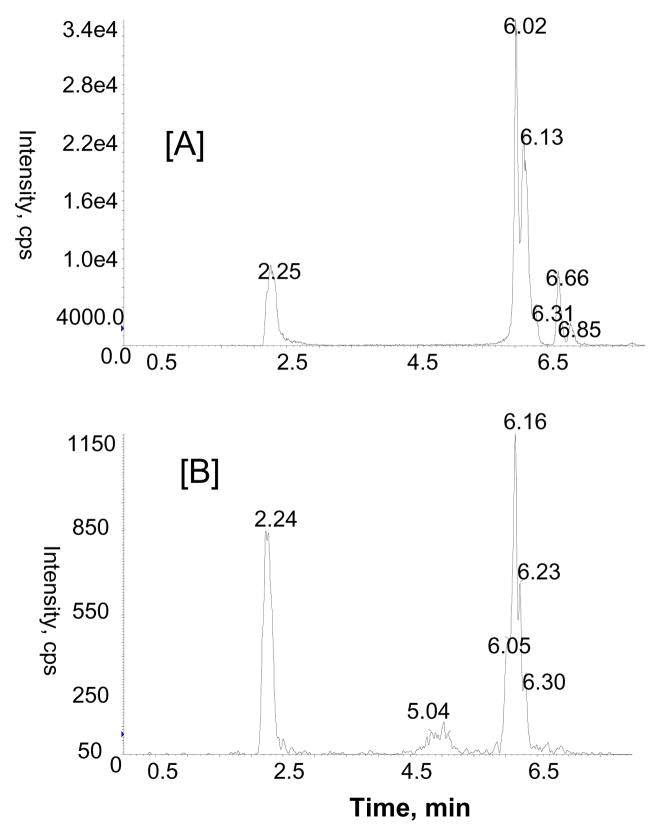
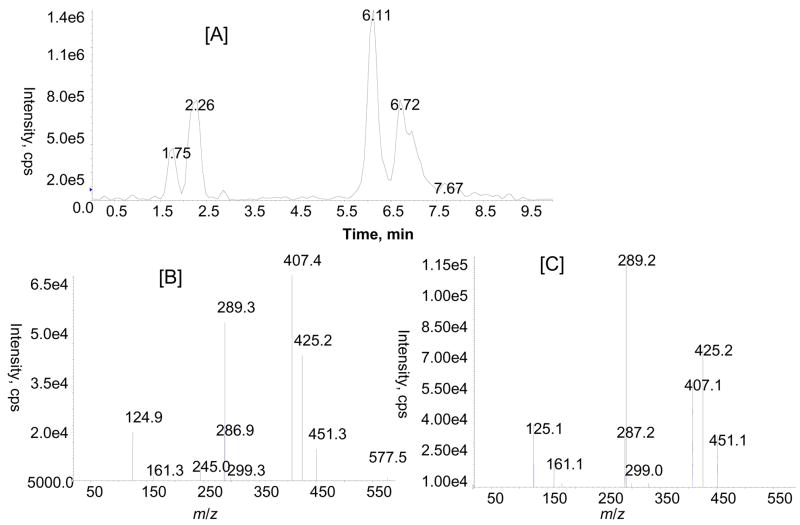
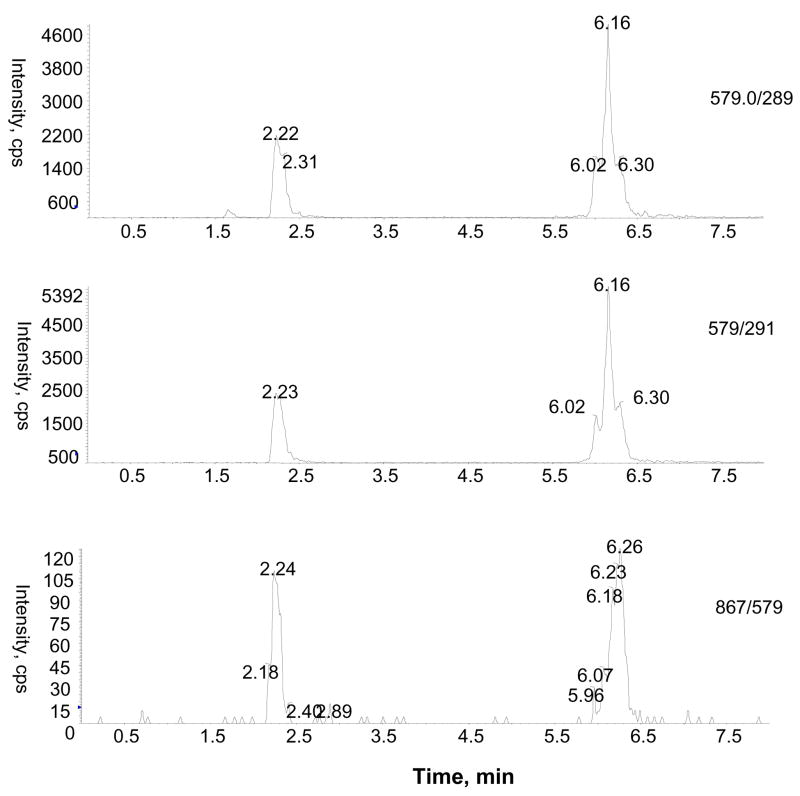
LC-MS/MS with ESI turbo ionspray analysis of rat urine after enzymatic hydrolysis was performed using multiple MS/MS experiments (MRM and MS/MS) in negative and positive ion modes to identify the flavan-3-ol and proanthocyanidin metabolites. MRM analysis with the mass transitions m/z 291/139 (catechin or epicatechin), 305/139 (methyl catechin or epicatechin), 579/291 (dimers), 579/289 (dimers) and 867/579 (trimers) indicated the presence of monomeric catechin or epicatechin, methylated catechin or epicatechin, and proanthocyanidin dimers and proanthocyanidin trimers in urine collected over 16 h after GSE treatment. Identity of monomeric catechins (catechin and epicatechin) was further confirmed by comparison with product ions and retention time (tR) of standards in LC-MS/MS experiments (data not shown). Proanthocyanidins were only tentatively identified based on extensive interpretation of their product ion spectrum and previously published references (Tsang et al., 2005; Yamaguchi et al., 1999). For the identification of proanthocyanidins, we compared the product ion profile of metabolites with the proanthocyanidins of GSE administered to the rats. As can be seen in Fig 3A, there are at least four major peaks with a mass transition m/z 579/289 at tR 2.2, 6.0, 6.1 and 6.6 min in the MRM analysis, indicating the presence of proanthocyanidin dimers. Similarly, a MRM ion chromatogram with mass transition m/z 867/577 showed three peaks at tR 2.2, 5.0 and 6.1 min (Fig. 3B), corresponding to isomers of proanthocyanidin trimers in the GSE-treated urine sample. Further identification of proanthocyanidin dimmers was obtained from LC-MS/MS analysis of m/z 577 [M-H]−. As anticipated, a number of peaks were observed in the LC-MS/MS analysis of m/z 577 (Fig. 4). The mass spectra resulting from MS/MS experiments of m/z 577 at tR 2.2 and 6.1 min in the negative ion mode show product ions at m/z 451 (M-H-126)−, 425 (M-H-152)−, 407 (M-H-170)−, 289, and 287 which are consistent with those of proanthocyanidin dimers previously reported in grape seed (Sun et al., 1999). MS/MS spectra again demonstrated that the relative abundance of major product ions (e.g. m/z 289 and 407) varies indicating that they represent different isomeric forms and that arise from variation in the positioning of monomeric flavan-3-ol units. At this point, chemical structures of individual proanthocyanidins could not be characterized precisely due to unavailability of authentic standards.
Figure 3.
Representative MRM ion chromatograms showing proanthocyanidin di and trimers with mass transitions m/z 579/289 [A] and 867/577 [B], respectively in rat urine after GSE treatment.
Figure 4.
Total ion chromatogram obtained from LC-MS/MS of m/z 577 in GSE treated rat urine [A]. Product ion spectra of m/z 577 at tR 2.2 [B] and 6.1 min [C].
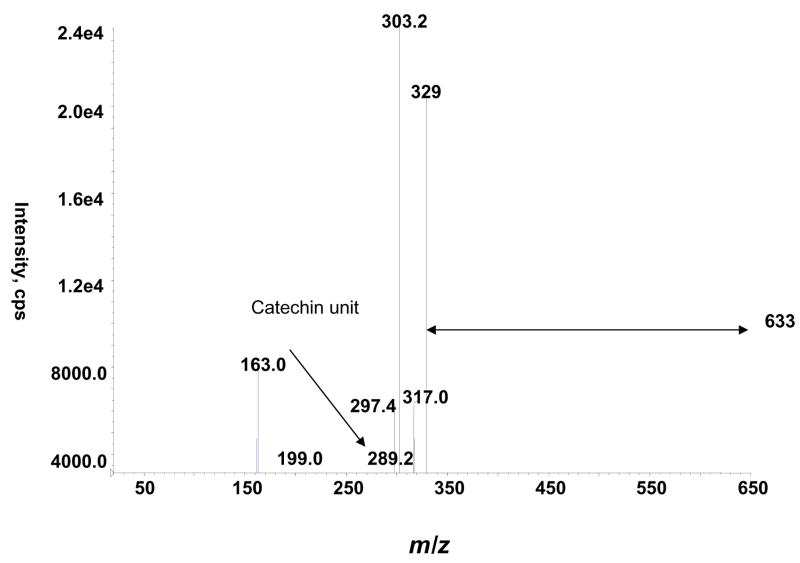
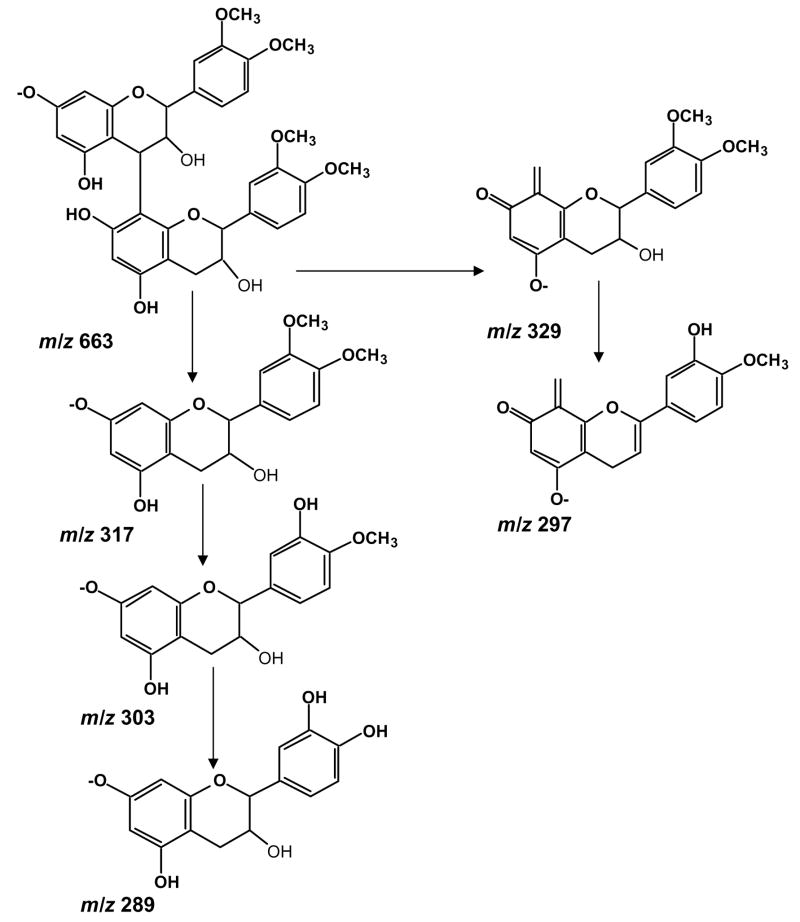
We also detected a m/z 633 ion which on fragmentation yielded the following product ions, m/z 329, 303, 289 and 163 (Fig. 5). The most obvious fragmentation pathway is the breakage of the inter-flavan bond of m/z 633 leading to the formation of m/z 317 which corresponds to dimethylated (epi)catechin (Fig. 6). This ion on further fragmentation generates m/z 303 (monomethylated (epi)catechin) and (epi)catechin m/z 289. The product ion m/z 329 could be the result of heterocyclic ring fission followed by further cleavage of the C3–4 position of upper unit of (epi)catechin. A neutral loss of water (−18 Da) and methyl group from the product ion m/z 329 is suggested for the generation of product ion m/z 297. These pieces of information led us to propose that the ion m/z 633 corresponds to a dimeric structure made up of two dimethylated (epi)catechins as shown in Fig. 6.
Figure 5.
Product ion spectrum of m/z 633 in rat urine.
Figure 6.
Proposed fragmentation pathway for tetramethylated proanthocyanidin dimmer m/z 633.
Plasma metabolites
Flavan-3-ol, monomeric catechins (catechin and epicatechin), their monomethylated metabolites, and proanthocyanidin dimers and pronathocyanidin trimers were detected using LC-MS/MS in the MRM mode. The plasma concentration of (+)-catechin was below the limit of quantification. Interestingly, proanthocyanidins up to trimers were detected in 4h after the GSE-treated plasma samples. As can been seen in Fig. 7, two well separated peaks with mass transitions m/z 579/291, 579/289 and m/z 867/579, indicated the occurrence of proanthocyanidin dimers and proanthocyanidin trimers, respectively.
Figure 7.
Representative MRM ion chromatograms resulting from the analysis of GSE treated rat plasma. Mass transitions were m/z 579/280 and 579/291 [A and B] and m/z 867/579 [C] for the detection of proanthocyanidin dimers and trimers, respectively.
Identification and quantification of GSE metabolites in rat brain
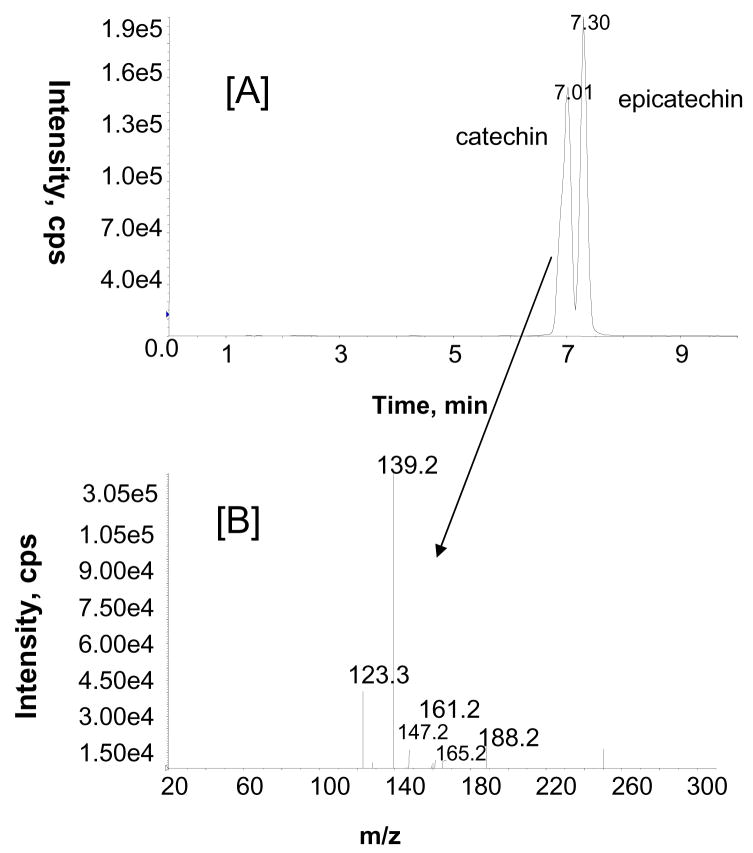
Two brain samples were pooled, extracted (as described in the experimental section) and analyzed for GSE metabolites using LC-MS/MS. Interestingly, the MRM analysis of brain samples indicated the presence of catechin and epicatechin (Fig. 8A). The structures of catechin and epicatechin were further confirmed by comparing their product ion spectra with those of standards (Fig. 8B). (+)-Catechin concentration was found to be approximately 53.16 ng/gram wet tissue, in the final brain extract.
Figure 8.
Representative MRM ion chromatogram obtained from the methanolic extract of brain sample from a GSE-treated rat with mass transitions m/z 291/139 [A]. Product ion spectrum of m/z 291 at tR 7.0 min [B].
DISCUSSION
A previous study has shown that GSE is a rich source of monomeric (epi)catechins and oligomeric proanthocyanidins (Reed et al., 2005). In the present study, proanthocyanidins up to nonamers and substantial but undetermined high molecular weight polymeric proanthocyanidins were detected. One of the major questions has been whether the high molecular weight proanthocyanidins are bioavailable in detectable amounts (Donovan et al., 2002). It has been suggested that the effects of dietary proanthocyanidins are unlikely to be due to proanthocyanidins themselves or monomeric metabolites with the intact flavonoid-ring structure, as they do not exist at detectable concentrations in vivo. However, emerging evidence has shown many health benefits of GSE including neuroprotection (Feng et al., 2007; Deshane et al., 2004).
In the present investigation, the mean amounts of (+)-catechin and (−)-epicatechin fed to each rat by gavage were 10.2 and 4.25 mg, respectively. In addition, the GSE contained a substantial amount of proanthocyanidins. The plasma concentration of catechin 4 h after GSE administration was below the limit of quantification. This observation is in accord with the previous report which indicated that catechins level in human plasma reach maximum 2 h after ingestion and decrease substantially after 3h (Masukawa et al., 2006). The concentration of methyl (epi)catechin could not be estimated due to lack of standards. Proanthocyanidins up to trimers were detected in the blood with high specificity using the LC-MS/MS method. Previous studies by Soji et al. (2006) indicated absorption of proanthocyanidins from the digestive tract and their presence in the rat plasma after the treatment with the proanthocyanidin oligomeric fraction of apple. Similarly, Baba et al. (2002) and Sano et al. (2003) reported the detection of proanthocyanidin dimers in rat and human plasma using LC-MS. Although Tsang et al. (2005) reported proanthocyanidin dimer and trimer in GSE treated urine, this is the first report identifying proanthocyanidins up to trimers in blood and urine after GSE administration in rats. Unavailability of pure proanthocyanidins, complexity caused by different polymerization pattern and the isomers for each oligomer represent some of the problems in quantification of grape proanthocyanidin metabolites.
The low plasma concentrations of catechins and detection of proanthocyanidins indicates that proanthocyanidins were not necessarily degraded into only monomeric metabolites 4 h after administration. The absorption of higher oligomeric proanthocyanidins cannot be ignored, since the LC-MS/MS method is not sensitive enough to detect proanthocyanidins larger than trimers when the concentration is very low in biological samples. A previous report that proanthocyanidins are degraded to compounds with lower molecular weights by the colonic microflora in vitro warrants further confirmation in vivo (Deprez et al., 2000). Studies by Kahle at al. (2007) have indicated that ninety percent of the consumed proanthocyanidins were recovered in the ileostomy effluent and therefore would reach the colon under physiologic circumstances.
LC-MS/MS analysis of the urine from rats treated with GSE indicated the presence of monomeric catechins, proanthocyanidin dimers and proanthocyanidin trimers. As can be seen in Fig. 3A, there were four major peaks with retention times of 2.2, 6.0, 6.1 and 6.7 min, indicating four isomers of proanthocyanidin dimers. These results are in agreement with previous reports, in which proanthocyanidin B1, B2, B3 and B4 dimers were detected in urine after GSE treatment (Tsang et al., 2005).
A number of flavonoid metabolites containing ortho-dihydroxy (catechol) groups are reported to be substrates for catechol-O-methyltransferase (COMT) in vitro (Nagai et al., 2004). Several reports have shown the methylated metabolites of catechin, epicatechin and quercetin (all having catechol-like structure) (Donovan et al., 1999; Hong et al., 2004). To the best of our knowledge, this is the first report identifying a new tetramethylated metabolite of proanthocyanidin dimer (m/z 633) by LC-MS/MS, although Garcia-Ramirez et al. (2006) identified a dimeric proanthocyanidin consisting of two dimethylated epicatechins linked by an ethyl bridge in plasma after oral administration of synthetic oligomeric proanthocyanidins.
Recent studies have suggested that GSE might exert neuroprotective properties, so it was necessary to investigate whether GSE components are accessible to the brain to exert these effects (Feng et al., 2007). In this study monomeric catechin and epicatechin were shown to have crossed the blood brain barrier and gained entry to the brain in GSE-treated rats. Although El Mohsen et al. (2002) have claimed the presence of the epicatechin glucuronide and 3′-O-methyl epicatechin glucuronide in the rat brain following oral ingestion of epicatechin, more definitive analytical data were needed, particularly MS/MS product ions that truly correspond to glucuronidated metabolites of epicatechin and methyl catechin. Other flavonoids such as naringenin and puerarin have been detected in rat brain samples (Peng et al., 1998; Prasain et al., 2004).
In our study, to ensure that the detected metabolites had actually crossed the brain endothelial cell barrier and passed to the brain, brain tissues from animals that were perfused through the heart using ice cold saline were used. The results show the presence of unconjugated catechin and epicatechin in the brain tissue. Thus the catechins found in the brain were located intracellularly or interstitially and not intravascularly.
In conclusion, monomeric catechins and proanthocyanidin up to trimers are absorbed from the digestive tract and present in blood and urine. The present data also demonstrate that unconjugated catechin and epicatechin are bioavailable to the brain in rats. More quantitative studies are needed to investigate absorption, metabolism and excretion of oligomeric proanthocyanidins.
Acknowledgments
These studies were supported in part by grants from the National Center for Complementary and Alternative Medicine and the National Institutes of Health Office of Dietary Supplements (5P50 AT-00477 – to the Purdue University-UAB Botanicals Center for Age-Related Disease, Connie Weaver, PI), UAB Alzheimer Disease Research Center (1P50AG16582-08) and UAB Memory Disorder Research center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the National Institutes of Health.
The mass spectrometer was purchased by funds from a NIH/NCRR Shared Instrumentation Grant (S10 RR19231) and from this institution. Operation of the UAB Comprehensive Cancer Center Mass Spectrometry Shared Facility has been supported in part by a NCI Core Research Support Grant to the UAB Comprehensive Cancer (P30 CA13148). We also wish to thank Kikkoman, Inc., for providing the grape seed extract for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baba S, Osakabe N, Natsume M, Terao J. Absorption and urinary excretion of procyanidin B 2 [epicatechin-(4β-8)-epicatechin] in rats. Free Rad Biol Med. 2002;33:142–148. doi: 10.1016/s0891-5849(02)00871-7. [DOI] [PubMed] [Google Scholar]
- Boukharat M. Procyanidines galloylées du sarment de vigne (Vitis vinifera) Séparation et identification par chromatographie liquide haute performance et chromatographie en phase gazeuse. Chromatography. 1988;455:406–409. [Google Scholar]
- Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid Redox Signal. 2001;3:957–67. doi: 10.1089/152308601317203503. [DOI] [PubMed] [Google Scholar]
- Déprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C, Scalbert A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr. 2000;130:2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- da Silva JMR, Rigaud J, Cheynier V, Cheminat A, Moutonet M. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. [Google Scholar]
- Deshane J, Chaves L, Sarikonda KV, Isbell S, Wilson L, Kirk M, Grubbs C, Barnes S, Meleth S, Kim H. Proteomics analysis of rat brain protein modulations by grape seed extract. J Agric Food Chem. 2004;52:7872–7883. doi: 10.1021/jf040407d. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Bell JR, Kasim-Karakas S, German JB, Walzem RL, Hansen RJ, Waterhouse AL. Catechin is present as metabolites in human plasma after consumption of red wine. J Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- Donovan JL, Manach C, Rios L, Morand C, Scalbert A, Rémésy C. Procyanidins are not bioavailable in rats fed a single meal containing a grape seed extract or the procyanidin dimer B 3. Br J Nutr. 2002;87:299–306. doi: 10.1079/bjnbjn2001517. [DOI] [PubMed] [Google Scholar]
- El Mohsen MMA, Kuhnler G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Rad Biol Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- Faria A, Mateus N, de Freitas V, Calhau C. Modulation of MPP+ uptake by procyanidins in Caco-2 cells: involvement of oxidation/reduction reactions. FEBS Lett. 2006;580:155–160. doi: 10.1016/j.febslet.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Feng Y, Liu YM, Leblanc MH, Bhatt AJ, Rhodes PG. Grape seed extract given three hours after injury suppresses lipid peroxidation and reduces hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2007;61:295–300. doi: 10.1203/pdr.0b013e318030c92d. [DOI] [PubMed] [Google Scholar]
- García-Ramírez B, Fernandez-Larrea J, Salvadó MJ, Ardèvol A, Arola L, Bladé C. Tetramethylated dimeric procyanidins are detected in rat plasma and liver early after oral administration of synthetic oligomeric procyanidins. J Agric Food Chem. 2006;54:2543–2551. doi: 10.1021/jf0527753. [DOI] [PubMed] [Google Scholar]
- Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B 2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- Hong YJ, Mitchell AE. Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. J Agric Food Chem. 2004;52:6794–801. doi: 10.1021/jf040274w. [DOI] [PubMed] [Google Scholar]
- Kahle K, Huemmer W, Kempf M, Scheppach W, Erk T, Richling E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J Agric Food Chem. 2007;55:10605–14. doi: 10.1021/jf071942r. [DOI] [PubMed] [Google Scholar]
- Koga T, Moro K, Nakamori K, Yamakoshi J, Hosoyama H, Kataoka S, Ariga T. Increase in oxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seeds. J Agric Food Chem. 1999;47:1892–1897. doi: 10.1021/jf9810517. [DOI] [PubMed] [Google Scholar]
- Masukawa Y, Matsui Y, Shimizu N, Kondou N, Endou H, Kuzukawa M, Hase T. Determination of green tea catechins in human plasma using liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;834:26–34. doi: 10.1016/j.jchromb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab Dispos. 2004;32:497–504. doi: 10.1124/dmd.32.5.497. [DOI] [PubMed] [Google Scholar]
- Pataki T, Bak I, Kovacs P, Bagchi D, Das DK, Tosaki A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–899. doi: 10.1093/ajcn/75.5.894. [DOI] [PubMed] [Google Scholar]
- Peng HW, Cheng FC, Huang YT, Chen CF, Tsai TH. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;714:369–374. doi: 10.1016/s0378-4347(98)00204-7. [DOI] [PubMed] [Google Scholar]
- Piskula MK, Terao J. Accumulation of (−)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J Nutr. 1998;128:1172–1178. doi: 10.1093/jn/128.7.1172. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52:3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–784. [Google Scholar]
- Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66:2248–2263. doi: 10.1016/j.phytochem.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- Sano A, Yamakoshi J, Tokutake S, Tobe K, Kubota Y, Kikuchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- Shoji T, Masumoto S, Moriichi N, Akiyama H, Kanda T, Ohtake Y, Goda Y. Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem. 2006;54:884–92. doi: 10.1021/jf052260b. [DOI] [PubMed] [Google Scholar]
- Sun B, Belchior GP, Ricardo da Silva JM, Spranger MI. Isolation and purification of dimeric and trimeric procyanidins from grape seeds. J Chromatogr A. 1999;841:115–121. [Google Scholar]
- Tsang C, Auger C, Mullen W, Bornet A, Rouanet JM, Crozier A, Teissedre PL. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. Br J Nutr. 2005;94:170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F, Yoshimura Y, Nakazawa H, Ariga T. Free radical scavenging activity of grape seed extract and antioxidants by electron spin resonance spectrometry in an H2O2/NaOH/DMSO system. J Agric Food Chem. 1999;47:2544–2548. doi: 10.1021/jf9806762. [DOI] [PubMed] [Google Scholar]
- Yamakoshi J, Saito M, Kataoka S, Tokutake S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J Agric Food Chem. 2002;50:4983–4988. doi: 10.1021/jf0201632. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]