Abstract
Background
Thyroid nodules are commonly encountered problems in clinical practice. For patients who have a thyroid nodule, the fine-needle aspiration biopsy (FNAB) is the most important test, as it is the most reliable diagnostic method for distinguishing between benign thyroid nodules and cancerous nodules. FNAB is able to be performed either via an ultrasound (USG) or alone and is the first choice when it comes to diagnosing thyroid nodules, given that it is cheap, safe and provides accurate results.
Objective
In this study-a retrospective analysis of FNAB via USG - our aim is to evaluate the multiple variables related to FNAB procedures, including the experience of the person performing the biopsy, the age and gender of the patient, the number of nodules, the size of the nodule(s) and the number of lams recorded from the cytopathology report on non-diagnostic rates, conducted at an invasive radiology clinic and at a general surgery clinic.
Materials and methods
A total of 1062 patients involving 1869 nodules, examined using FNAB via USG, were reviewed retrospectively from records dated between November 2011 and July 2014 and from pathology reports taken from the ANEAH General Surgery clinic and Interventional Radiology clinic. Cytopathology results were classified according to the 2007 Bethesda System for Reporting. Gender, age, number of nodules, diameter of the nodules, biopsied nodules, location of the nodules, number of lams, symptoms and the date of biopsies were the parameters used to examine the factors involved in non-diagnostic cytopathology invasive radiology. These parameters were inspected at both of the clinics (ANEAH General Surgery clinic and Interventional Radiology clinic). In analyzing the results, the statistical significance level was set at 0.05, where in cases that the p value was under 0.05 (p < 0.05), it was determined that no significant relationship existed. In this study, data were analyzed using SPSS 20 software.
Results
Of the nodules reviewed, 1620 were found on females and 249 on males. The age of the patients ranged from 10 to 87 years, with the mean age being 50 years. In the general surgery clinic, 470 nodules of 341 patients were aspirated, and in the interventional radiological clinic, 1399 nodules of 721 patients were aspirated. In the literature review conducted to compare statistical assessments of FNAB via USG, no significant difference was found between the ANEAH General Surgery clinic and the Invasive Radiology clinic (p > 0.05). In the invasive radiology clinic, non-diagnostic rates decreased with the increase in experience of the person who conducted the biopsy (p = 0.001).
Conclusion
The results from both of the clinic's rates of non-diagnostic FNAB, performed via USG, were found to be acceptable. Our study also demonstrates that USG-guided FNAB can be performed with a low non-diagnostic rate as experience grows.
Keywords: FNAB, Non-diagnostic, Thyroid biopsy, Thyroid USG
Highlights
-
•
Thyroid nodules are commonly encountered problems in clinical practice.
-
•
FNAB is the most reliable diagnosis method for distinguishing between benign and malignant thyroid nodules.
-
•
The non-diagnostic rates may decrease with the increase of experience in administering USG-guided thyroid biopsies.
1. Introduction
Thyroid nodules are a commonly seen problem in clinical practices around the world. Thyroid nodules are observed in 5% of females and in 1% of males in non-endemic areas that are supplied with sufficient iodine. However, thyroid nodules can be identified in 19–67% of randomly selected individuals, particularly women and older individuals, through a high resolution USG [1], [2]. A fine-needle aspiration biopsy (FNAB) is recommended in cases of dominant or painless growing nodules, solid nodules and nodules larger than 1 cm in diameter, due to the 5–10% malignancy risk in multinodular goiters [2].
An FNAB, which can be performed either with or without an USG, is the most important examination method for patients with thyroid nodules [3]. It is generally accepted that FNAB is the most reliable diagnosis method for distinguishing between benign and malignant thyroid nodules. In addition, as it is a safe, reliable and less costly method, providing accurate results and less complications, and is well-tolerated by patients, FNAB is the first choice when it comes to diagnosing thyroid nodules [4]. FNAB was first described by Martin and Ellis in 1930 [5]. In the 1950s, it began to be more commonly used, particularly in Sweden and other Scandinavian countries [6]. For the past two decades, it has enjoyed widespread use throughout the world [5], [6]. The performance of FNABs accompanied with an USG has recently been increasing, and it has been reported that the execution of an FNAB under ultrasound imaging has a diagnostic value higher than that of an FNAB without an USG. The USG-aided FNAB has certain advantages, such as enabling the needle to be advanced to the desired location, avoiding any major vascular fields, and allowing the needle to access the remote fields of central necrosis areas [7].
Today, the main purpose of FNABs is to determine whether a thyroid nodule requires surgical treatment or not and thereby possibly avoid the major complications and high costs associated with thyroidectomies [5], [6], [7]. The most significant diagnosis problem related to FNABs is the failure to extract sufficient material during the procedure; therefore, adequate training is necessary for sample making and preparation [2], [7].
Some studies show that when FNABs are made by the same person, the prepared and reported thyroid FNAB's results are better than others [7], [8]. The ideal situation would be that the reporting is conducted by an experienced cytopathologist for FNABs that have been prepared by trained hands [8]. In patients who have received a non-diagnostic FNAB, the FNAB should be made again with the aid of USG. Important studies conducted on this topic have reported that repeated cytological examinations are able to provide more accurate diagnoses [9], [10].
The purpose of our study was to conduct a retrospective analysis of USG-guided thyroid FNAB results in both a general surgery clinic and an interventional radiology clinic and to evaluate the relationship among prognostic factors, such as non-diagnostic rates of biopsy, the experience of the person administering the FNAB, age, gender, number and size of nodes and number of lam.
2. Materials and methods
2.1. Patients and ethics
This study was conducted in the general surgery and interventional radiology clinics at Health Sciences University Adana Numune Training and Research Hospital during the period from October 2011 and July 2014. A total of 1869 nodules involving 1062 patients who had received an aspirated biopsy for thyroid nodules were eligible for the study. The data of patients and pathology reports were evaluated retrospectively, and the cytopathology results were classified according to the 2007 Bethesda System for Reporting [8].
The prognostic parameters, such as the patient's age, gender, number of nodules, number of biopsies taken, location of nodule, nodule size, number of lam, date of procedure and the clinics that performed the FNAB were evaluated in order to research the biopsy patients who had non-diagnostic (insufficient diagnosis) cytopathology results. These parameters were evaluated in both clinics. The Ethical Committee of our center approved the study protocol (ANEAH.EK2014.62).
2.2. Thyroid fine needle aspiration biopsy (FNAB)
All of the biopsies were taken by the same physician in their respective clinics. The on-site rapid assessment method was performed on the first 155 patients in the general surgery clinic, but was not performed on the other 315 nodules. At the interventional radiology clinic, the on-site rapid assessment method was not performed. All patients underwent a thyroid USG prior to being administered the FNAB by the radiology clinic (Fig. 1).
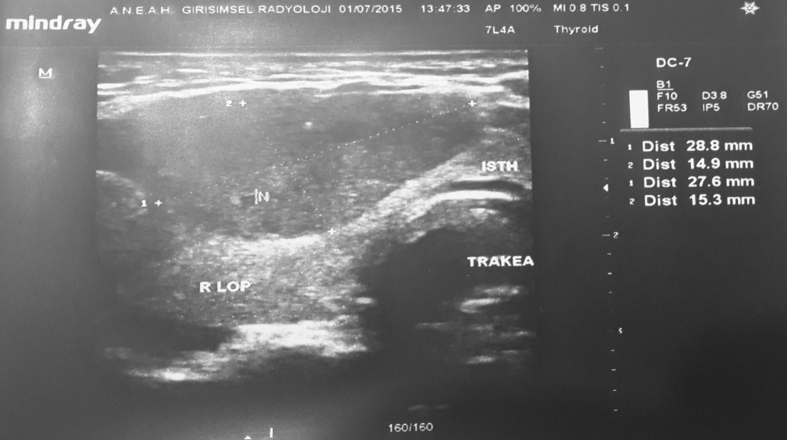
Fig. 1.
The appearance of thyroid nodule with USG (ANEAH archive).
The size of the thyroid gland, number of solitary or multiple nodules, underlying disease, size of nodule, characteristics of nodule limit, the presence of halo, calcification features, sonographic characteristics of the nodule, and distinction of solid or cystic were evaluated with USG (Fig. 2).
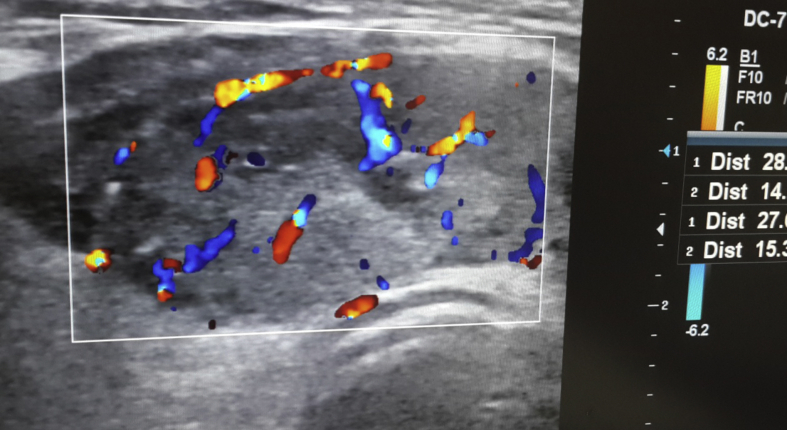
Fig. 2.
The appearance of thyroid nodule with Doppler-USG (ANEAH archive).
The biopsies were taken by the Mindray DC-7 USG and with a 7.5 and 10 MHz linear probe. All interventions were made in a biopsy room, where the patient was placed in the appropriate position prior to the FNAB being administered. This position involved the placement of a pillow under the patient's shoulders and the hyperextension of the neck while they were in prone position. Betadine or alcohol was applied on the patient's neck before administering lidocaine HCl (1 ml ampule, subcutaneous) to the patient as a local anesthetic.
The FNAB was conducted using a 10 cc syringe with 22G (black points) or 21G (green tip) needles. To begin, 1–2 cc of air was taken into the syringe; then, the projection of nodules on the skin to be biopsied were detected with USG (Fig. 5). Next, the tip of the needle was entered into the nodule guided by USG. Negative pressure was created by taking up 10 cc of air into the syringe. The procedure continued with the movement of the millimeter needle back and forth in nodules to remove cells (Fig. 6). Once the material was extracted, the absence of hemorrhaging and the material of the needle tip was noted. The extracted material was sprayed onto the lam after removal of the needle tip and then spread to the other lam with the aid of second lam (Fig. 7). The lams were sent to the pathology lab after being air dried or undergoing alcohol fixation process (Fig. 8).
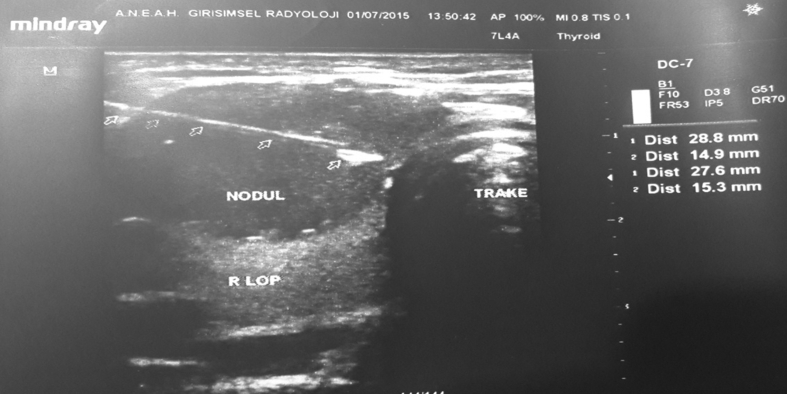
Fig. 5.
Thyroid FNAB with USG-guided (ANEAH archive).
Fig. 6.
The needle in thyroid nodule during FNAB with USG-guided (ANEAH archive).
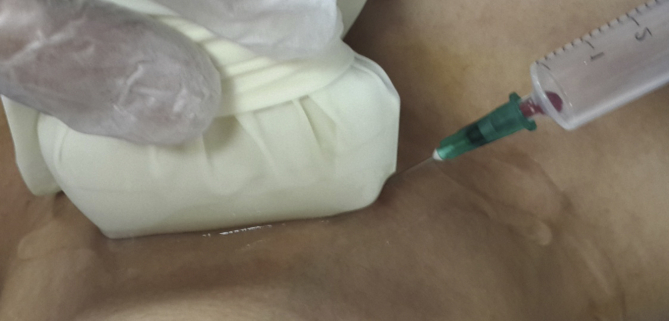
Fig. 7.
Preparation before the FNAB (ANEAH archive).
Fig. 8.
The fixation of lam in alcohol and air after FNAB (ANEAH archive).
2.3. Inclusion and exclusion criteria
2.3.1. Inclusion criteria13
-
1.
Patients willing to give written informed consent.
-
2.
Of diameter larger than 1.0 cm that is solid and hypo-echoic on USG.
-
3.
Of any size with USG findings suggestive of extra-capsular growth or metastatic cervical lymph nodes.
-
4.
Of any size with patient history of neck irradiation in childhood or adolescence; PTC (papillary thyroid carcinoma), MTC (medullary thyroid carcinoma), or MEN 2 (multiple endocrine neoplasm 2) in first-degree relatives; previous thyroid surgery for cancer; increased calcitonin levels in the absence of interfering factors.
-
5.
Of diameter smaller than 10 mm along with USG findings associated with malignancy include micro-calcifications, marked hypo-echogenicity, an irregular or microlobulated margin, a longitudinal dimension larger than the cross-sectional dimension, intrinsic vascularity, direct tumor invasion of adjacent soft tissue, and metastasis to one or more lymph nodes.
-
6.
If a radioisotope scan is available, do not biopsy hot areas.
-
7.
In the presence of multiple nodules, FNAB was performed for suspicious features of thyroid USG above described criteria.
-
8.
In the presence of complex (solid-cystic) thyroid nodule(s), FNAB was taken the solid component of the lesion according to the suspicious USG findings.
-
9.
When initial FNA biopsy was nondiagnostic, it was repeated with USG guidance.
-
10.
No infection at time of FNAB in biopsy location.
2.3.2. Exclusion criteria
-
1.
Patients willing not to give written informed consent.
-
2.
Uncooperative patients or psychiatric disorders.
-
3.
A severe bleeding disorders such as INR (International ratio) > 1.4, prothrombin time > 15 s, platelet account > 50,000/mm3.
-
4.
Nodules that are hot on scintigraphy should be excluded from FNA biopsy.
2.4. On-site (bedside) rapid assessment of FNAB
On-site rapid assessment involves the rapid staining of the preparation during the aspiration. This procedure ensures that enough material is taken, preventing the possibility of taking a deficient amount of material. When the lesion has been rapidly evaluated and requires the use of diagnostic immunohistochemistry (IHC), flow cytometry or molecular methods, then the material needs to be prepared according to the respective method. The information provided after each aspiration helps correct other aspiration methods that have been administered [11], [41].
2.5. Assessment of thyroid FNAB results
FNABs are useful methods for conducting assessments of thyroid nodules. The main purposes of FNABs are to facilitate a process for disease management and to determine whether surgery is necessary, and if so, to identify the correct surgical procedure to perform. As there is no general consensus on the reporting of thyroid FNAB, several classification systems are suggested by various authors, according to personal/corporate experience, as well as a number of clinical organizations (Papanicolaou Society, American Thyroid Association, the American Association of Clinical endocrinologist) [13]. Recent studies have revealed that among pathologists and clinicians there is incompatibility in the diagnostic terminology [8]. Many studies have reported on the use of a sequential system for thyroid FNAB classification. In this effort to create a common system of reporting, basic schemes have been developed to formulate various diagnostic categories, such as benign, unidentified significance lesion (atypia), follicular neoplasm, suspicious, malign and inadequate (not optimal), to name several. Ideally, the goal is to create a system that enables a reporting process whereby the risk of malignancy can be verbally articulated. As a result of the above mentioned problems associated with reporting, the NCI (National Cancer Institute) has supported a new sequential system, which was presented at a 2007 conference. This new system, named the ‘Bethesda Reporting System’, has recently begun to be increasingly used throughout the world [8].
2.6. Bethesda system for reporting
The Bethesda Reporting System has identified 6 categories in thyroid FNAB reporting. The main purpose of this system is to aid clinicians during patient examinations, particularly with regards to determining benign/malign potential. In addition, a common language is used, with the aim of minimizing disparity in reporting between pathologists and clinicians [8].
2.7. Classification of groups
The groups were classified into six categories according to the 2007 Bethesda Reporting System.
-
1.
Bethesda 1 group (Non-diagnostic or inadequate): Only cyst fluid, acellular sample and others (artifact, covering the ground bleeding, etc.). A total of 221 patients in the two clinics were determined to be in this group.
-
2.
Bethesda 2 group (Benign): Compatible with benign follicular nodules (adenomatoid nodules, colloidal nodules, etc.); and in cases of clinical compatibility, compatible with lymphocytic (Hashimoto's) thyroiditis; compatible with granulomatous (subacute) thyroiditis. A total of 1556 patients in the two clinics were determined to be in this group.
-
3.
Bethesda 3 group (Undetermined significant atypia or undetermined significant follicular lesion). A total of 35 patients in the two clinics were determined to be in this group.
-
4.
Bethesda 4 group (Follicular neoplasm or suspicion of follicular neoplasm): Hurthle cell (oncocytic) type must be specified. A total of 17 patients in the two clinics were determined to be in this group.
-
5.
Bethesda 5 group (Suspicion of malignancy): Suspicion of papillary carcinoma (Fig. 3), medullary, metastatic, lymphoma and others. A total of 16 patients in the two clinics were determined to be in this group.
-
6.
Bethesda 6 group (Malign): Thyroid papillary carcinoma (Fig. 4), poorly differentiated carcinoma, medullary thyroid carcinoma, undifferentiated (anaplastic) carcinoma, squamous cell carcinoma, mixed featured carcinoma, metastatic carcinoma, non-Hodgkin's lymphoma. A total of 24 patients in the two clinics were determined to be in this group.
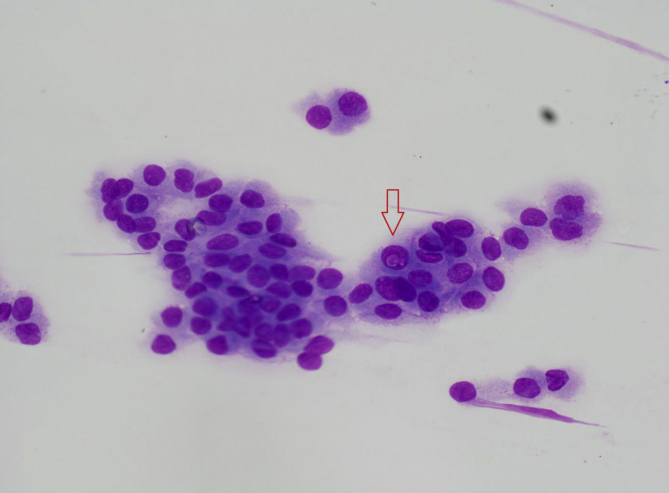
Fig. 3.
Intranuclear cytoplasmic pseudo-inclusion in Thyroid papillary carcinoma after FNAB (ANEAH archive).
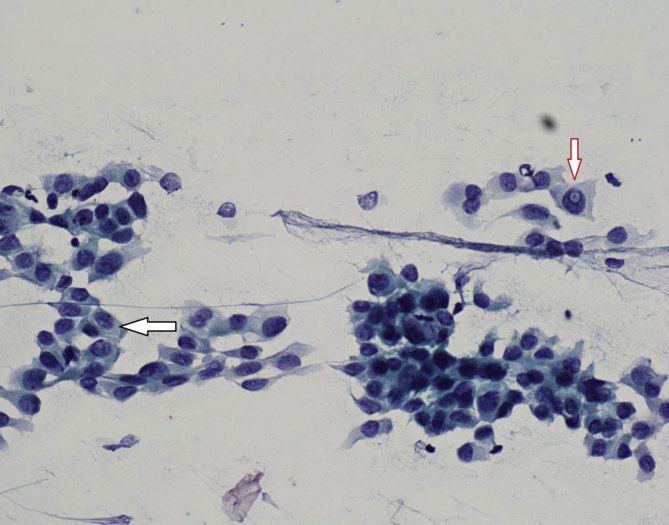
Fig. 4.
Intranuclear cytoplasmic pseudo-inclusion (red arrow) and nuclear cleavage (black arrow) in Thyroid papillary carcinoma after FNAB (ANEAH archive).
Bethesda scores were compared according to the pathology results in the two clinics. The factors affecting the Bethesda Reporting System classification were evaluated in each clinic.
2.8. Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences 20.0 for Windows (SPSS Inc., Chicago, IL, United States). Two-group comparisons were performed by using the Mann-Whitney U test, and the Kruskal-Wallis H test was employed for comparisons involving 3 or more groups. The Post-Hoc Multiple Comparison test was used in cases of the occurrence of significant differences in the Kruskal Wallis-H test. Results were expressed as mean ± standard deviation (min-max). The correlation between the variables was examined using Fisher's Exact Test and Monte Carlo simulation, with the help of the Pearson Chi-square analysis. The significance level was set at 0.05, and if p < 0.05, there was significant difference, whereas if p > 0.05, there was no significant difference.
3. Results
A total of 1062 patients involving 1869 nodules that underwent a USG-guided FNAB were evaluated. Each nodule was identified as a case and compared with a total of 1869 cases. The female patients had 1620 thyroid nodules, the male patients had 249 thyroid nodules and the mean age was 50 years (minimum 10 years and maximum 87 years old). In the general surgery clinic, 341 patients and 470 nodules were aspirated, while in the interventional radiology clinic, 721 patients and 1339 nodules were aspirated.
In Bethesda 1, 2, 3, 4, 5 and 6 groups, the mean ages were 50 (±13, 14), 50 (±13, 85), 47(±15, 13), 43(±9), 49 (±15), and 43 (±16) years, respectively. The minimum age was 10 years and the maximum age was 87 years. In Bethesda 1, 2, 3, 4, 5 and 6 groups, the mean nodule sizes were 18 (±10), 18(±8), 20(±9), 21(±8), 18(±6) and 24(±17) mm in diameter, respectively (Tables 1 and 2).
Table 1.
Relationship of nodules features with Bethesda groups.
| Bethesda |
Average queue | Mann-Whitney U test |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | Min | Max | ss | z | p | |||
| Age | Others | 1648 | 49,79 | 50 | 10 | 86 | 13,92 | 924,03 | 0,871 | 0,384 |
| Bethesda 1 | 221 | 50,45 | 51 | 13 | 87 | 13,14 | 957,51 | |||
| Total | 1869 | 49,87 | 50 | 10 | 87 | 13,83 | ||||
| Diameter of nodule (mm) | Others | 1648 | 18,49 | 16 | 3,5 | 95 | 8,99 | 936,91 | −0,418 | 0,676 |
| Bethesda 1 | 221 | 18,81 | 16 | 4 | 60 | 10,31 | 920,76 | |||
| Total | 1869 | 18,52 | 16 | 3,5 | 95 | 9,16 | ||||
| Number of lam | Others | 1648 | 4,68 | 4 | 1 | 16 | 2,38 | 936,06 | −0,244 | 0,807 |
| Bethesda 1 | 221 | 4,75 | 4 | 0 | 14 | 2,71 | 927,08 | |||
| Total | 1869 | 4,69 | 4 | 0 | 16 | 2,42 | ||||
| Bethesda |
Chi-square test (χ2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Others |
Bethesda 1 |
Total |
|||||||
| n | % | n | % | n | % | χ2 | p | ||
| Gender | Female | 1433 | 87 | 187 | 84,6 | 1620 | 86,7 | 0,92 | 0,337 |
| Male | 215 | 13 | 34 | 15,4 | 249 | 13,3 | |||
| Total | 1648 | 100 | 221 | 100 | 1869 | 100 | |||
| Number of nodule | Only a nodule | 423 | 25,7 | 42 | 19 | 465 | 24,9 | 4,629 | 0,031 |
| Multiple nodule | 1225 | 74,3 | 179 | 81 | 1404 | 75,1 | |||
| Total | 1648 | 100 | 221 | 100 | 1869 | 100 | |||
| Aspiration date | First half | 810 | 49,2 | 124 | 56,1 | 934 | 50 | 3,774 | 0,052 |
| Last half | 838 | 50,8 | 97 | 43,9 | 935 | 50 | |||
| Total | 1648 | 100 | 221 | 100 | 1869 | 100 | |||
In the Bethesda 2 group, 82.4 percent of the patients had only one nodule and 83.5 of the patients had multiple nodules. A significant difference was found between Bethesda groups and nodule groups (p = 0.001).
Table 2.
Comparison of number of nodules, age, gender and lam in Bethesda groups.
| Numbers of Nodule |
Chi-square test (χ2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Only nodule |
Multiple nodule |
Total |
|||||||
| n | % | n | % | n | % | χ2 | p | ||
| Bethesda | Bethesda 1 | 42 | 9 | 179 | 12,7 | 221 | 11,8 | 22,251 | 0,001 |
| Bethesda 2 | 383 | 82,4 | 1173 | 83,5 | 1556 | 83,3 | |||
| Bethesda 3 | 14 | 3 | 21 | 1,5 | 35 | 1,9 | |||
| Bethesda 4 | 9 | 1,9 | 8 | 0,6 | 17 | 0,9 | |||
| Bethesda 5 | 7 | 1,5 | 9 | 0,6 | 16 | 0,9 | |||
| Bethesda 6 | 10 | 2,2 | 14 | 1 | 24 | 1,3 | |||
| Total | 465 | 100 | 1404 | 100 | 1869 | 100 | |||
| Bethesda |
Average queue | Kruskal Wallis-H Test |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | Min | Max | ss | H | p | |||
| Age | Bethesda 1 | 221 | 50,45 | 51 | 13 | 87 | 13,14 | 957,51 | 11,149 | 0,049 |
| Bethesda 2 | 1556 | 50,02 | 50 | 12 | 86 | 13,85 | 933,06 | |||
| Bethesda 3 | 35 | 47,17 | 48 | 18 | 76 | 15,13 | 837,26 | |||
| Bethesda 4 | 17 | 43,47 | 43 | 30 | 60 | 9,19 | 649,41 | |||
| Bethesda 5 | 16 | 49,07 | 45 | 29 | 75 | 15,96 | 876,40 | |||
| Bethesda 6 | 24 | 43,67 | 42 | 10 | 82 | 16,02 | 693,65 | |||
| Total | 1869 | 49,87 | 50 | 10 | 87 | 13,83 | binary comparison 4-2 4-1 6-2 6-1 | |||
| Nodule size (mm) | Bethesda 1 | 221 | 18,81 | 16 | 4 | 60 | 10,31 | 920,76 | 5,127 | 0,401 |
| Bethesda 2 | 1556 | 18,4 | 16 | 3,5 | 90 | 8,82 | 933,40 | |||
| Bethesda 3 | 35 | 20,06 | 18 | 6 | 40 | 9,71 | 1019,51 | |||
| Bethesda 4 | 17 | 21,35 | 21 | 8 | 40 | 8,14 | 1174,71 | |||
| Bethesda 5 | 16 | 18,06 | 17 | 8 | 33 | 6,53 | 978,66 | |||
| Bethesda 6 | 24 | 19,88 | 14,5 | 9 | 95 | 17,63 | 847,54 | |||
| Total | 1869 | 18,52 | 16 | 3,5 | 95 | 9,16 | ||||
| Number of lam | Bethesda 1 | 221 | 4,75 | 4 | 0 | 14 | 2,71 | 927,08 | 7,274 | 0,201 |
| Bethesda 2 | 1556 | 4,63 | 4 | 1 | 12 | 2,33 | 928,28 | |||
| Bethesda 3 | 35 | 5,2 | 4 | 2 | 12 | 2,75 | 1052,00 | |||
| Bethesda 4 | 17 | 5,59 | 4 | 2 | 12 | 2,72 | 1123,44 | |||
| Bethesda 5 | 16 | 5 | 4 | 2 | 10 | 2,83 | 981,63 | |||
| Bethesda 6 | 24 | 6 | 5 | 2 | 16 | 3,62 | 1108,65 | |||
| Total | 1869 | 4,69 | 4 | 0 | 16 | 2,42 | ||||
The Bethesda 4 and 6 groups were younger than the Bethesda 1 and 2 groups, and there were significant differences among the groups according to age (p = 0.049).
A total of 61 patients (13%) were in the general surgery clinic, and a total of 221 patients (11.4%) were in the interventional radiology clinic. No significant difference was found according to Bethesda groups in comparisons conducted between the general surgery clinic and the interventional radiology clinic (p > 0.05) (Table 3).
Table 3.
Comparisons of Bethesda system for reporting in groups.
| Groups |
Chi-square test (χ2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| General surgery |
Interventional radiology |
Total |
|||||||
| n | % | n | % | n | % | χ2 | p | ||
| Bethesda |
Bethesda 1 | 61 | 13 | 160 | 11,4 | 221 | 11,8 | 6,837 | 0,233 |
| Bethesda 2 | 384 | 81,7 | 1172 | 83,8 | 1556 | 83,3 | |||
| Bethesda 3 | 6 | 1,3 | 29 | 2,1 | 35 | 1,9 | |||
| Bethesda 4 | 6 | 1,3 | 11 | 0,8 | 17 | 0,9 | |||
| Bethesda 5 | 3 | 0,6 | 13 | 0,9 | 16 | 0,9 | |||
| Bethesda 6 | 10 | 2,1 | 14 | 1 | 24 | 1,3 | |||
| Total |
470 |
100 |
1399 |
100 |
1869 |
100 |
|||
|
General Surgery |
|||||||||
|
Female |
Male |
Total |
|||||||
|
n |
% |
n |
% |
n |
% |
||||
| Bethesda |
Bethesda 1 | 55 | 12,7 | 6 | 15,8 | 61 | 13 | a | 0,737 |
| Bethesda 2 | 353 | 81,7 | 31 | 81,6 | 384 | 81,7 | |||
| Bethesda 3 | 5 | 1,2 | 1 | 2,6 | 6 | 1,3 | |||
| Bethesda 4 | 6 | 1,4 | 0 | 0 | 6 | 1,3 | |||
| Bethesda 5 | 3 | 0,7 | 0 | 0 | 3 | 0,6 | |||
| Bethesda 6 | 10 | 2,3 | 0 | 0 | 10 | 2,1 | |||
| Total |
432 |
100 |
38 |
100 |
470 |
100 |
|||
|
Interventional Radiology |
|||||||||
|
Female |
Male |
Total |
|||||||
|
n |
% |
n |
% |
n |
% |
||||
| Bethesda | Bethesda 1 | 132 | 11,1 | 28 | 13,3 | 160 | 11,4 | a | 0,838 |
| Bethesda 2 | 998 | 84 | 174 | 82,5 | 1172 | 83,8 | |||
| Bethesda 3 | 25 | 2,1 | 4 | 1,9 | 29 | 2,1 | |||
| Bethesda 4 | 10 | 0,8 | 1 | 0,5 | 11 | 0,8 | |||
| Bethesda 5 | 12 | 1 | 1 | 0,5 | 13 | 0,9 | |||
| Bethesda 6 | 11 | 0,9 | 3 | 1,4 | 14 | 1 | |||
| Total | 1188 | 100 | 211 | 100 | 1399 | 100 | |||
Monte Carlo Simulation was performed because of 20% of smaller than 5 the expected value in the cell.
In the general surgery clinic, 12.7% of patients were female and 15.8% were male. There were no significant differences between the Bethesda groups in the general surgery group in terms of gender (p > 0.05) (Table 1). In the interventional radiology clinic, 11.1% of patients were female and 13.3% were male. No significant difference was found according to gender in comparisons conducted among the Bethesda groups at the interventional radiology clinic (p > 0.05) (Table 1).
In the Bethesda 1 group, 19% of the patients had only one nodule and 81% of the patients had multiple nodules. In the Bethesda 2 group, 82.4% of the patients had only one nodule and 83.5 of the patients had multiple nodules. A significant difference was found between Bethesda groups and nodule groups (p < 0.05) (Table 2). The Bethesda 4 and 6 groups were younger than the Bethesda 1 and 2 groups, and there were significant differences among the groups according to age (p < 0.05) (Table 2). A significant difference was not found between general surgery and interventional radiology groups within the Bethesda 1 group (p > 0.05). In the Bethesda 1 group, 13% of the patients were from general surgery and 11.4% of the patients were from interventional radiology (Table 4).
Table 4.
Comparison of all Bethesda groups between surgery and radiology.
| Group |
Chi-square test (χ2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| General surgery |
Interventional radiology |
Total |
|||||||
| n | % | n | % | n | % | χ2 | p | ||
| Bethesda | Others | 409 | 87 | 1239 | 88,6 | 1648 | 88,2 | 0,802 | 0,37 |
| Bethesda 1 | 61 | 13 | 160 | 11,4 | 221 | 11,8 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
| Bethesda | Others | 86 | 18,3 | 227 | 16,2 | 313 | 16,7 | 1,083 | 0,298 |
| Bethesda 2 | 384 | 81,7 | 1172 | 83,8 | 1556 | 83,3 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
| Bethesda | Others | 464 | 98,7 | 1370 | 97,9 | 1834 | 98,1 | 0,819 | 0,365 |
| Bethesda 3 | 6 | 1,3 | 29 | 2,1 | 35 | 1,9 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
| Bethesda | Others | 464 | 98,7 | 1388 | 99,2 | 1852 | 99,1 | Fisher's exact | 0,398 |
| Bethesda 4 | 6 | 1,3 | 11 | 0,8 | 17 | 0,9 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
| Bethesda | Others | 467 | 99,4 | 1386 | 99,1 | 1853 | 99,1 | Fisher's exact | 0,774 |
| Bethesda 5 | 3 | 0,6 | 13 | 0,9 | 16 | 0,9 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
| Bethesda | Others | 460 | 97,9 | 1385 | 99 | 1845 | 98,7 | 2,692 | 0,101 |
| Bethesda 6 | 10 | 2,1 | 14 | 1 | 24 | 1,3 | |||
| Total | 470 | 100 | 1399 | 100 | 1869 | 100 | |||
The ages of the patients in the Bethesda 1 group were older than other Bethesda groups. There were no significant differences between the Bethesda 1 group and other Bethesda groups in terms of age (p > 0.05). When the groups were compared according to nodule size (diameter), the nodules in the Bethesda 1 group were smaller than those found in other Bethesda groups (p > 0.05). The Bethesda 1 group had fewer lams than other groups, and a significant difference was detected between the Bethesda 1 group and other Bethesda groups in terms of number of lam (p > 0.05) (Table 5). There were no significant differences between the Bethesda 1 group and other Bethesda groups with respect to gender and aspiration date (p > 0.05). In the Bethesda 1 group, 84.6% of the patients were female and 15.4% were male. In terms of aspiration date, 56.1% of the Bethesda 1 group had aspirations performed in the first half of the study (Table 1). The composition of the Bethesda 1 group included 13% of patients from general surgery and 11.4% from interventional radiology. A significant difference was not found between general surgery and interventional radiology in terms of Bethesda groups (p > 0.05) (Table 4).
Table 5.
Relationship of Bethesda groups with aspiration date between surgery and radiology groups.
| General surgery |
Chi-square test (χ2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| First half |
Second half |
Total |
|||||||
| n | % | n | % | n | % | χ2 | p | ||
| Bethesda | Others | 210 | 89,4 | 199 | 84,7 | 409 | 87 | 2,279 | 0,131 |
| Bethesda 1 | 25 | 10,6 | 36 | 15,3 | 61 | 13 | |||
| Total | 235 | 100 | 235 | 100 | 470 | 100 | |||
| Bethesda | Others | 41 | 17,4 | 45 | 19,1 | 86 | 18,3 | 0,228 | 0,633 |
| Bethesda 2 | 194 | 82,6 | 190 | 80,9 | 384 | 81,7 | |||
| Total | 235 | 100 | 235 | 100 | 470 | 100 | |||
| Bethesda | Others | 230 | 97,9 | 234 | 99,6 | 464 | 98,7 | Fisher's exact | 0,216 |
| Bethesda 3 | 5 | 2,1 | 1 | 0,4 | 6 | 1,3 | |||
| Total | 235 | 100 | 235 | 100 | 470 | 100 | |||
| Bethesda | Others | 232 | 98,7 | 232 | 98,7 | 464 | 98,7 | Fisher's exact | 1,00 |
| Bethesda 4 | 3 | 1,3 | 3 | 1,3 | 6 | 1,3 | |||
| Total | 235 | 100 | 235 | 100 | 470 | 100 | |||
| Bethesda | Others | 234 | 99,6 | 233 | 99,1 | 467 | 99,4 | Fisher's exact | 1,00 |
| Bethesda 5 | 1 | 0,4 | 2 | 0,9 | 3 | 0,6 | |||
| Total | 235 | 100 | 235 | 100 | 470 | 100 | |||
| Bethesda |
Others | 228 | 97 | 232 | 98,7 | 460 | 97,9 | 0,92 | 0,338 |
| Bethesda 6 | 7 | 3 | 3 | 1,3 | 10 | 2,1 | |||
| Total |
235 |
100 |
235 |
100 |
470 |
100 |
|||
|
Interventional radiology |
|||||||||
|
First half |
Second half |
Total |
|||||||
|
n |
% |
n |
% |
n |
% |
||||
| Bethesda | Others | 600 | 85,8 | 639 | 91,3 | 1239 | 88,6 | 10,252 | 0,001 |
| Bethesda 1 | 99 | 14,2 | 61 | 8,7 | 160 | 11,4 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
| Bethesda | Others | 128 | 18,3 | 99 | 14,1 | 227 | 16,2 | 4,472 | 0,034 |
| Bethesda 2 | 571 | 81,7 | 601 | 85,9 | 1172 | 83,8 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
| Bethesda | Others | 686 | 98,1 | 684 | 97,7 | 1370 | 97,9 | 0,138 | 0,71 |
| Bethesda 3 | 13 | 1,9 | 16 | 2,3 | 29 | 2,1 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
| Bethesda | Others | 691 | 98,9 | 697 | 99,6 | 1388 | 99,2 | 1,472 | 0,225 |
| Bethesda 4 | 8 | 1,1 | 3 | 0,4 | 11 | 0,8 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
| Bethesda | Others | 697 | 99,7 | 689 | 98,4 | 1386 | 99,1 | 4,958 | 0,026 |
| Bethesda 5 | 2 | 0,3 | 11 | 1,6 | 13 | 0,9 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
| Bethesda | Others | 693 | 99,1 | 692 | 98,9 | 1385 | 99 | 0,071 | 0,79 |
| Bethesda 6 | 6 | 0,9 | 8 | 1,1 | 14 | 1 | |||
| Total | 699 | 100 | 700 | 100 | 1399 | 100 | |||
There were significant differences found between Bethesda 1, 2 and 5 groups in the interventional radiology clinic in terms of aspiration date (p < 0.05).
A significant difference was not detected among any of the Bethesda groups in the general surgery clinic in terms of aspiration date (p > 0.05) (Table 5). There were, however, significant differences found between Bethesda 1, 2 and 5 groups in the interventional radiology clinic in terms of aspiration date (Table 5).
No major complications were seen in either of the clinics. A localized intranoduler hemorrhage occurred in one patient in the general surgery group. Hemostasis was achieved by applying pressure with fingers to the biopsy area for about 15 min after aspiration. A control USG was done on some patients for whom there was suspicion of bleeding following application of pressure.
4. Discussion
In the present study, we aimed to compare the results of thyroid FNABs performed by general surgery physicians and those performed by interventional radiology physicians and more importantly to investigate the factors that influence the emergence of Bethesda 1 results. We did not detect any difference in terms of Bethesda 1 results or in terms of sufficiency in either of the groups at the end of study. Related to this finding, it was observed that when the experience of the person responsible for administering the FNAB increased in the interventional radiology group, the Bethesda 1 results decreased. However, no significant difference was seen in the general surgery groups with respect to experience. Considering the features of the thyroid nodule, we detected an increase in the number of these features in Bethesda 1 and 2 groups compared to the Bethesda 4 and 6 groups when the patient's age increased.
Many studies have been conducted to investigate the effectiveness of FNAB in the follow-up and treatment management of thyroid nodules. In a meta-analysis conducted by Bongiovanni et al. [12] on the effectiveness of the Bethesda system in reporting on thyroid nodules for thyroid cytopathology, it was found that the rate of non-diagnostic FNABs in Bethesda 1 was 13% (2–24%), and in the follow-up of these patients, the malignancy rate was 16.8%.
In order to decrease the non-diagnostic rates in FNAB, many factors have been investigated within the last three decades, including USG-guided aspiration biopsies [14], [15], [16], [17], [18], [19], [20], [21], [22], the number of needle interventions [23], [24], [25], the type and width of needle [26], [27], [28], [29], [30], the experience of the physician [30], [31] and the performance of on-site assessments [32], [33]. In our study, all of these factors were investigated within the context of the FNAB performed by a surgeon and a radiologist.
USG-guided FNAB is a commonly accepted procedure, as it decreases non-diagnostic FNAB rates and facilitates the performance of an aspiration biopsy through the development of imaging techniques [14], [15], [16], [17], [18], [19], [20], [21], [22]. In our study, all of the FNABs were administered with USG-guided imaging in both general surgery and interventional radiology clinics. USG is a reliable tool, particularly for deep and small thyroid nodules, and it helps to predict the presence of malignancy in a nodule using sonographic features (nodules that include micro calcification, a solid component, abnormal cervical lymph nodes etc.) [34], [35], [36], [37], [38], [39]. In a systemic review and meta-analysis carried out by Schmidt et al. [40], the efficacy of on-site assessments in obtaining diagnostic FNAB rates was investigated. In their study, it was found that the results of the rapid on-site assessment improved by 12%. In the present study, the on-site rapid assessment was not made by the radiologist but rather an on-site rapid assessment of the first 155 of 479 nodules was performed by a general surgeon. No statistically significant difference, however, was detected between an FNAB performed with on-site rapid assessment and others when carried by a surgeon in terms of non-diagnostic rates. The effect of diameter of thyroid nodule to adequacy of FNAB and rates of non-diagnostic has been evaluated in many studies, but no significant relationship has been clearly established.
In a study conducted by Martin et al. [41] on USG-guided thyroid FNABs, where a cytological analysis was performed, no significant differences were found between nodules larger than 1 cm in diameter and those less than 1 cm in diameter in terms of non-diagnostic cytology rates. In our study, we did not observe any statistically significant differences between Bethesda 1 group and other Bethesda groups in respect to diameter of nodule (p > 0.05). We believe that all thyroid biopsies should be USG-guided, so as to prevent unnecessary biopsies and complications and to reduce non-diagnostic sitology rates (Bethesda 1).
While all biopsy procedures may involve some complications, USG-guided FNABs have less complication than those that are not USG-guided. These complications include hemorrhages, local infections and vasovagal syncope [42]. In the present study, only one controlled localized intranoduler hemorrhage was observed in a patient. We believe that the reasons for the reduced rate of complications in our study are related to the routine performance of FNABs in a biopsy room, the use of antiseptics, the directing of the Doppler USG towards target nodule, the application of pressure to the biopsy area with fingers for about 15 min after aspiration, and the routine performance of control USGs to prevent cervical hematoma in at-risk patients with intrathyroidal hemorrhage [42], [43], [44], [45].
The results of USG-guided thyroid FNAB can vary among physicians. The most significant disadvantage associated with the USG-guided FNABs is that their success is largely dependent on the person responsible for performing the aspiration biopsy. Seiberling, an otolaryngologist, reported a non-diagnostic rate of 9.6% in his thyroid FNAB study, where he also found the non-diagnostic rate to be similar with radiology [46]. In a study by Bohacek et al. [47], a non-diagnostic rate of 6% was reported for USG-guided FNABs performed by surgeons. In the literature, the results of USG-guided FNAB range between 3.6 and 13% when performed by surgeons and between 8 and 23% when performed by radiologists [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56]. In the present study, the general surgeons had non-diagnostic rates of 13%, and in the intervention group, the non-diagnostic rate was 11.4% (Table 5). When general surgery and intervention radiology groups were compared in terms of non-diagnostic FNAB rate, no significant difference was found (p > 0.05).
There are some limitations of our study. Firstly, the on-side rapid assessment method was not performed all of patients so if the on-side rapid assessment was made to the whole patients, the non-diagnostic rate could be decreased. Secondly, not all thyroid nodules underwent surgery and paraffin bloc was not prepared for pathologic examination without cytology. FNAB results were based on USG and cytology, which may cause some false negative results. Thirdly, our non-diagnostic rates are similarly with literature but our results need to be confirmed with prospective randomized studies.
5. Conclusion
Although surgeons are not experts in the use of USG, they nonetheless benefit from the use of USG in their clinical practice (57). In our study, the non-diagnostic rate did not change according to number of patients; however, in the radiology clinic, there were significant differences between first half and second half aspirations with respect to the non-diagnostic rate. We found the non-diagnostic results of USG-guided FNAB to be similar in surgeons and radiologists, and these results are in agreement with those found in the literature.
Since all of the FNABs performed were USG-guided, which increased the probability of hitting the needle, there were no significant differences between nodule size and non-diagnostic FNAB rate. Finally, both radiologists and surgeons had low but acceptable non-diagnostic rates with USG-guided thyroid FNAB. We believe that the non-diagnostic rates may decrease with the increase of experience in administering USG-guided thyroid biopsies. Further prospective studies will still need to be done to verify our results and to improve experience techniques for low non-diagnostic FNAB rates.
Sources of funding
No funding.
Author contribution
Hilmi Bozkurt: Data collections, data analysis and writing.
Oktay İrkörücü: Study design, interpretation.
Mehmet Aziret: Practise, writing.
Enver Reyhan: Study design, practise.
Mehmet Kemal Okuyan: Practical, design.
Conflicts of interest
There is not any conflict of interest.
Guarantor
Mehmet Aziret.
Research registration unique identifying number (UIN)
Researchregistry867.
References
- 1.Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid Cancer the American thyroid association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid. Cancer Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Lal G., Clark O.H. Thyroid and parathyroid. In: Brunicardi F.C., Schwartz S.I., editors. Schwartz's Principle of Surgery. 9 th Ed. Mc Graw Hill; New York: 2010. pp. 1344–1373. [Google Scholar]
- 3.Morgan J.L., Serpell J.W., Cheng M.S. Fine needle aspiration cytolgy of throid nodüles: how useful is it? Aust. N. Z. J. Surg. 2003;73:480. doi: 10.1046/j.1445-1433.2003.02670.x. [DOI] [PubMed] [Google Scholar]
- 4.Theoharis C.G., Schofield K.M., Hammers L., Udelsman R., Chhieng D.C. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19(11):1215–1223. doi: 10.1089/thy.2009.0155. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen G.K., Ginsberg J., Crockford P.M. Fine-needle aspiration biopsy cytology of the thyroid. Its value and limitations in the diagnosis and management of solitary thyroid nodules. Pathol. Annu. 1991;26:63–69. [PubMed] [Google Scholar]
- 6.Piromalli D., Martelli G., Del Prato I. The role of fine needle aspiration in the diagnosis of thyroid nodules: analysis of 795 consecutive cases. J. Surg. Oncol. 1992;50:247–250. doi: 10.1002/jso.2930500410. [DOI] [PubMed] [Google Scholar]
- 7.Baskin H.J. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules and multinodular goiters. Endocr. Pract. 2004;10:242–245. doi: 10.4158/EP.10.3.242. [DOI] [PubMed] [Google Scholar]
- 8.Cibas E.S., Ali S.Z. Nci thyroid FNA state of the science conference. The Bethesda system for reporting thyroid cytopathology. Am. J. Clin. Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 9.Baloch Z., LiVolsi V., Jain P. Role of repeat fine-needle aspiration biopsy (FNAB) in management of thyroid nodules. Diagn Cytopathol. 2003;29:203–206. doi: 10.1002/dc.10361. [DOI] [PubMed] [Google Scholar]
- 10.Hundahl S.A., Fleming I.D., Fremgen A.M., Menck H.R. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the USA, 1985-1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Burlingame O.O., Kessé K.O., Silverman S.G., Cibas E.S. On-site adequacy evaluations performed by cytotechnologists: correlation with final interpretations of 5241 image-guided fine needle aspiration biopsies. Cancer Cytopathol. 2011;120:177–184. doi: 10.1002/cncy.20184. [DOI] [PubMed] [Google Scholar]
- 12.Bongiovanni M., Spitale A., Faquin W.C., Mazzucchelli L., Baloch Z.W. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56:333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 13.Gharib H., Papini E., Paschke R., Duick D.S., Valcavi R., Hegedüs L., Vitti P. AACE/AME/ETA task force on thyroid nodules. American association of clinical endocrinologists, Associazione medici Endocrinologi,and european thyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J. Endocrinol. Investig. 2010;33(5 Suppl):1–50. [PubMed] [Google Scholar]
- 14.Mavec P. Cytologic diagnosis from tumor tissue using the “quick method” during operation. Acta Cytol. 1967;11:229–230. [PubMed] [Google Scholar]
- 15.Godwin J.T. Rapid cytologic diagnosis of surgical specimens. Acta Cytol. 1976;20:111–115. [PubMed] [Google Scholar]
- 16.Silverman J.F., West R.L., Finley J.L., Larkin E.W., Park H.K., Swanson M.S., Fore W.W. Fine-needle aspiration versus large-needle biopsy or cutting biopsy in evaluation of thyroid nodules. Diagn Cytopathol. 1986;2:25–30. doi: 10.1002/dc.2840020107. 51. [DOI] [PubMed] [Google Scholar]
- 17.Danese D., Sciacchitano S., Farsetti A., Andreoli M., Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fineneedle aspiration biopsy of thyroid nodule. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 18.Leenhardt L., Hejblum G., Franc B., Fediaevsky L.D., Delbot T., Le Guillouzic D., Menegaux F., Guillausseau C., Hoang G., Turpin G., Aurengo A. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J. Clin. Endocrinol. Metab. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 19.Lin J.D., Huang B.Y. Comparison of the results of diagnosis and treatment between solid and cystic well-differentiated thyroid carcinoma. Thyroid. 1998;8:661–666. doi: 10.1089/thy.1998.8.661. [DOI] [PubMed] [Google Scholar]
- 20.Khurana K.K., Richards V.I., Chopra P.S., Izquierdo R., Rubens D., Mesonero C. The role of ultrasonography-guided fine-needle aspiration biopsy in the management of nonpalpable and palpable thyroid nodules. Thyroid. 1998;8:511–515. doi: 10.1089/thy.1998.8.511. [DOI] [PubMed] [Google Scholar]
- 21.Sabel M.S., Haque D., Velasco J.M., Staren E.D. Use of ultrasound-guided fine needle aspiration biopsy in the management of thyroid disease. Am. Surg. 1998;64:738–741. [PubMed] [Google Scholar]
- 22.Carmeci C., Jefferey R.B., McDougall I.R., Nowels K.W., Weigel R.J. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8:283–289. doi: 10.1089/thy.1998.8.283. [DOI] [PubMed] [Google Scholar]
- 23.Moller K., Papanikolaou I.S., Toermer T. EUS-guided FNA of solid pancreatic masses: high yield of 2 passes with combined histologic-cytologic analysis. Gastrointest. Endosc. 2009;70:60–69. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Cleveland P., Gill K.R.S., Coe S.G. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest. Endosc. 2010;71:1194–1199. doi: 10.1016/j.gie.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 25.LeBlanc J.K., Ciaccia D., Al-Assi M.T. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest. Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto H., Kitano M., Komaki T. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J. Gastroenterol. Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal S., Mir R.N., Sohn W. Endoscopic ultrasound-guided fine-needle aspiration using 22- and 25-gauge needles alternately. Endoscopy. 2009;41:87. doi: 10.1055/s-0028-1103444. [DOI] [PubMed] [Google Scholar]
- 28.Imazu H., Uchiyama Y., Kakutani H. A prospective comparison of EUS-guided FNA using 29-gauge and 22-gauge needles. Gastroenterol. Res. Pract. 2009;2009:546390. doi: 10.1155/2009/546390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui A.A., Lyles T., Avula H. Endoscopic ultrasoundguided fine needle aspiration of pancreatic masses in a veteran population: comparison of results with 22- and 25-gauge needles. Pancreas. 2010;39:685–686. doi: 10.1097/MPA.0b013e3181c5c597. 52. [DOI] [PubMed] [Google Scholar]
- 30.Ljung B.M., Drejet A., Chiampi N. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93:263–268. doi: 10.1002/cncr.9040. [DOI] [PubMed] [Google Scholar]
- 31.Choi S.H., Han K.H., Yoon J.H. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin. Endocrinol. 2011;74:776–782. doi: 10.1111/j.1365-2265.2011.04011.x. [DOI] [PubMed] [Google Scholar]
- 32.Klapman J.B., Logrono R., Dye C.E. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am. J. Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias-Garcia J., Dominguez-Munoz J.E., Abdulkader I. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am. J. Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 34.Dray M., Mayall F., Darlington A. Improved fine needle aspiration (FNA) cytology results with a near patient diagnosis service for breast lesions. Cytopathology. 2000;11:32–37. doi: 10.1046/j.1365-2303.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghofrani M., Beckman D., Rimm D.L. The value of onsite adequacy assessment of thyroid fine-needle aspirations is a function of operator experience. Cancer. 2006;108:110–113. doi: 10.1002/cncr.21715. [DOI] [PubMed] [Google Scholar]
- 36.Hamill J., Campbell I.D., Mayall F. Improved breast cytology results with near patient FNA diagnosis. Acta Cytol. 2002;46:19–24. doi: 10.1159/000326710. [DOI] [PubMed] [Google Scholar]
- 37.Lachman M.F., Cellura K., Schofield K. On-site adequacy assessments for image-directed fine needle aspirations: a study of 341 cases. Conn. Med. 1995;59:657–660. [PubMed] [Google Scholar]
- 38.Saleh H.A., Khatib G. Positive economic and diagnostic accuracy impacts of on-site evaluation of fine needle aspiration biopsies by pathologists. Acta Cytol. 1996;40:1227–1230. doi: 10.1159/000333985. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt R.L., Walker B.S., Cohen M.B. When is rapid on-site evaluation cost-effective for fine-needle aspiration biopsy? PLoS One. 2015 Aug 28;10(8):e0135466. doi: 10.1371/journal.pone.0135466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin E., O'Malley M.D., Michalle M.W. Us-guided fine NeedleAspiration biopsy of thyroid nodüles:adequacy of cytologic material and procedure time with and without i?mmediate cytologic analysis. Vasc. İnterv. Radiol. 2002;222:383–387. doi: 10.1148/radiol.2222010201. [DOI] [PubMed] [Google Scholar]
- 41.Baloch Z.W., LiVolsi V.A. Fine-needle aspiration of thyroid nodules: past, present, and future. Endocr. Pract. 2004;10:234–241. doi: 10.4158/EP.10.3.234. [DOI] [PubMed] [Google Scholar]
- 42.Rozycki G.S. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann. Surg. 1998;228:557–567. doi: 10.1097/00000658-199810000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cakmak G.K., Emre A.U., Tascılar O., Gultekin F.A., Ozdamar S.O., Comert M. Diagnostic adequacy of surgeon-performed ultrasound-guided fine needle aspiration biopsy of thyroid nodules. J. Surg. Oncol. 2013;107:206–210. doi: 10.1002/jso.23212. [DOI] [PubMed] [Google Scholar]
- 44.Oktay I., Reyhan E., Kamuran C.D. Surgeon-performed ultrasound-guided thyroid fine-needle aspiration biopsy: evaluation of 470 biopsies. Hell. J. Surg. 2013;85(6):380–385. 53. [Google Scholar]
- 45.Seiberling K.A., Dutra J.C., Gunn J. Ultrasound-guided fine needle aspiration biopsy of thyroid nodules performed in the office. Laryngoscope. 2008;118:228–231. doi: 10.1097/MLG.0b013e318157465d. [DOI] [PubMed] [Google Scholar]
- 46.Bohacek L., Milas M., Mitchell J., Siperstein A., Berber E. Diagnostic accuracy of surgeon-performed ultrasound-guided fine-needle aspiration of thyroid nodules. Ann. Surg. Oncol. 2012;19:45–51. doi: 10.1245/s10434-011-1807-z. [DOI] [PubMed] [Google Scholar]
- 47.Sabel M.S., Haque D., Velasco J.M., Staren E.D. Use of ultrasoundguided fine needle aspiration biopsy in the management of thyroid disease. Am. Surg. 1998;64:738–741. [PubMed] [Google Scholar]
- 48.Eedes C.R., Wang H.H. Cost-effectiveness of immediate specimen adequacy assessment of thyroid fine-needle aspirations. Am. J. Clin. Pathol. 2004;121:64–69. doi: 10.1309/XLND-TE28-9WAQ-YK0Y. [DOI] [PubMed] [Google Scholar]
- 49.Bhatki A.M., Brewer B., Robinson-Smith T., Nikiforov Y., Steward D.L. Adequacy of surgeon-performed ultrasoundguided thyroid fine-needle aspiration biopsy. Otolaryngol. Head. Neck Surg. 2008;139:27–31. doi: 10.1016/j.otohns.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Khurana K.K., Richards V.I., Chopra P.S., Izquierdo R., Rubens D., Mesonero C. The role of ultrasonography-guided fineneedle aspiration biopsy in the management of nonpalpable and palpable thyroid nodules. Thyroid. 1998;8:511–515. doi: 10.1089/thy.1998.8.511. [DOI] [PubMed] [Google Scholar]
- 51.Tambouret R., Szyfelbein W.M., Pitman M.B. Ultrasoundguided fine-needle aspiration biopsy of the thyroid. Cancer. 1999;87:299–305. doi: 10.1002/(sici)1097-0142(19991025)87:5<299::aid-cncr10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 52.Mittendorf E.A., Tamarkin S.W., McHenry C.R. The results of ultrasound-guided fine-needle aspiration biopsy for evaluation of nodular thyroid disease. Surgery. 2002;132:648–653. doi: 10.1067/msy.2002.127549. [DOI] [PubMed] [Google Scholar]
- 53.Mehrotra P., Viswanathan H., Johnson S.J., Wadehra V., Richardson D.L., Lennard T.W. Ultrasound guidance improves the adequacy of our preoperative thyroid cytology but not its accuracy. Cytopathology. 2006;17:137–144. doi: 10.1111/j.1365-2303.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 54.Li W.B., Zhu Q.L., Zhang B., Jiang Y.X., Yang D. Ultrasound-guided fine needle aspiration in the diagnosis of thyroid nodule. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2010;32:76–80. doi: 10.3881/j.issn.1000-503X.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Law M.T., Taylor M., Bennett I.C. Surgeon-performed ultrasound guided needle biopsy of the thyroid: a safe and effective diagnostic procedure. World J. Endocr. Surg. 2011;3:116–121. [Google Scholar]
- 56.Rozycki G.S. Surgeon-performed ultrasound: its use in clinical practice. Ann. Surg. 1998;228:16–28. doi: 10.1097/00000658-199807000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]