Abstract
Glutamate-like receptors (GLRs) is a highly conserved family of ligand-gated ion channels, which have been associated with various physiological and developmental processes. Here, we investigated the evolutionary pattern of GLRs in plants. We observed that tandem duplications occupied the largest proportion of the plant GLR gene family expansion. Based on a phylogenetic tree, we suggested a new subfamily, GLR4, which is widespread in angiosperm but absence on Brassicales. Meanwhile, because GLR1 and GLR2 subfamilies were potential sister clades, we combined them into the GLR1&2 subfamily. A comparative analysis of plant GLR subfamilies revealed that selective forces shaped the GLR1&2 repertoires in the stems of eudicotyledons with distinct functional preferences. Moreover, GLR1&2 formed a species-specific highwoody-expanded subfamily, with preferential expression in the cambial-enriched and shoot apical meristem fractions of the highwood species. Together, these findings lay the foundation for evolutionary analysis of plant GLRs over the entire plant timescale and identified unique targets for manipulating the woody-growth behaviours of plant GLRs.
Amino acids are formed from incorporation of inorganic nitrogen, and they serve as nitrogen signaling molecules in animals and plants. Plant glutamate-like receptors (GLRs), counterparts of mammalian ionotropic glutamate receptors (iGluRs), are assumed to be the prime amino acid sensors in plants. A parsimony phylogenetic analysis showed that plant GLRs preceded the divergence of animal and protokaryotic iGluR classes, and in Arabidopsis can be divided into three clades, GLR1, GLR2 and GLR31. Moreover, the GLR1 and GLR2 subfamilies are thought to have a sister relationship in the parsimony tree of Arabidopsis GLRs, which was supported by their overlapping expression patterns1.
Despite the classification, plant GLRs as predicted ligand-gated ion channels share the basic structures of the channel-forming domains and ligand-binding sites2. The channel-forming domain consists of three complete trans-membrane domains (M1, M3 and M4) plus a partial trans-membrane domain that forms a pore-loop structure (M2-Pore)3. The predicted pore structure has functional cation channels and transplantation of AtGLR1.1 and AtGLR1.4 pore domains into rat GluR1 and GluR6 chimeras, respectively, led to conductance of cations4. A ligand-binding domain consists of two lobes, GlnH1 and GlnH2, which are related to channel-gating through agonist-induced conformational changes5. Moreover, plant GLRs have an additional modulatory region, the amino-terminal domain (ATD), which in iGluRs can regulate channel activities by binding a wide range of ions and molecules6. Furthermore, the resolved crystal structure of animal iGluRs revealed that ATDs regulate channel activities through interactions between the subunits to determine the channel composition7.
Analogous to mammalian iGluRs, plant GLRs conduct many cations into the cell in the presence of extracellular amino acids, such as AtGLR3.4, which are highly selective to calcium ions (Ca2+)8. As Ca2+ is a main signal messenger in plants, GLRs could play fundamental roles in mediation of diverse cellular responses in plant cells. Indeed, AtGLR3.1 is preferentially expressed in stomatal guard cells, and AtGLR3.1 over-expression elicited in impaired external Ca2+-induced stomatal closure, suggests that AtGLR3.1 is correlated with the cytosolic Ca2+ concentration that regulates stomatal movements9. AtGLR3.5-mediated Ca2+ signaling promotes seed germination by counteracting the inhibitory effects of abscisic acid through the repression of ABI410. Applying exogenous glutathione to Arabidopsis leaves triggered a transient rise in cytosolic Ca2+ concentration, which is highly sensitive to GLR antagonists, and was also impaired in loss-of-function atglr3.3 mutants11. These mutants showed enhanced susceptibility to bacterial pathogens, lower expression of pathogen-induced defence marker genes and similar phenotype to the glutathione biosynthesis defective mutant pad211. Under D-Ser activation, AtGLR1.2 mediated Ca2+ influx into pollen tube cells as they extended in the pistil. The Ca2+ signature is largely altered in glr1.2 pollen tubes12.
Highwood species’ properties differ from those of other plant species and are mainly determined by the relative abundance of wood. Wood forms during secondary growth through activity of the vascular cambium, a layer of meristematic cells on the outer periphery of the stem. Cambial initials produce xylem mother cells13,14, differentiate into mature xylem cells, including vessels and fibres. Xylem differentiation and cambial activity are spatially and temporally regulated at the transcriptional level and involve a complex hierarchical signal transduction network15. Ca2+ has positive effects on wood formation in coniferous trees16. Moreover, increases in Ca2+ in cambium, xylem and phloem occur with increased Ca2+ supply in nutrient solution17. Likewise, stable isotope labelling showed 60–70% of Ca2+ content in vascular tissue, suggesting that long-distance transport Ca2+ into woody tissue18. Furthermore, under high titre Ca2+ stress, poplar wood formation can be strongly inhibited shown in decreased vessel sizes and wood increments, decreased specific performances in reduced layers of cambial and xylem differentiation zones in the radial direction, and decreased lengths of young libriform fibres17. Secondary ion mass spectrometry showed a strikingly temporary increase in Ca2+ concentration in the cambium during the reactivation period19, suggesting that Ca2+ acts as signaling messenger, triggering cambium activation. Indeed, phenotypic investigation of poplar stem tissues under increasing Ca2+ concentrations in cambium showed a much wider cambial zone with larger vacuoles, which is typical of active vascular cambium contrasting to cells filled with dense cytoplasm and decreasing cambial width with a reduced Ca2+ supply17. Additionally, Ca2+ reportedly increased in the apical meristem during bud flush and the beginning of cell division20. These results further show that cambium activation relies on Ca2+ signaling. Thus, we suggest that potential Ca2+-transporters could participate in Ca2+ long-distance transport and responses to wood formation.
At least two AtGLR (GLR3.4 and GLR3.2) proteins are enriched primarily in the phloem and play fundamental roles in Ca2+ signal transduction21. In parallel, using non-invasive electrodes, Mousavi et al. mapped surface potential changes in Arabidopsis after wounding leaves and found membrane depolarization domains in leaves distal to the wounds22. Furthermore, mutations in several GLR3 genes (3.2, 3.3 and 3.6) alleviated wound-induced surface potential changes and correlated with jasmonate-response gene expression, which was more attenuated in a glr3.3 glr3.6 double mutant22. This suggests that electrical impulses act as intracellular signals and that plant GLRs are potent regulators in this long-distance communication in wound-induced defence responses. These results support that long-distance signaling exists in plants and indicates that plant GLRs analogous to those important for synaptic activity in animals have roles in organ-organ Ca2+ signal transmission.
Plant GLRs, as extracellular amino acid sensors, play fundamental roles in regulating various biological processes. However, knowledge of this gene family is confined to Arabidopsis thaliana. Thus, further investigations of GLRs in other plant species are necessary to determine more gene family profiles. Rosaceae, as a core plant lineage, and composing many highwood species, have had their genomes extensively sequenced23,24,25,26,27,28. This provides an opportunity to further analyse the GLR family in highwood species. We collected genome data and used Rosaceae as a model for insight into plant GLR evolution. We combined comparative genomics, molecular evolutionary studies and expression analyses to examine the evolution of plant GLRs and investigate characteristics of highwood GLRs.
Results
Identification, classification and characterization of plant GLRs
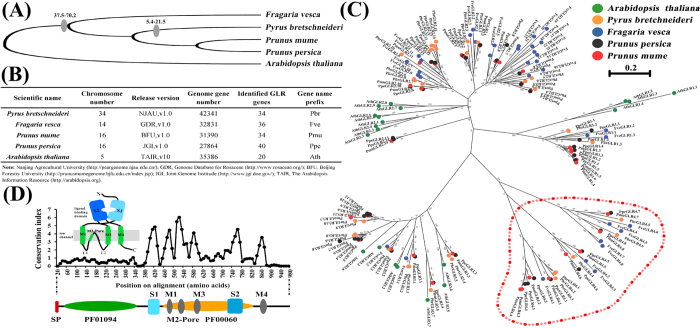
There were 167 candidate GLRs identified in four Rosaceae species (Fig. 1A), and 23 were classified as pseudogenes because they contained only short gene fragments or contained frameshift mutations and/or premature stop codons. Consequently, 144 complete GLRs were used for analysis (Fig. 1B and Table S1). Phylogenetic analysis suggested that analogous to Arabidopsis, most Rosaceae GLRs were divided into three traditional phylogenetic clades (GLR1, GLR2 and GLR3). Moreover, we observed a well-supported new clade (bootstrap values of 82–100%; Fig. 1C), independent of traditional clades, and named this clade as GLR4 subfamily.
Figure 1. GLR gene family in four Rosaceae species.
(A) Species tree of four Rosaceae species and Arabidopsis. The gray oval indicates the occurrence of WGD. Numbers in the figure show species divergence time. Unit: MYA. The data were downloaded from NCBI Taxonomy common tree (http://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi) and the tree was constructed by MEGA5. (B) Table of genome information and GLR genes number identified in four Rosaceae species and Arabidopsis. (C) Phylogenetic tree of four Rosaceae species and Arabidopsis GLRs protein. Roseceae GLRs have been given preliminary names is similar to those of Arabidopsis. Protein phylogenetic analysis using the Maximum Likelihood method in MEGA5 for 164 GLR amino acid sequences from pear (orange circular), strawberry (blue circular), plum (black circular), peach (red circular) and Arabidopsis (green circular). The sequences were aligned using MUSCLE. The bootstrap consensus tree was generated using the JTT matrix-based model with discrete gamma distribution [five categories (+G, parameter = 1.0524)] by MEGA5 from 1000 bootstraps. The scale bar is 0.2. The red dotted box shows that the subfamily group of Roseceae species more than Arabidopsis, we define it as GLR4 subfamily. (D) Histogram showing the mean conservation index (number of conserved physico-chemical properties) for sliding window (20 aa window, 10 aa slide) line chart of protein aligned from Rosaceae species and Arabidopsis GLRs. The protein domain organization of GLRs is shown in cartoon form above the histogram and in linear form below it.
GLR4 members shared a similar modular organization to other Rosaceae and Arabidopsis GLRs (Fig. S1), consisting of a receptor family ligand binding region (PFAM ID: PF01094) and ligand-gated ion channel domain (PFAM ID: PF00060). In detail, it contained an extracellular ATD, four trans-membrane domains (M1-M4), a putative ligand-binding domain with two lobes (GlnH1 and GlnH2) that were separated by an ion channel domain, and a short cytoplasmic C terminus (Fig. 1D). Then, we aligned GLR4 proteins together with other subfamilies GLRs to examine the mean conservation index (number of conserved physico-chemical properties) using a sliding window (20 amino acid window, 10 amino acid slide) in four Rosaceae species and Arabidopsis. The results showed that the all annotated domains performed high conservation indexes, instead, the ATD (PF101904) was considerably more variable (Fig. 1D). The most conserved domain was the M2-Pore region (highest mean conservation index of 6.05), which determined the selective transduction of cations by their residues29. We generated a protein alignment for this region. The residues that determine the selective transduction of cations were highly uniform among Rosaceae and Arabidopsis GLRs included GLR4 subfamily (Fig. S2). Hence, GLR4 subfamily members are consistent with other Rosaceae GLRs that possess potential conserved plant GLR channel specificities. However, the principal issue of the GLR4 subfamily distribution in plant evolution needs to be addressed.
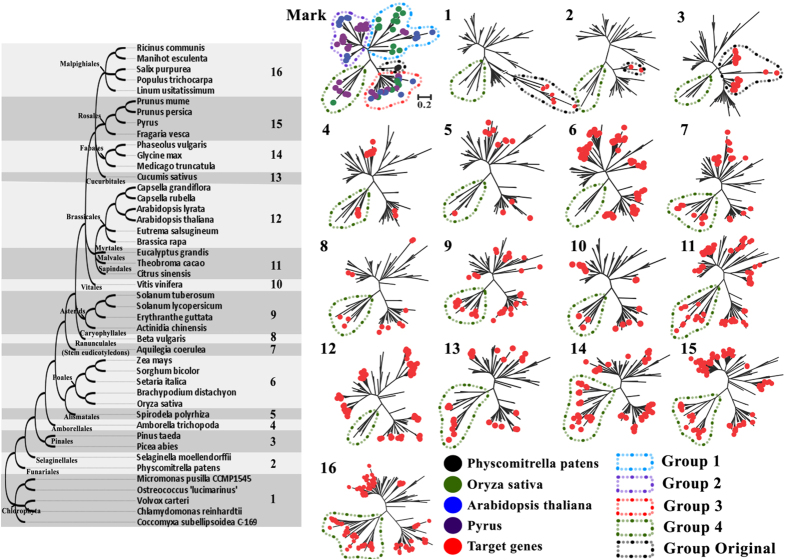
For this purpose, we comparatively analysed GLRs in 45 plant species. We separated these plant species into 16 parts based on plant taxonomy (http://www.ncbi.nlm.nih.gov/Taxonomy/CommonTree/wwwcmt.cgi) and selected moss (lower plant), rice (monocotyledonous plant), Arabidopsis (dicotyledonous herbs), and pear (dicotyledonous woody) GLRs as anchors for construction of a phylogenetic tree to cluster the remaining plant GLRs (Fig. 2). Then, a collection of 881 sequences from 45 plant species was classified (Table S2). The GLR4 genes were widespread in all analysed angiosperms. They first appeared in Amborella, which is the earliest known angiosperm species30. However, this subfamily has not been found in Brassicales (Fig. 2 and Table S2). This could explain the missing GLR4 subfamily in the genome of Arabidopsis.
Figure 2. Phylogenetic analysis for plant GLRs evolutionary history.
Protein phylogenetic analysis using the Maximum Likelihood, The sequences were aligned using MUSCLE. The bootstrap consensus tree was generated using the JTT matrix-based model with discrete gamma distribution by MEGA5 from 1000 bootstraps. An unscaled tree showing the phylogenetic relationships of 45 plant species was illustrated on the left, we divided this plant system into 16 parts according to the plant taxonomy. The right part is phylogenetic analysis for each part of plant species’ GLRs, we take moss (lower plant), rice (endogen plant), Arabidopsis (dicotyledonous herbs), pear (dicotyledonous woody) as marker genes to construct phylogenetic tree for each part plant species, then novel 881 sequences were collected and all plant GLR genes subfamily were classified. The scale bar is 0.2 for all phylogenetic trees. The non-circular labelled branches in 1~16 part phylogenetic trees are mark genes in Mark phylogenetic trees, all these mark genes in 1~16 part phylogenetic trees are location consistency with Mark phylogenetic trees.
Evolutionary history of plant GLRs
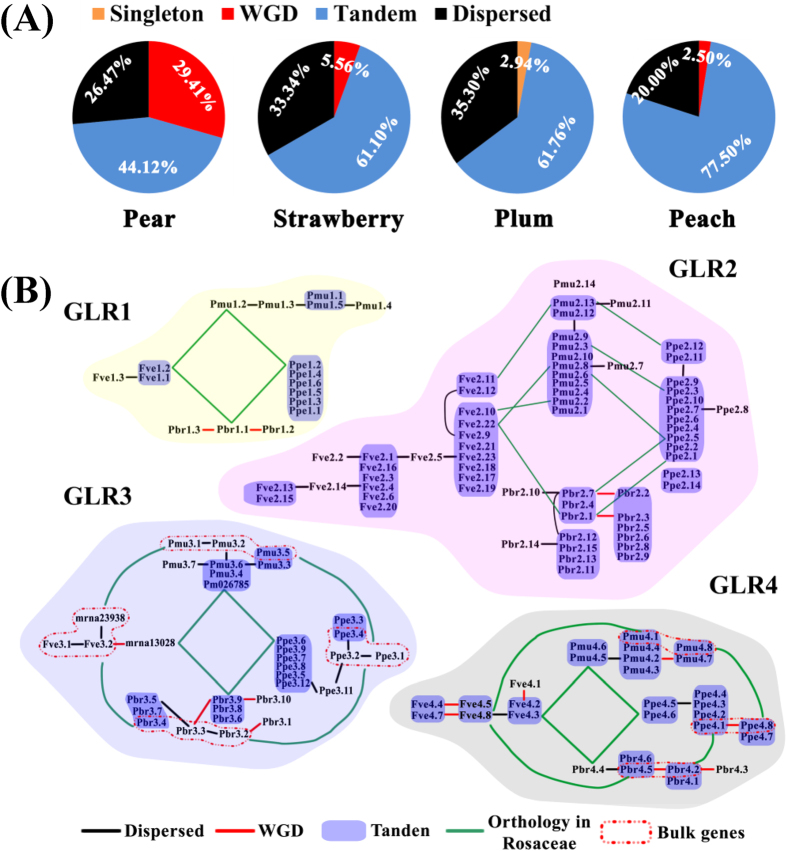
To investigate the evolutionary processes of plant GLR repertoires, we used Rosaceae GLRs as examples to determine the potential original, expansion and divergence of this gene family. First, we detected the duplication modes for GLRs in four Rosaceae genomes using the MCScanX package. Tandem duplications occupied the largest proportion of the GLR family’s expansion in Rosaceae (Fig. 3A, Fig. S3 and Table S3). Dispersed duplication was secondary contributor to expansion of this gene family in Rosaceae. Interestingly, the recent whole genome duplication (WGD) events (30–45 MYA) occurred in pear27 likely resulted in the observed higher proportion of WGD/segmental-type GLR gene duplications (Fig. 1A). Together, these observations support that GLRs evolved mainly from tandem duplications in Rosaceae. Then, we assembled the duplicated gene pairs to form a linear expansion in each Rosaceae species based on hypothesis tests on the nearest duplicated gene pair’s performance characteristics, which included the highest identity (Table S4), maximum number of flanking genes in segments and the lowest synonymous mutations (Table S5). Finally, we identified the ancestral genes of the Rosaceae GLR families by collinearity and synteny analyses (Table S6). We constructed a schematic diagram of the development history of the Rosaceae GLR family (Fig. 3B).
Figure 3. Expansion analysis of GLR gene family in Rosaceae genomes.
(A) The quantification statistics for different duplication events in Roseceae GLRs. Initially, the BLASTP algorithm was used to search for potential homologous GLR gene pairs (E < 1 e−5, top five matches) across entire genome. Secondly, these homologous pairs were used and inputted into MCScanX to identify syntenic chains. The GLRs locate in a syntenic block were defined as WGD/segments duplication; The GLRs neighbouring less than 10 genes were defined as tanden duplication; and the remainders of homologous GLRs were defined dispersed duplication. (B) Orthology and duplicate path analysis of GLR genes in four Roseceae species. We assembled the duplicated gene pairs to form a linear expansion in each Rosaceae species based on hypothesis tests on the nearest duplicated gene pair’s performance characteristics, which included the highest identity (Table S4), maximum number of flanking genes in segments and the lowest synonymous mutations (Table S5). Orthology GLRs were identified in MCScanX program.
This schematic diagram illustrated that five series of Rosaceae ancestral genes, two bulk orthologous genes in GLR3 and GLR4 subgroups, that were ordinal linked in four species. In contrast, the GLR2 subfamily did not complete an orthology series, but was substituted by a more abundant and shorter series among two or three Rosaceae species. Meanwhile, there were three fragmented GLRs (mrna13028 and mrna23938 in strawberry, and Pm026785 in plum) that participated in evolution of the Rosaceae GLR3 subfamily, indicating that these genes contained frameshift mutations and/or premature stop codons after the duplication events. In general, Rosaceae GLRs were taken several series of ancestral genes as the anchors during expansion; however GLR2 subfamily had undergone a gene elimination during evolution. Once ‘born’, new GLRs presumably further expanded by tandem duplications, revealing this as a tremendous driving force for Rosaceae GLR gene expansion.
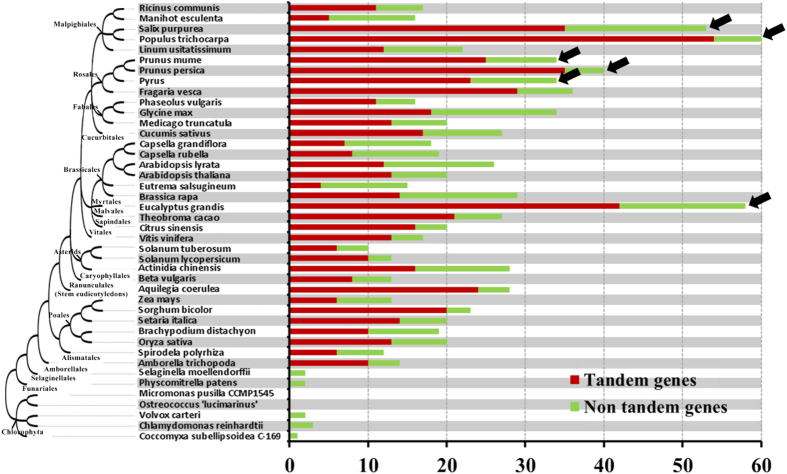
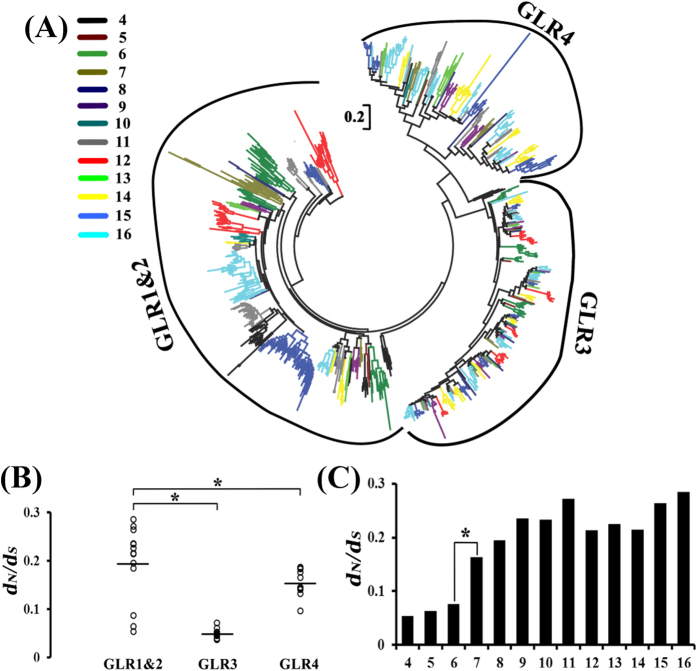
Analysis of GLR evolutionary patterns over the entire plant timescale showed 581 GLRs, accounting for 65.95% of all analysed plant GLRs, were tandem-related genes (Fig. 4 and Table S7). It indicated that in all plant species, not just Rosaceae, tandem duplication is a major expansion force for GLR families. Exploring the evolutionary origin of plant GLRs by phylogeny analysis showed that the plant GLR family had an original group. We named this group GLR0, and it was closest to the GLR3 group (Fig. 2). GLR0 appeared first in algae, then began to differentiate and expand with evolution of Pinales, and finally differentiated into three stable and distinguishable subgroup structure, GLR3, GLR4 and a larger and highly spread-out cluster (GLR1 and GLR2 subfamilies), from the Amborellales, the earliest angiosperm species known (Fig. 2).
Figure 4. A broad number and tandem duplication event survey of GLR genes in plants.
Histogram of the number of tandem genes (red) and non-tandem genes (green) sequences identified in the in the indicated plant species. An unscaled tree showing the phylogenetic relationships of 45 plant species was illustrated on the left. X axis indicated the number of genes. The black arrow indicated the highwood species in analysis plants.
Plant GLR1 and GLR2 subfamilies formed sister clades
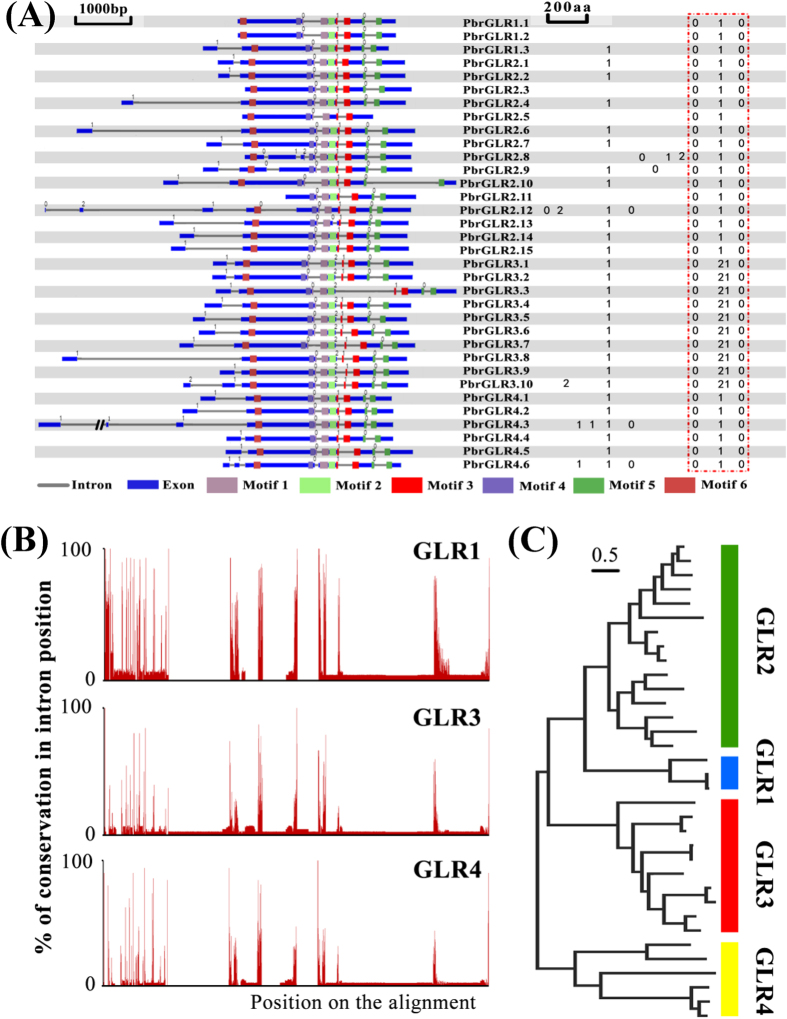
Marker-based phylogenetic analysis shows that GLR1 and GLR2 combined into a distinguish cluster occurred in all of tested plant orders (Fig. 2), suggesting GLR1 and GLR2 subfamilies could be homologous and from a common gene. Consistent with the conservation analysis, GLR2 had the most similar primary sequence to GLR1 in four Rosaceae species (Table S4). Taken pear GLRs as example, we tested the genes structure among subfamilies, the result showed the numbers of introns in GLRs were random (Fig. S1 and Table S1). Indeed, using the server GSDraw (Gene Structure Draw server) to detect the gene structure based on a genomic sequence alignment using the main domain, showing that these highly dynamic introns were concentrated at the N-terminus (Fig. 5A). However, the intron positions after motif 4 were conserved among pear GLR1 and GLR2 members (Fig. 5A). In addition, the highest identity value (43.12%) for the intron sequence after motif 4 between GLR1 and GLR2 subfamilies in pear (Fig. 5B). In comparison, there was only 25.37% identity between GLR2 and GLR3 subfamilies, and 20.23% identity between GLR2 and GLR4 subfamilies (Fig. 5B). Moreover, a phylogram of these intron sequences also revealed a greater similarity of GLR2 to GLR1 receptors than to other subfamily receptors (Fig. 5C). Likewise, similar results were obtained in other Rosaceae species (data not shown). Together, these observations demonstrated GLR1 and GLR2 formed sister clades and integrated into a single clade, which we named GLR1&2.
Figure 5. Evolutionary origin of pear GLRs.
(A) Gene structure analysis of pear GLR genes using PIECE. The left part is pear GLRs gene structure performed by view gene structure in genomic sequences alignment with the main domain. 0, 1, 2 denote the phase of the intron. The blue part indicates the exons, the gray line indicates the intron and different colors indicate different motifs. The right part is gene structure performed by view type of gene structure in alignment protein sequences. The red dotted line represents conserved intron phase among all pear GLRs. (B) Map of the conserved intron sequences, which represents by red dotted line in (A), of pear GLR2 subfamily with other pear GLR subfamilies. Selected intron sequences were aligned using PRANK, and the scattered sequences in the alignments were cleaned manually to obtain final high-quality alignments. (C) Phylogram based on the sequence of introns in the same subset of sequences as (B). Maximum likelihood tree was built by applying the JTT model with 1000 bootstraps in MEGA5.
GLR1&2 subfamily has more tandem gene arrays accompanied by domain loss events
Comparative analysis of the evolutionary patterns for expansion of GLR subfamilies showed distinct characteristics for GLR1&2. The degree of tandem duplication events contributed to the expansion for GLR1&2 and GLR4 subfamily were similar, 75.67 and 76.02%, respectively, which significantly higher than the overall average value (65.95%) (Fig. S4 and Table S7). In contrast, tandem duplication genes only accounted for 44.44% of the GLR3 subfamily and none of the GLR0 subfamily. The results indicated that tandem expansion force was mainly in the GLR1&2 and GLR4 subfamilies. The average of 2.6 tandem gene arrays was significantly higher for the GLR1&2 than the averages of 1.43 and 1.88 for GLR3 and GLR4, respectively (Fig. S4). If duplicated genes had similar functions, then there were more functional differentiation events involved in the gene expansion process in the GLR1&2 subfamily.
However, many domain loss events accompanied GLR1&2 gene expansion. Surveying conserved domains showed that Rosaceae GLRs were missing one or more domains, with loss rates from 3.47% (GlnH1) to 15.97% (ATD) (Fig. S1 and Table S8). Among these, the missing ATD rate was significantly higher than those of other domains, especially in the strawberry. We further compared the domain loss rate in each GLR subfamily. The domain loss events in GLR1&2 subgroups accounted for reach up to 78.57% of all loss events (Table S9). Meanwhile, the percentage lacking the ATD reached 11.03% in plant GLRs, with 74% in the GLR1&2 subfamily (Table S10). Together, these clues indicated that during the expansion process, the GLR1&2 subfamily incurred many domains loss event, especially in the ATD.
Selective forces acting on the GLR1&2 subfamily in stem eudicotyledonous species
GLR1&2 subfamily showed a much broader distribution than other subfamilies (Fig. 2). Indeed, amino acid sequences of GLR1&2 subfamily members had the lowest identities among the analysed plants (Table S11). Then, we reconciled the gene phylogeny by analysing GLR1&2 in all the plant species. There were no obvious orthologous relationships for these genes among species, but a number of species-specific clades (Fig. 6A). Thus, we refer to the GLR1&2 subfamily genes as species-specificity divergent GLRs.
Figure 6. Species-specificity of GLR1&2 subfamily was evolved under selective forces.
(A) Phylogenetic relationships of the all angiosperm GLRs shown in (Table S2). Each colour represents a part of GLR proteins as description in Fig. 2. The sequences were aligned with PRANK and the ML tree was built with MEGA5 under JTT matrix-based model with 1000 bootstraps. The scale bar is 0.2. GLR1&2 subfamily showed a species-specific distribution in this phylogenetic analysis. (B) Distribution of selective forces of three GLR subfamilies estimated for all angiosperm species. Selective forces acting on plant GLRs by calculating the ratio of nonsynonymous to synonymous nucleotide substitution rates (dN/dS, ω). The median dN/dS values of GLR1&2 were significantly higher than GLR3 and GLR4 (p < 0.01, Wilcoxon rank-sum tests). The horizontal line representative the median dN/dS values. (C) Histogram of dN/dS rates of GLR1&2 subfamily estimated for all angiosperm species which was devised into 13 parts as description in Fig. 2. The ω were remarkable elevated beginning from Ranunculales (p < 0.01, Wilcoxon rank-sum tests), which was the first stem eudicotyledonous order of plant species analysed.
We studied the selective forces acting on plant GLRs by calculating the ratio of nonsynonymous to synonymous nucleotide substitution rates (dN/dS, ω). All tested GLR subfamily genes evolved under strong purifying selection (ω ≪ 1) and the GLR1&2 subfamily had the highest median ω of 0.194 (Fig. 6B), indicating that GLR1&2 subfamily evolved under weaker purifying selection and/or contained more sites shaped by positive selection. To gain insights into species diversification of the GLR1&2 subfamily, we compared ω of these genes in plant development lineage (Fig. 6C). Among these, Amborellales, Alismatales and Poales had the lowest ω of 0.053–0.096, consistent with their high sequence conservation levels in the plant lineage. Notably, the median ω in the following taxonomic tree was statistically higher than those of Amborellales, Alismatales and Poales. The ω were elevated beginning from Ranunculales (ω = 0.183), which was the first stem eudicotyledonous order of plant species analysed. Certain orders had more greatly diverged ω, such as Myrtales, Rosales and Malpighiales, which all are contain highwood species. Thus, we inferred that selective forces acting on GLR1&2 were correlated with those of the stem tissue genes.
GLR1&2 subfamily expansion in highwood species
In the present study, the majority of plant species contained ~20 GLR members. Moreover, all analysed highwood species (pear, peach, plum, salix, populous and eucalyptus) contained significantly more GLRs (Fig. 4), and we assessed contributions of subfamilies to GLR expansion in highwood species. The GLR3 subfamily population was roughly equal in all species (Fig. S4). Salix and Populous had more GLR4 genes than the other species. In contrast, all highwood species had more GLR1&2 genes (Fig. S4), suggesting that this subfamily was the major contributor to expansion. Therefore, we inferred that the GLR1&2 subfamily potentially regulated aspects of woody growth in highwoods that may be less developed in the other species.
High transcript abundance of GLR1&2 in cambium
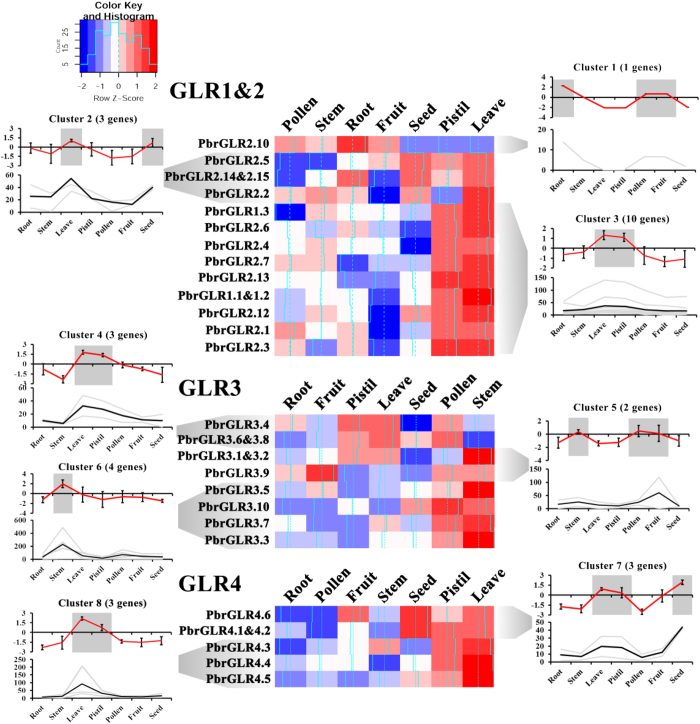
To explore whether the GLR1&2 subfamily was specifically expressed in the stem, we analysed expression profiles of the pear GLR family in seven tissues or organs using reverse transcription-quantitative real-time PCR (RT-qPCR). However, no PbrGLR2.8, PbrGLR2.9 and PbrGLR2.11 transcripts were detected. The remain 31 genes were classified into eight clusters based on transcript abundance patterns (Fig. 7). The gene family was predominantly expressed in leaves and pistils. Thirteen GLRs were highly expressed in stem tissue. Surprisingly, not only several of the GLR1&2 genes but also other subfamilies genes were predominantly expressed in the stem tissue. We also analysed GLRs from poplar, another highwoody plant species, for RNAseq transcript abundance in six tissues (Fig. S5). The results were consistent with the observations in pear.
Figure 7. Heat map of the qRT-PCR transcript abundance pattern of the 31 GLRs from pear in 7 different tissues clustered in 7 expression groups using K-means.
Genes and samples were hierarchically clustered according to their transcript abundance (expressed in relation to the mean of all samples and log2-transformed). For each gene, its name is shown to the left of the heatmap. Next to each cluster have two graphs that up graph with the mean transcript abundance (red line) base on standardized log2-relative expression value in RT-qPCR ± SD for the entire cluster, and down graph with the each genes relative expression value in RT-qPCR (gray line) and they mean transcript abundance value (black line) ± SD for the entire cluster. The Y-axis represents log2-relative expression value and relative expression value in RT-qPCR respectively. PbrGLR2.8, PbrGLR2.9 and PbrGLR2.11 could not be detected by RT-qPCR in 7 different tissues/organs, because their expression levels were too low to detect by normal RT-qPCR. There are 5 pair genes can’t be distinguish because of high identify in sequences. An amplified PbrTub-2 and PbrGADPH were used as internal control.
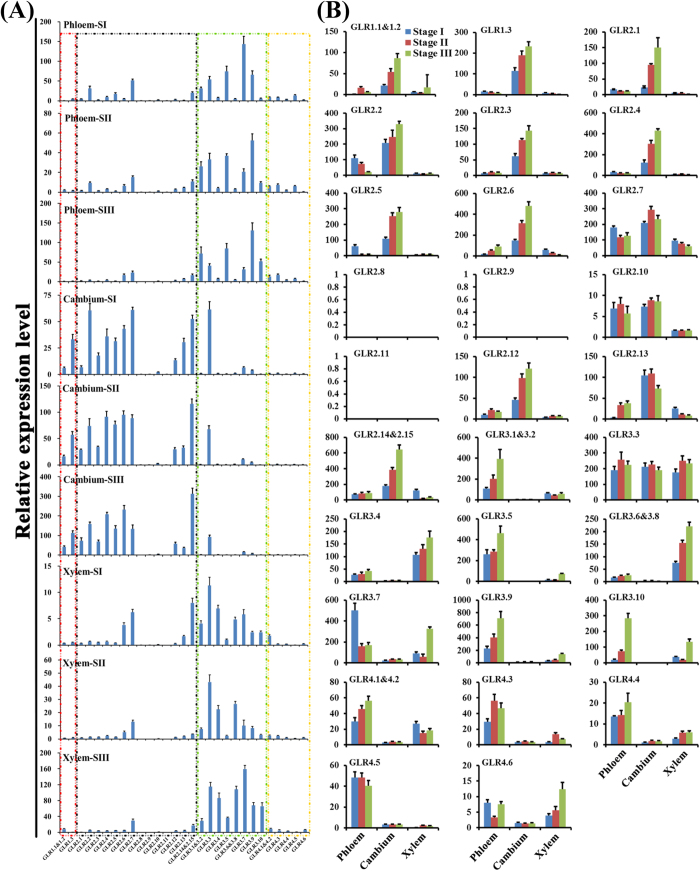
Indeed, to investigate specific temporal and spatial function of GLRs in stem, we selected xylem, phloem and cambium samples from three phases stems, which having increased lignification, to determine different PbrGLR expression levels (Fig. S6). GLRs expressions were grouped into three main patterns (Fig. 8), showing that GLR1&2 members had nearly identical expression patterns, and were preferentially expressed in cambium. This was consistent with previous results showing that plant GLR1 and GLR2 subfamilies are sister clades. GLR3 subfamily genes were preferentially expressed in phloem or xylem samples, and GLR4 genes had relatively low transcript levels in analysed vascular tissues but were preferentially expressed in phloem. Indeed, PbrGLRs’ transcript abundance gradually increased in vascular tissues during lignification-enhanced stages. Coincidentally, we also found similar GLR expression patterns in hypocotyl from Arabidopsis which producing secondary xylem with vessels and fibres similar to those of woody species (Fig. S7A). Arabidopsis GLR3 was prominently expressed in all phloem-type cells containing phloem companion, phloem pole pericycle, protophloem and metaphloem, and also in xylem cells. However, GLR1 and GLR2 of Arabidopsis were preferentially expressed in procambium cells. In addition, we found that most of GLRs’ expression levels significantly increased during Arabidopsis maturation (Fig. S7B). Poplar GLRs showed similar expression patterns to pear and Arabidopsis in phloem and xylem tissues, although we did not find available expression data for poplar cambium tissue (Fig. S8). These observations support the hypothesis that GLRs perform distinct specific temporal and spatial functional roles in vascular tissues, and that GLR3 and GLR4 genes participate in xylem and phloem, while GLR1&2 genes is involved in the cambium.
Figure 8. Temporal-spacial expression patterns of pear GLRs in stem vascular tissues by qRT-PCR.
Xylem, phloem and cambium samples from three stages stems, which having increased lignification, were selected to determine different PbrGLRs expression levels. (A) The pear GLR gene family’s expression patterns in a single tissue. SI, SII and SIII denoted Stage I, Stage II and Stage III, respectively. Stage I: The stem above cotyledons from 90 days old pear tree; Stage II: The stem from the current growth branches of 8 years old pear tree. Stage III: The stem from the last year the branches of 8 years old pear tree. The stages were detail described in Fig. S5. The different color of dotted boxes were represented four subfamilies of pear GLRs. (B) The expression patterns of a single pear GLR gene in all analyzed vascular tissues. An amplified PbrTub-2 and PbrEF1α were used as internal control. Samples were taken in at least triplicate reduplicate of biological, the average of which is show.
Meanwhile, we detected transcript abundance profiles of GLRs during sprouting in the shoot tip tissues of pear and poplar. Nearly all pear GLR1&2 genes that were highly expressed in cambium also showed increased expression in sprouting dormant shoot tips (Fig. S9), with similar results in poplar (Fig. S10). These results corroborate a possible dual role in meristematic modulation, and many genes can regulate both the shoot apical meristem and vascular cambium31,32.
Discussion
Evolutionary history of plant GLRs
Comprehensive survey of plant GLR genes allowed us to develop a model of their evolution (Fig. 2). Phylogenetic relationships, sequence similarities, gene structures and expression patterns suggested that GLR1 and GLR2 subfamilies were sister clades, thus we combined them into a common subfamily, GLR1&2. We hypothesized that GLRs originated from the GLR0 plant subfamily, closely related to the GLR3 subfamily, and that they subsequently diverged into the GLR1&2, GLR3 and GLR4 subfamilies, with the earliest occurring in Amborellales, which is the first angiosperm species30 (Fig. 2), estimated 160 million years ago. However, the GLR4 subfamily was absent in Brassicales. Thus, if the GLR4 subfamily evolved in a common ancestor of plant and existed in the ancient species, it must have subsequently been lost in Brassicales for an unknown reason.
GLR1&2 subfamily exhibited distinct characteristics
Comparative analysis of evolutionary patterns for GLR subfamily expansion in plant, revealed distinct characteristics for GLR1&2. Tandem duplications as the main driving force with a concomitant more tandem gene arrays were observed in expansion of this subfamily, indicating functional differentiation in the gene expansion process. Furthermore, low sequence identities, high selective forces and highly spread instead orthologous phylogenetic relationships of GLR1&2, suggested that this subfamily diverged in a species-specific pattern. The reason for this divergence is unknown, but it implies a reduced evolutionary constraint on co-expression, partially redundant ion channel genes undergo the selection for higher diversity in ion transport sequences to recognise more variable ligands in the environment. Indeed, the higher selective forces acting of GLR1&2 subfamily start at stem eudicotyledonous plants and this subfamily members occurred remarkable extended in highwood plant species, inferring that the GLR1&2 subfamily is potentially involved in regulating aspects of highwood woody growth.
Meanwhile, many plant GLRs had loss events in the ATD and 74% occurred in the GLR1&2 subfamily. The ATD is a discernible and divergent domain preceding the GlnH1 domain in the N-terminus and is involved in the assembly of GLR subunits into heteromeric complexes in animal iGluRs and Arabidopsis GLRs (Table S10)2,7,33. Moreover, expression of the lack-ATD gene (PbrGLR2.11) was not detected in any pear samples (Figs 7 and 8), indicating that the ATD lesion may be an elimination mechanism for gene redundancy in the GLR1&2 repertoire. Additionally, 80.22% of this type of GLR was involved in tandem duplication (Table S2). Thus, these results led to the hypothesis that retroposition events eliminated the gene redundancy to meet the frequent tandem events. However, 3.23% of highwood GLR repertoires performed retropositioning, significantly lower than the overall 11.03% (Table S10). It highlighted that family expansion and retain more functional GLRs was required in highwood species. In this light, we excluded ATD-lacking GLRs and recounted the number of plant GLR members (Fig. S11). There were more distinctly for GLR members in highwood higher than other plant species. This addressed why herbaceous strawberry had so many GLRs (Fig. 4).
GLRs perform distinct specialization functions in vascular tissues, and GLR1&2 is involved in cambium activate functions
Although there is no direct experimental evidence that plants GLRs have a role in xylem, ample evidence demonstrates that transcription and post-transcription modification of key enzymes in the nitrogen assimilation pathway are influenced by the xylem flow of amino acids34,35,36. Moreover, many factors, such as light cycle, nitrogen supply and stress, can induce the amino acid profile changes in xylem sap, which is a continuum of the apoplasmic space33,37,38. It is tempting to assume that plant GLRs may be involved in Ca2+ signaling responses in amino acid profiles in the xylem. Coincidentally, results in this study corroborate previous work in expression profiles of Arabidopsis, poplar and pear (Fig. 8, Figs S7 and S8), in which GLR3 genes were preferentially expressed in phloem or xylem cells, indicating that GLR3 genes could be involved in regulation of long-distance signaling in the vasculature, particularly phloem and xylem.
Preferential expression of GLR1&2 in cambium cells is a plausible explanation for why GLR1&2 channels function in the cambium and promote wood formation. In this scenario, this is a feedback loop invoked to expand the GLR1&2 subfamily in highwood species. In fact, over their long lifespans, highwood plants require more sophisticated mechanisms and more genetic redundant-copy control to adapt to seasonal variations and expansive length of cambial activity, and to separate the juvenile and mature wood during secondary tree growth. This does not occur in vascular transmission regions, such as phloem and xylem, nor in short-lived annuals like Arabidopsis. Thus, it also aids understanding why highwood plants contain a high percentage of retained tandem repeats during evolution for the purpose of responding to variety of environmental stimuli39. Indeed, a possibility is that cambial activation through GLR channels affects wood development via Ca2+ signaling in the cambium. The functions of GLR1&2 subgroups should, however, be further investigated using reverse genetic approaches in woody model plants to characterize their roles.
Studies conducted thus far suggest that GLR subfamily genes perform distinct specialized functions in vascular tissues. GLR3 and GLR4 having a role in ion transport in xylem and phloem, and GLR1&2 in the activate functions of cambium.
Co-expression is attributed to functional diversity via hereotetramer in plant GLRs
The resolved crystal structures of animal iGluRs are well characterized and unequivocally show that functional ligand-gated channels are formed from either four heterologous or homologous subunits within the same agonist class40. Although the picture of subunit composition in plant GLRs is far from complete, a preliminary analysis of GLR compositions is being elucidated. Co-expression analysis using single-cell sampling documented at least five GLRs co-expressed in Arabidopsis leaf epidermal or mesophyll cells41. Therefore, heterologous tetramer formation is quite likely. Moreover, a biophotonic assay demonstrated that AtGLR3.2/AtGLR3.4 heteromeric interactions were stronger than homomeric interactions. Additionally, the heterogenous expression of AtGLR3.2/AtGLR3.4 channels in human embryonic kidney cells resulted in a more sensitive current voltage compared with AtGLR3.4-only channels21. These results corroborate the previous hypothesis. In this scenario, the large scale of plant GLR co-expression in vascular tissues (Fig. 8, Figs S7 and S8) should be considered a model mechanism to defend against various environmental stresses via incorporation of a third or fourth species in the tetramer, which creates the type of highly dynamic functional diversity attributable to hereotetramer in plant GLRs. In this hypothesized mechanism, having different subunits strengthens the complex.
We analysed the genomes of 45 plant species to determine the comprehensive evolution of GLRs. This led to identification of the GLR1&2 subfamily expansion in the highwood plants analyzed by tandem duplication. Moreover, species-specificity and selective forces shaped the GLR1&2 repertoires’ distinct functional preferences in the stems of eudicotyledons. Indeed, expression studies showed that this subfamily was preferentially expressed in the cambium. This study represents a starting point for characterization of newly described GLR functions to better understand their roles in plants, especially highwood species.
Materials and Methods
Gene identification and annotation
Genomic and available annotated protein databases for pear (Pyrus bretschneideri) were downloaded from the pear genome project (http://peargenome.njau.edu.cn/). Conifer data were downloaded from the conifer genome project (http://congenie.org/). Data for other plant species were downloaded from Phytozome (http://phytozome.jgi.doe.gov/pz/portal.html). Multiple strategies were used to search for members of the GLR family in plants. First, the keyword ‘Glutamate Receptor’ was used to search available annotated protein databases. Secondly, a Hidden Markov Model (HMM) search was performed with the GLR domain’s HMM profile (PF00060), which has a ligand-gated ion channel-based function for the GLRs, and all significant hits (HMMER E value < e-5) were subsequently used as query. Finally, A. thaliana GLR protein sequences were used as queries in exhaustive BLAST algorithm-based searches with standard parameters until convergence occurred for each plant species. All identified sequences (below an arbitrary threshold E value < e-5) were then used as queries in TBLASTN searches of genomic DNA databases. Furthermore, all of the obtained GLR protein sequences were again analysed in the InterProScan database (http://www.ebi.ac.uk/interpro/search/sequence-search)42 to verify the presence of ligand-gated ion channel domains, and protein sequences lacking the PF00060 domain were removed.
Phylogenetic analyses
Protein tree building
The selected GLRs’ amino acid sequences were aligned using MUSCLE43 and examined in Jalview44. ProtTest45 was used to evaluate the best model of substitution to infer the optimal phylogeny. The trees were calculated with MEGA546, applying the best model of amino acid substitution. Approximate likelihood ratio tests were used for estimating bootstrap values. The trees were viewed and graphically edited with Mesquite, FigTree or Firework.
Intron sequence tree building
Selected intron sequences were aligned using PRANK47, and the scattered sequences in the alignments were cleaned manually to obtain final high-quality alignments. A maximum likelihood tree was built by applying the JTT model with 1000 bootstraps.
Taxonomy tree building
The taxonomy tree of analysed plant species was obtained by downloading data from the NCBI (http://www.ncbi.nlm.nih.gov/). The tree was constructed using MEGA5.
Chromosomal locations and gene structures of Rosaceae GLRs
The chromosomal location of GLRs was obtained from Rosaceae genome annotation documents. Then, the data were plotted using Circos software48. The gene structures of the Rosaceae GLRs were drawn by Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/). Website Plant Intron Exon Comparison and Evolution database49 (PIECE, http://wheat.pw.usda.gov/piece/FAQ.php) was used to conduct a motif analysis of the Rosaceae GLR genes and proteins. The figure, with six motifs, was plotted using coding sequences against the genomic sequence.
Duplication model and synteny analysis
The analysis of synteny among the four Rosaceae genomes was conducted locally using a method analogous to that developed for the plant genome duplication database (http://chibba.agtec.uga.edu/duplication/)50. Initially, the BLASTP algorithm was used to search for potential homologous GLR gene pairs (E < 1 e−5, top five matches) across multiple genomes. Secondly, these homologous pairs were used and inputted into MCScanX51 to identify syntenic chains among the four Rosaceae genomes. Further, MCScanX also was used to identify singletons, WGD/segments, and tandem and dispersed duplications in the GLR families. Then, the information from the duplication model was plotted using Circos software.
Calculating d N /d S
We estimated the dN/dS ratio (ω) using maximum likelihood as performed in PAML52. All PAML analyses were implemented three times using different input parameters to refrain from local optima. We used MUSCLE to create multiple amino acid sequence alignments of orthologous genes. Then, these results were used to guide the nucleotide coding region alignments implemented by custom-written software. The columns with gaps were deleted for ω calculations. We selected model M0 to calculate the global selective pressure acting on the different subfamilies of GLRs based on their phylogenetic tree.
RNAseq expression analysis
RNAseq data of Arabidopsis and poplar were obtained from the Arabidopsis eFP Browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi) and PopGenIE (http://popgenie.org/), respectively, except for the expression data of poplar xylem, which was obtained from the Poplar eFP Browser (http://bar.utoronto.ca/efppop/cgi-bin/efpWeb.cgi). Absolute expression data were normalized by the GeneChip operating software method in these databases, with a target intensity value of 100. Most tissues were sampled in triplicate. Then, these values were log2 transformed and standardized using EXPANDER 6 53. The K-means clustering in EXPANDER 6 grouped the genes into different main clusters with unique expression patterns.
RT-qPCR
The confirmation of primers were shown in Table S12. Total RNA was extracted using TRIzol reagent (TaKaRa, Japan) according to the manufacturer’s method. RNA samples were assessed with OD 260/280 > 2.0 and OD 260/230 > 1.8. Equal amounts of total RNA (2 μg) from all of the samples were treated with DNase I to eliminate genomic DNA contamination and were then used for cDNA synthesis with a PrimeScriptTM RT Reagent Kit (Perfect Real Time; TaKaRa). Purified cDNA samples were diluted 1:20 with RNase-free water before use as templates in the RT-qPCR process. RNA extraction and cDNA synthesis from all of the samples was performed with four biological replicates.
Then, 20 μl solution system, corresponding to 5 ng of total cDNA, was used in per PCR (TaKaRa SYBR PrimeScript RT-PCR Kit for Perfect Real Time). The reaction was carried out in a RT-qPCR cycler (Roche LightCycler® 480II) according to the manufacturer’s method (TaKaRa). Reaction mixtures were incubated for 10 min at 95 °C for pre-incubation, followed by 45 amplification cycles of 15 s at 9 °C, 15 s at 60 °C and 20 s at 72 °C. After that, a dissociation curve was generated (at 60–95 °C) to check the specificity of the amplicon. Three biological replicates were amplified for all samples. Lin-RegPCR was used to calculate the efficiency of the RT-qPCR primers. An electrophoresis analysis of the PCR product sizes was also carried out to determine their amplicon specificity. The expression levels of the GLRs in all samples were determined by their quantification cycle values (Cqs). Then, these expression values transformed into log2 values and were standardized using EXPANDER 6. The K-means clustering in EXPANDER 6 grouped the genes into different main clusters with unique expression patterns. An MIQE checklist contains the essential information about qPCR analysis in this study provided (MIQE checklist). All indexes met the MIQE guidelines54.
Plant growth conditions
All materials were collected from P. bretschneideri Rehd. cultivar ‘Dangshansu’ growing in a natural environment in Nanjing, China. The harvested seeds were germinated for 3 d and transferred to pots containing soil and vermiculite. Roots, stems and leaves were harvested 90 d after transfer. The mature seeds were obtained 13 weeks after flowering. Flowers were harvested a few days before anthesis, and the styles were then detached, weighed and stored in liquid nitrogen. Anthers were also dehisced, dried in bottles containing desiccant, and stored at −20 °C in coated silica gel until needed. Mature pear pollen was cultivated in liquid for germination and growth. The incubation medium contained the following components (mM): 5 2-(N-morpholino) ethanesulfonic acid hydrate, 440 sucrose, 0.55 Ca(NO3)2, 1.60 MgSO4, 1.60 H3BO3, and 1.00 KNO3 at pH 6.0–6.2 (adjusted with Tris). The pollen was incubated in small Petri dishes at 25 ± 1 °C for 3 h. Then, the samples were centrifuged, the supernatants removed and the pollen tube precipitates frozen in liquid nitrogen. The three typical stages of phloem, cambium and xylem samples were described in Fig. S6. Stage I: stem above cotyledons from 90-d-old pear trees; Stage II: stem from the current growth branches of 8-year-old pear trees. Stage III: stem from last year’s branches of 8-year-old pear trees. All shoot tip tissues were harvested from 8-year-old pear trees and divided into two phases; dormant (February) and sprouted (April). All materials harvested were used for extraction of total RNA.
Data access
Genomic and available annotated protein databases for pear (Pyrus bretschneideri) was downloaded from the pear genome project (http://peargenome.njau.edu.cn/). Conifers were downloaded from conifer genome project (http://congenie.org/). The other plant species were downloaded from the sources phytozome (http://phytozome.jgi.doe.gov/pz/portal.html). And also, the accession number was listed at the supplemental data document (Table S2).
Additional Information
How to cite this article: Chen, J. et al. Evolutionary and Expression Analysis Provides Evidence for the Plant Glutamate-like Receptors Family is Involved in Woody Growth-related Function. Sci. Rep. 6, 32013; doi: 10.1038/srep32013 (2016).
Supplementary Material
Acknowledgments
This work was supported by National Key Technology R&D Program of the Ministry of Science and Technology of China (2014BAD16B03-4), National Natural Science Foundation of China (31522048 and 31272119), Jiangsu Province Science and Technology Support Program, China (BE2014334 and BE2014400) and the Fundamental Research Funds for the Central Universities (KYTZ201602 and KYLH201502-2).
Footnotes
Author Contributions Conceived, designed, analyzed and wrote this manuscript: J.C. Performed the experiments: Y.J. Contributed reagents: LXY; Contributed analysis tools: L.L. Contributed analysis tools: P.W. Contributed materials: S.Z. Contributed materials: H.Z., Conceived, designed, analyzed, wrote and modified this manuscript: J.W. All authors have read and approved the final manuscript.
References
- Chiu J. C. et al. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Molecular Biology & Evolution 19, 1066–1082 (2002). [DOI] [PubMed] [Google Scholar]
- Lam H.-M. et al. Glutamate-receptor genes in plants. Nature 396, 125–126 (1998). [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. & Jessell T. M. Principles of neural science. Vol. 4 (McGraw-Hill: New York, , 2000). [Google Scholar]
- Tapken D. & Hollmann M. Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation. Journal of molecular biology 383, 36–48 (2008). [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Shneider N. A. & Axel R. A family of glutamate receptor genes: evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron 5, 569–581 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A. et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences 94, 5923–5928 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R. et al. Crystal structure and association behaviour of the GluR2 amino-terminal domain. The EMBO Journal 28, 1812–1823 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill E. D., Bieck A. M. & Spalding E. P. Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant physiology 159, 40–46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D. et al. De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. The Plant Journal 58, 437–449 (2009). [DOI] [PubMed] [Google Scholar]
- Kong D. et al. Arabidopsis Glutamate Receptor Homolog 3.5 Modulates Cytosolic Ca2+ Level to Counteract Effect of Abscisic Acid in Seed Germination. Plant physiology 167, 1630–1642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. et al. Glutamate receptor-like channel3. 3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis. Plant physiology 162, 1497–1509 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E. et al. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332, 434–437 (2011). [DOI] [PubMed] [Google Scholar]
- Hertzberg M. et al. A transcriptional roadmap to wood formation. Proceedings of the National Academy of Sciences 98, 14732–14737 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.-H. Vascular tissue differentiation and pattern formation in plants. Annual Review of Plant Biology 53, 183–202 (2002). [DOI] [PubMed] [Google Scholar]
- Zhong R., Lee C. & Ye Z.-H. Evolutionary conservation of the transcriptional network regulating secondary cell wall biosynthesis. Trends in plant science 15, 625–632 (2010). [DOI] [PubMed] [Google Scholar]
- Dünisch O. & Bauch J. Influence of mineral elements on wood formation of old growth spruce (Picea abies [L.] Karst.). Holzforschung-International Journal of the Biology, Chemistry, Physics and Technology of Wood 48, 5–14 (1994). [Google Scholar]
- Lautner S. et al. Calcium nutrition has a significant influence on wood formation in poplar. New Phytologist 173, 743–752 (2007). [DOI] [PubMed] [Google Scholar]
- Kuhn A., Bauch J. & Schröder W. In Nutrient Uptake and Cycling in Forest Ecosystems 135–150 (Springer, 1995). [Google Scholar]
- Follet-Gueye M.-L., Verdus M.-C., Demarty M., Thellier M. & Ripoll C. Cambium pre-activation in beech correlates with a strong temporary increase of calcium in cambium and phloem but not in xylem cells. Cell calcium 24, 205–211 (1998). [DOI] [PubMed] [Google Scholar]
- Lautner S. & Fromm J. Calcium-dependent physiological processes in trees. Plant Biology 12, 268–274 (2010). [DOI] [PubMed] [Google Scholar]
- Vincill E. D., Clarin A. E., Molenda J. N. & Spalding E. P. Interacting glutamate receptor-like proteins in phloem regulate lateral root initiation in Arabidopsis. The Plant Cell 25, 1304–1313 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S. A., Chauvin A., Pascaud F., Kellenberger S. & Farmer E. E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426 (2013). [DOI] [PubMed] [Google Scholar]
- Kalyanaraman A. et al. The genome of the domesticated apple (Malus 9 domestica Borkh.). Nat Genet 42, 833839Verardo (2010). [DOI] [PubMed] [Google Scholar]
- Shulaev V. et al. The genome of woodland strawberry (Fragaria vesca). Nature genetics 43, 109–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. et al. The genome of Prunus mume. Nature Communications 3, 187–190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde I. et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nature genetics 45, 487–494 (2013). [DOI] [PubMed] [Google Scholar]
- Wu J. et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome research 23, 396–408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagné D. et al. The draft genome sequence of European pear (Pyrus communis L.‘Bartlett’). PloS one 9, e92644 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko V. A., Glasser C. R. & Mayer M. L. Structural similarities between glutamate receptor channels and K+ channels examined by scanning mutagenesis. The Journal of general physiology 117, 345–360 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert V. A. et al. The Amborella genome and the evolution of flowering plants. Science 342, 1241089 (2013). [DOI] [PubMed] [Google Scholar]
- Schrader J. et al. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. The Plant Cell 16, 2278–2292 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A. T. What genes make a tree a tree? Trends in plant science 10, 210–214 (2005). [DOI] [PubMed] [Google Scholar]
- Mayer M. L. Emerging models of glutamate receptor ion channel structure and function. Structure 19, 1370–1380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincentz M., Moureaux T., Leydecker M. T., Vaucheret H. & Caboche M. Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginifolia leaves by nitrogen and carbon metabolites. The Plant Journal 3, 315–324 (1993). [DOI] [PubMed] [Google Scholar]
- Fritz C., Mueller C., Matt P., Feil R. & Stitt M. Impact of the C-N status on the amino acid profile in tobacco source leaves. Plant, cell & environment 29, 2055–2076 (2006). [DOI] [PubMed] [Google Scholar]
- Sulieman S., Fischinger S. A., Gresshoff P. M. & Schulze J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiologia Plantarum 140, 21–31 (2010). [DOI] [PubMed] [Google Scholar]
- Rosnitschek-Schimmel I. The influence of nitrogen nutrition on the accumulation of free amino acids in root tissue of Urtica dioica and their apical transport in xylem sap. Plant and cell physiology 26, 215–219 (1985). [Google Scholar]
- Lam H.-M. et al. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. The Plant Cell 7, 887 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Zou C., Lehti-Shiu M. D., Shinozaki K. & Shiu S.-H. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant physiology 148, 993–1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Stern-Bach Y. & Stevens C. F. The tetrameric structure of a glutamate receptor channel. Science 280, 1596–1599 (1998). [DOI] [PubMed] [Google Scholar]
- Roy S. et al. Investigating glutamate receptor-like gene co-expression in Arabidopsis thaliana. Plant, cell & environment 31, 861–871 (2008). [DOI] [PubMed] [Google Scholar]
- Quevillon E. et al. InterProScan: protein domains identifier. Nucleic acids research 33, W116–W120 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 32, 1792–1797 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M., Clamp M. & Barton G. J. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abascal F., Zardoya R. & Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104–2105 (2005). [DOI] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löytynoja A. & Goldman N. webPRANK: a phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC bioinformatics 11, 579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M. et al. Circos: an information aesthetic for comparative genomics. Genome research 19, 1639–1645 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. PIECE: a database for plant gene structure comparison and evolution. Nucleic acids research 41, D1159–D1166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-H., Tang H., Wang X. & Paterson A. H. PGDD: a database of gene and genome duplication in plants. Nucleic acids research 41, D1152–D1158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic acids research 40, e49–e49 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Molecular biology and evolution 24, 1586–1591 (2007). [DOI] [PubMed] [Google Scholar]
- Ulitsky I. et al. Expander: from expression microarrays to networks and functions. Nature protocols 5, 303–322 (2010). [DOI] [PubMed] [Google Scholar]
- SA B. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry 55, 611–622 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.