Abstract
Hereditary pulmonary alveolar proteinosis (PAP) caused by mutations in CSF2RA or CSF2RB, which encode GM-CSF receptor α and β respectively, is a rare disease. Although some experimental therapeutic strategies have been proposed, no clinical evidence has yet been reported. We herein describe the clinical course and recurrence of hereditary PAP after lung transplantation. A 36-year-old woman developed PAP of unknown etiology. She underwent bilateral lung transplantation from living donors at the age of 42 years because of severe respiratory failure complicated by pulmonary fibrosis. However, PAP recurred after 9 months, and we found that donor-origin alveolar macrophages had been almost completely replaced with recipient-origin macrophages. We performed a genetic analysis and identified a point deletion in the CSF2RB gene that caused a GM-CSF receptor-mediated signaling defect. PAP progressed with fibrosis in both transplanted lungs, and the patient died of respiratory failure 5 years after the lung transplantation. Distinct from recent reports on pulmonary macrophage transplantation in mice, this case suggests that human alveolar macrophages might not maintain their population only by self-renewal but may depend on a supply of precursor cells from the circulation. Bone marrow transplantation should be considered for treatment of severe PAP with GM-CSF receptor gene deficiency.
Keywords: Hereditary pulmonary alveolar proteinosis, Lung transplantation, Recurrence
Abbreviations: aPAP, autoimmune pulmonary alveolar proteinosis; hPAP, hereditary pulmonary alveolar proteinosis
1. Introduction
Pulmonary alveolar proteinosis (PAP) is a rare disease characterized by abnormal accumulation of surfactant within alveoli and terminal bronchioli, and this surfactant interferes with gas exchange [1]. About 90% of patients with PAP have autoimmune PAP (aPAP), in which autoantibodies against granulocyte-macrophage colony stimulating factor (GM-CSF) inhibit the differentiation of alveolar macrophages, causing impaired surfactant clearance. Hereditary and unclassified PAP account for less than 1% in adult PAP [2]. Of these cases, genetic aberration of the GM-CSF receptor has been rarely reported [3], [4], [5], [6]. Whole-lung lavage is only palliative and symptomatic, but it remains the only option currently available. Some experimental therapeutic strategies have been proposed for hereditary PAP (hPAP); however, these treatments are not ready for practical application [6], [7].
Most cases of aPAP can be successfully treated by whole-lung lavage and/or GM-CSF inhalation therapy. Therefore, patients with PAP are not generally candidates for lung transplantation. We previously described a patient with unclassified PAP who underwent bilateral lung transplantation from living donors for rescue from rapidly progressing respiratory failure complicated by pulmonary fibrosis [8]. Several months after that lung transplantation, we identified a nonsense mutation in CSF2RB in this patient and diagnosed adult-onset hPAP [3]. We herein report the recurrence of PAP after this lung transplantation and discuss its mechanism.
2. Case report
The above-described 36-year-old woman noticed dyspnea on exertion and was diagnosed with PAP. Despite repeated whole-lung lavage and GM-CSF inhalation therapy, her respiratory condition had become critical 6 years after the onset of PAP. After deliberate consideration, she underwent bilateral lung transplantation with right and left lungs from her husband and brother, respectively [8]. At the time of transplantation, the etiology of PAP was unknown other than negativity for serum GM-CSF autoantibodies.
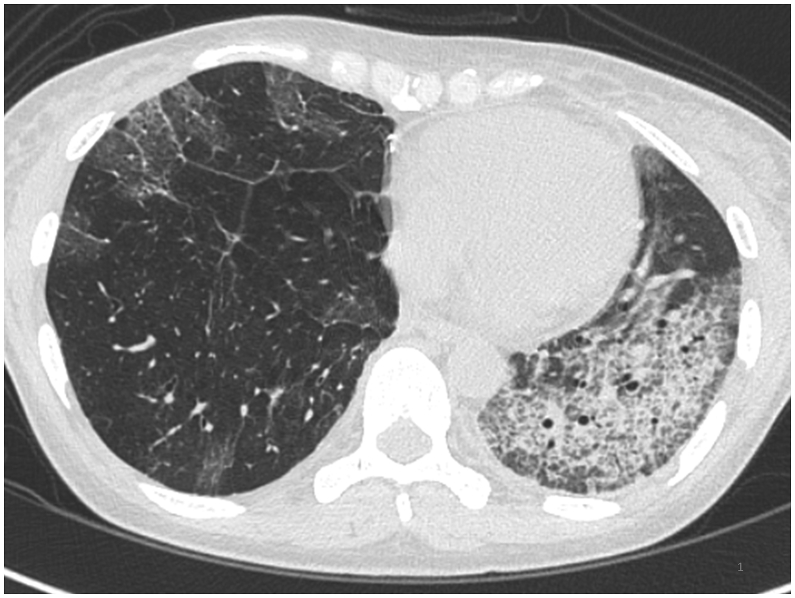
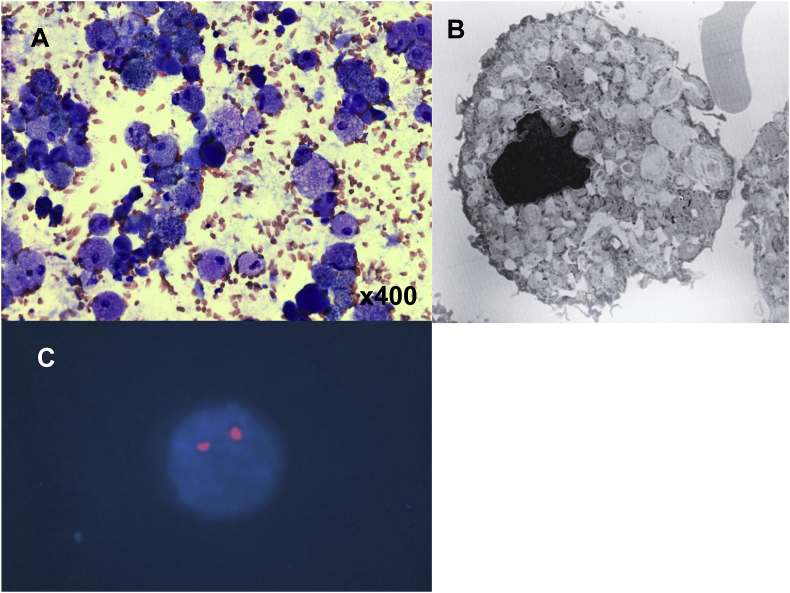
After lung transplantation, she was treated with tacrolimus, mycophenolate mofetil, and prednisolone. Nine months after the transplantation, she developed mild dyspnea and ground glass opacity that predominantly progressed in the left lung (Fig. 1). Sixteen months after the lung transplantation, we obtained bronchoalveolar lavage fluid from the bilateral lungs and found bilateral recurrence of PAP (Fig. 2A and B). Fluorescence in situ hybridization (FISH) analysis demonstrated that more than 99% of the alveolar macrophages from both lungs did not harbor the Y chromosome, indicating that the phenotype of the alveolar macrophages had reversed to the recipient type in the bilateral lungs (Fig. 2C).
Fig. 1.
High-resolution computed tomographic appearance of the chest 31 months after transplantation. The crazy paving appearance is consistent with recurrence of pulmonary alveolar proteinosis, and left dominance was seen from the beginning of recurrence.
Fig. 2.
Results of bronchoscopic analysis. (A) Bronchoalveolar lavage cells showing foamy alveolar macrophages (Diff-Quik stain). (B) Snapshot image of foamy alveolar macrophages under a scanning electron microscope. (C) FISH analysis demonstrated that 99% of the alveolar macrophages had XX sex chromosomes.
The recurrence of PAP and replacement of donor-origin alveolar macrophages led us to intensively investigate gene abnormalities because the patient was suspected to have functional defects in alveolar macrophages that are known to be derived from bone marrow. Consequently, we found a homozygous one-point deletion in the CSF2RB gene with an intracellular STAT5 signaling defect as reported previously [3]. We thoroughly discussed the feasibility of bone marrow transplantation with hematologists but could not obtain their consent to perform such an operation. We informed the patient about the risks and benefits and determined not to perform bone marrow transplantation. This decision was based on the fact that the patient had already received transplants from two different donors and had several serious risk factors for a poor prognosis in receiving bone marrow transplantation, such as reactivated hepatitis B virus infection, renal dysfunction, respiratory dysfunction, and having received immunosuppressive therapy after the lung transplantation.
Thereafter, she underwent whole-lung lavages five times and twice for the left and right lungs, respectively, during the 4 years post-transplantation. She gradually developed respiratory failure with progression of PAP, then required home oxygen therapy. Furthermore, 4 years after the lung transplantation, her forced expiratory volume in 1 s (FEV1.0) decreased from 1.95 to 1.13 L for 3 months. Based on the clinical diagnosis of bronchiolitis obliterans syndrome without donor-specific human leukocyte antigen antibodies, we started two courses of steroid pulse therapy and increased immunosuppressants. Even though she inhaled prophylactic amphotericin B, she developed new multiple nodular shadows and elevation of 1,3-β-d-glucan and galactomannan antigen. Aspergillus fumigatus, Aspergillus terreus, and Aspergillus flavus were cultured from bronchoalveolar lavage, and we started intensive antifungal therapy for invasive pulmonary aspergillosis. Although nodular shadows shrank and her condition transiently stabilized, she gradually deteriorated. Unfortunately, she eventually died of respiratory failure due to PAP recurrence and progression to bronchiolitis obliterans syndrome.
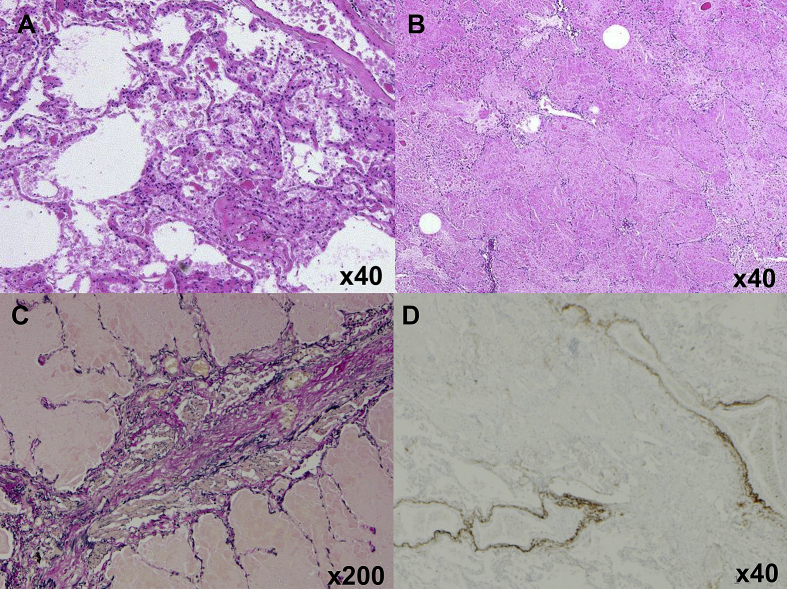
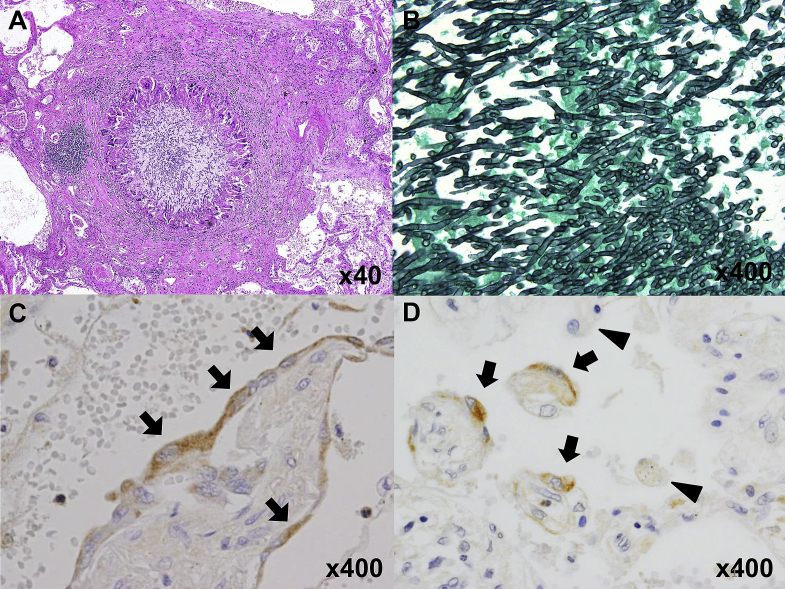
Autopsy showed recurrence of PAP accompanied by focal fibrosis. In accordance with computed tomography findings, pathological PAP findings were also predominant in the left lung. Pulmonary emphysema was seen only in the right lung (Fig. 3A and B). Chronic airway rejection, which was pathologically detected as bronchiolitis obliterans, and chronic vascular rejection with C4d deposition confirmed by immunohistochemistry were also found. (Fig. 3C and D). Furthermore, Aspergillus infection remained in both lungs despite intensive antifungal therapy (Fig. 4A and B). The GM-CSF common β receptor was not expressed on alveolar macrophages but on hyperplastic alveolar epithelial cells in the bilateral lungs (Fig. 4C and D).
Fig. 3.
Histological examination of autopsied lungs. (A) The right lung, donated by the husband, and (B) the left lung, donated by the brother, showed accumulation of surfactant proteins (hematoxylin and eosin stain). (C) Chronic airway rejection that manifested as bronchiolitis obliterans was histologically confirmed (Elastica van Gieson stain). (D) Chronic vascular rejection was confirmed by C4d deposition on vascular endothelial cells (immunohistochemical stain).
Fig. 4.
Histological examination of autopsied lungs. (A, B) Granulomas and Aspergillus were detected (hematoxylin and eosin stain and Grocott’s methenamine silver stain). Immunohistochemical analysis showed expression of the GM-CSF common β receptor in right lung (C) and left lung (D) epithelial cells (arrow), but not alveolar macrophages (arrowhead).
3. Discussion
In this case report, we describe the outcome of lung transplantation in a patient with hPAP caused by deficient expression of the GM-CSF receptor common β subunit. As stated above, this gene defect was identified after the lung transplantation; therefore, a poor outcome was attained. Our decision to perform lung transplantation at that time was inevitable and surely contributed to prolonging the patient's life. However, because some hPAP cases have been recently reported [3], [4], [5], [6], hPAP screening should be performed in patients with unclassified PAP, even adults, so that the feasibility of bone marrow transplantation can be discussed in the early stage of disease progression. One case of idiopathic PAP recurrence after lung transplantation was reported in the 1990s, although the etiology of the PAP was not clearly described [9].
The present case provides direct evidence that alveolar macrophages of donor origin replaced cells of recipient origin after lung transplantation. Although the origin of resident alveolar macrophages is still controversial, the leading hypothesis in the “steady state” indicates that resident alveolar macrophages are predominantly maintained by replication of local alveolar macrophages independent of circulating monocytes entering the lung [10]. A recent study has demonstrated that transplanted alveolar macrophages can maintain their population for a long period by local replication [7]. These findings raise the possibility of curative treatment option for hPAP by lung transplantation; however, the outcome of our case was otherwise unfortunate. In contrast, several studies have demonstrated that monocytes of bone marrow origin in chimeric mice replace alveolar macrophages after bone marrow transplantation [11], [12]. Furthermore, replacement of alveolar macrophages by bone marrow-derived donor-origin cells has been reported in both lung and bone marrow transplantations [13], [14]. This controversy might be explained by the assumption that lung or bone marrow transplantation itself alters the “steady state” of the lung. After any transplantation, many kinds of medication, especially immunosuppressive agents, are usually used. These agents can directly affect bone marrow and production of cytokines. Furthermore, immunosuppressive therapy could affect the function of alveolar macrophages and cause PAP after lung transplantation [15]. The interference of “steady state” after lung transplantation with several of the above-mentioned factors might allow replacement of alveolar macrophages by donor-origin cells. This hypothesis is consistent with the fact that the alveolar macrophages in this case study were almost completely replaced 16 months after the lung transplantation. Therefore, bone marrow transplantation might have been the most appropriate therapeutic option for this case of PAP [7]. Pulmonary macrophage transplantation therapy, which was recently reported in GM-CSF receptor β-deficient mice, might be a therapeutic option in the future [6].
The laterality of recurrence was notable in our case. The fact that the sibling's lung (not the husband's lung) was predominantly affected pathologically and radiologically after transplantation indicates the possible involvement of GM-CSF receptor β chain expression in the lung parenchyma. However, immunohistochemical analysis of alveolar epithelial cells showed at least the expression of the GM-CSF β receptor chain in the parenchyma of both lungs. Although we did not have a chance to perform genetic analysis on the blood from the sibling donor, the possibility of a heterozygous abnormality in the CSF2RB gene in her sibling remains. Alternatively, the right lung donated from her husband, an ex-smoker, showed emphysematous change that may have attenuated the disease expression.
4. Conclusion
Lung transplantation to treat hPAP due to an abnormality of the GM-CSF receptor resulted in recurrence of PAP after 9 months. We demonstrated that alveolar macrophages were almost completely replaced by cells of recipient origin by at least 16 months after lung transplantation. Lung transplantation itself in this case contributed to prolongation of the patient's life and was a necessary intervention at that time. Importantly, this case indicates that GM-CSF receptor analysis should be performed in cases of unclassified PAP to detect hPAP so that early consideration of bone marrow transplantation can be discussed in the treatment of hPAP.
Disclosures
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
We thank Mr. Akitoyo Ichinose for performing the electron microscopic examination. We also thank SRL, Inc. for performing the FISH analysis.
This report was partly supported by a grant, “Rare lung diseases (pulmonary alveolar proteinosis, congenital interstitial lung disease and hereditary hemorrhagic telangiectasia) {H24-Nanchitou(Nanchi)-Ippan-035},” from the Ministry of Health, Labour and Welfare, Japan.
References
- 1.Trapnell B.C., Whitsett J.A., Nakata K. Pulmonary alveolar proteinosis. N. Engl. J. Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 2.Ishii H., Seymour J.F., Tazawa R., Inoue Y., Uchida N., Nishida A. Secondary pulmonary alveolar proteinosis complicating myelodysplastic syndrome results in worsening of prognosis: a retrospective cohort study in Japan. BMC Pulm. Med. 2014;14:37. doi: 10.1186/1471-2466-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka T., Motoi N., Tsuchihashi Y., Tazawa R., Kaneko C., Nei T. Adult-onset hereditary pulmonary alveolar proteinosis caused by a single-base deletion in CSF2RB. J. Med. Genet. 2011;48:205–209. doi: 10.1136/jmg.2010.082586. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T., Maranda B., Sakagami T., Catellier P., Couture C.Y., Carey B.C. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur. Respir. J. 2011;37:201–204. doi: 10.1183/09031936.00090610. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T., Sakagami T., Rubin B.K., Nogee L.M., Wood R.E., Zimmerman S.L. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J. Exp. Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki T., Sakagami T., Young L.R., Carey B.C., Wood R.E., Luisetti M. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am. J. Respir. Crit. Care Med. 2010;182:1292–1304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T., Arumugam P., Sakagami T., Lachmann N., Chalk C., Sallese A. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagawa T., Yamasaki N., Tsuchiya T., Miyazaki T., Matsuki K., Tsuchihashi Y. Living-donor lobar lung transplantation for pulmonary alveolar proteinosis in an adult: report of a case. Surg. Today. 2011;41:1142–1144. doi: 10.1007/s00595-010-4411-0. [DOI] [PubMed] [Google Scholar]
- 9.Parker L.A., Novotny D.B. Recurrent alveolar proteinosis following double lung transplantation. Chest. 1997;111:1457–1458. doi: 10.1378/chest.111.5.1457. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maus U.A., Janzen S., Wall G., Srivastava M., Blackwell T.S., Christman J.W. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am. J. Respir. Cell Mol. Biol. 2006;35:227–235. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 12.Murphy J., Summer R., Wilson A.A., Kotton D.N., Fine A. The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 2008;38:380–385. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas E.D., Ramberg R.E., Sale G.E., Sparkes R.S., Golde D.W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192:1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- 14.Nakata K., Gotoh H., Watanabe J., Uetake T., Komuro I., Yuasa K. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood. 1999;93:667–673. [PubMed] [Google Scholar]
- 15.Gal A.A., Bryan J.A., Kanter K.R., Lawrence E.C. Cytopathology of pulmonary alveolar proteinosis complicating lung transplantation. J. Heart Lung Transpl. 2004;23:135–138. doi: 10.1016/s1053-2498(03)00032-9. [DOI] [PubMed] [Google Scholar]