Abstract
Asplenic patients are at increased risk for sepsis and fulminant infection. Sepsis in these patients is typically secondary to encapsulated bacteria, with Streptococcus pneumoniae being the most frequent pathogen. Rare complications of severe sepsis include purpura fulminans and bilateral adrenal hemorrhage (Waterhouse-Friderichsen syndrome).
We present the case of a 36-year-old woman, healthy except for splenectomy years prior for idiopathic thrombocytopenic purpura treatment, who presented with fever. Upon presentation to our hospital, three hours after symptoms onset, she had purpura fulminans and shock. Despite timely antimicrobials and maximal resuscitative efforts, her disease progressed and she expired 12 hours after symptoms onset. Autopsy revealed bilateral adrenal hemorrhage; acute adrenal crisis likely contributed to her refractory shock. Prior to her presentation, she had not received guideline-based post-splenectomy care.
Sepsis in asplenic patients can be fulminant and rapidly fatal. Streptococcus pneumoniae remains the most frequent cause, despite decreasing rates in recent years related to widespread pneumococcal vaccination. Guideline-based vaccinations and “pill-in-pocket” therapy can be life-saving for asplenic patients. Purpura fulminans represents an extreme manifestation of disseminated intravascular coagulation, is more common in asplenic patients, and portends a poor prognosis. Waterhouse-Friderichsen syndrome can be seen concurrently with purpura fulminans and further portends a poor prognosis; pre-mortem diagnosis requires a high index of suspicion.
Keywords: Streptococcus pneumoniae, Purpura fulminans, Asplenia, Waterhouse-Friderichsen syndrome, Sepsis
Introduction
Asplenic hosts are at increased risk of sepsis, which can be fulminant and rapidly progressive, with mortality rates estimated at 50% [1]. Streptococcus pneumoniae is the most common cause of sepsis in asplenic patients [2]. Although rates of pneumococcal sepsis have been decreasing since the widespread use of pneumococcal vaccines [3], overall rates of guideline-based post-splenectomy vaccinations and care remain low [4].
Purpura fulminans and Waterhouse-Friderichsen syndrome are rare, life-threatening complications of sepsis. Purpura fulminans represents severe disseminated intravascular coagulation with hemorrhagic skin necrosis. The classic presentation is diffuse, retiform purpura or angular purpuric lesions [5]. Waterhouse-Friderichsen syndrome was first described in 1911 as adrenal hemorrhage and subsequent adrenal crisis related to sepsis [6]. It is often accompanied by disseminated intravascular coagulation and purpura fulminans and also carries a poor prognosis. Treatment consists of stress-dose steroids and treatment of the underlying infection.
We report here a case of a young, asplenic but otherwise healthy woman who presented with fulminant sepsis, purpura fulminans, and Waterhouse-Friderichsen from Streptococcus pneumoniae blood stream infection. She had not received appropriate post-splenectomy interventions.
Case
A 36-year-old woman with a history of idiopathic thrombocytopenic purpura status post splenectomy presented with sudden onset of nausea, vomiting, diarrhea, and abdominal pain. Seven hours before presentation, she went to sleep in her usual state of health. Three hours before presentation, she awoke with sudden onset of low-grade fever, nausea, vomiting, diarrhea, abdominal pain, and worsening pain in all of her extremities. She then presented to our hospital with an evolving dark, purpuric rash on face and extremities. Her medications included lamotrigine and trazadone. She had an allergy to sulfonamide medications. She had two sons, 8 and 10 years old, worked at a daycare facility and lived in a wooded area in Massachusetts. One month previously, she had traveled to Martha’s Vineyard. She owned a dog, and did not smoke, drink alcohol, or use recreational drugs.
Upon initial physical examination, she appeared ill and in extremis. The blood pressure was 105/60 mmHg, pulse was 133 beats per minute, temperature 99.5∘F (37.5∘C), respirations 36 breaths per minute, and oxygen saturation 94% on room air. Examination was notable for rigors, tachycardia, tachypnea, diffusely tender extremities, and a purpuric rash on the face, tongue, palate, all four extremities, abdomen, and back. Physical examination was otherwise normal. Laboratory analysis was notable for white blood cell count 6.9 × 103/uL (reference range 4.0-11.0 × 103/uL) with 9% band forms, hemoglobin 10 g/dL (14.0-18.0 g/dL), and platelets 15 × 103/uL (150–450 × 103/uL). Serum bicarbonate was 8mEq/L (22–28 mEq/L) and serum creatinine was 3.3 mg/dL (0.5-1.2 mg/dL). Serum lactate was 8.7 mmol/L (0.5-2.0 mmol/L). ALT was 81 IU/L (0–40 IU/L), AST was 350 IU/L (0–40 IU/L), total bilirubin was 1.3 mg/dL (0.1-1.5 mg/dL) and alkaline phosphatase was 50 IU/L (39–117 IU/L). International Normalized Ratio (INR) was 5.0 (0.9-1.1) and prothrombin time was 49.6 s (22–35 s). A random cortisol level was 2.5ug/dL (5–20ug/dL). Initial chest X-ray showed no abnormalities, although a subsequent one several hours later showed puffy, bilateral infiltrates.
She developed progressive respiratory failure and was intubated. Subsequently, she developed progressive hypotension refractory to intravenous fluid boluses and was thus started on multiple vasopressors. The initial differential diagnosis included encapsulated organisms such as meningococcus and pneumococcus, Staphylococcus aureus, Capnocytophaga, Streptococcus pyogenes, and tick-borne pathogens including babesiosis. She was started promptly on empiric vancomycin, ceftriaxone, quinine, clindamycin, and doxycycline. She was given platelets, cryoprecipitate, and fresh frozen plasma for disseminated intravascular coagulation, and stress-dose steroids. Eight hours after presentation, the patient developed shock refractory to four vasopressors and intravenous fluids, her purpuric rash had significantly extended, and a bedside echocardiogram showed minimally functional myocardium. She suffered cardiac arrest soon thereafter and expired. An attempt was made to give her protein C concentrate, but this did not arrive before her death.
Blood cultures drawn in the Intensive Care Unit, post antimicrobial administration, were negative. No blood cultures prior to antimicrobial administration were processed. Serial blood smears were negative for intracellular organisms. Posthumously, on a blood smear used to rule-out babesiosis, we identified macrophages that had phagocytosed diplococci. Since the Wright stain stains all bacteria blue, it was unclear if these diplococci were Gram-positive or Gram-negative (Fig. 1). Pneumococcal antigen testing (antigen latex agglutination test) was then done and returned positive, while meningococcal antigen testing was negative, confirming the diagnosis of Streptococcus pneumoniae infection.
Fig. 1.
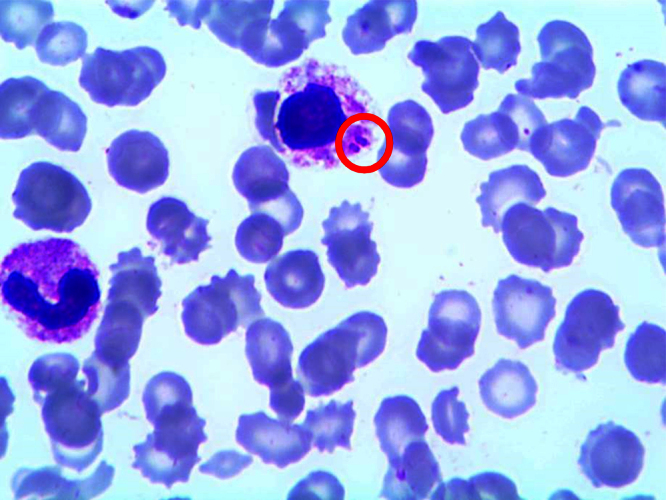
Peripheral blood smear obtained upon presentation to the Emergency Department. Two pairs of diplococci are seen (red circle) phagocytosed within a macrophage. Pneumococcal antigen testing (antigen latex agglutination test) was then done and returned positive, while meningococcal antigen testing was negative.
An autopsy was performed. Evidence of diffuse intravascular coagulation was found. Pulmonary examination was consistent with Acute Respiratory Distress Syndrome. Renal microscopic examination revealed extensive microvascular thrombotic disease. Bilateral adrenal glands showed evidence of extensive hemorrhage, consistent with the Waterhouse-Friderichsen syndrome (Fig. 2). Skin histology was consistent with diffuse disseminated intravascular coagulation (DIC) and purpura fulminans (Fig. 3).
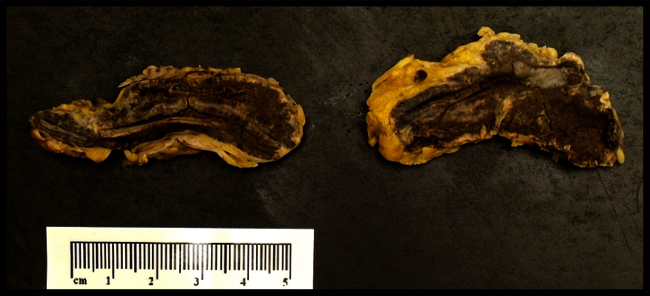
Fig. 2.
Bilateral adrenal glands were found on autopsy to have diffuse intra-parenchymal hemorrhage (dark brown areas), consistent with the Waterhouse-Friderichsen syndrome.
Fig. 3.
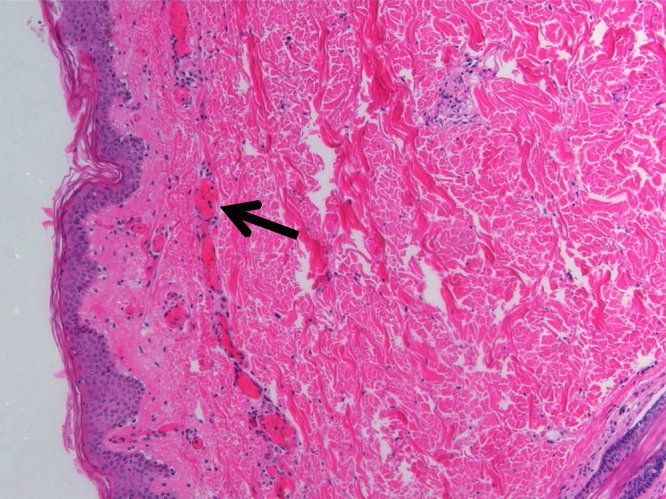
Biopsy of the skin taken at autopsy shows evidence of diffuse intravascular coagulation (DIC) and microthrombi within peripheral vessels (black arrow), consistent with purpura fulminans.
Discussion
Sepsis in the asplenic host can be fulminant and rapidly progressive, with mortality rates approaching 50% [1], [2]. Our case describes a previously healthy young woman, status-post splenectomy 15 years prior, who presented to our hospital after only a few hours of symptoms, and then rapidly declined, resulting in death within 12 hours of symptoms onset. She had evidence of extensive disseminated intravascular coagulation and purpura fulminans upon presentation. The major differential for infectious causes of purpura fulminans in the asplenic patient is Neisseria meningitidis, Capnocytophaga, Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, and Haemophilus influenzae; it is noteworthy to point out that babesiosis can present in a fulminant fashion in asplenic hosts [7], thus given our patient had recently been on Martha’s Vineyard and lived in an Ixodes scapularis tick endemic area, we empirically started empiric babesia therapy with quinine and clindamycin. Additionally, because she owned a dog, there was initial concern for a Capnocytophaga infection, which has been shown to cause fulminant infection in asplenic patients as well [8]. In the end, she was found to have evidence of pneumococcal sepsis.
Pneumococcal infection is the most common cause of fulminant infection in the asplenic host [2]. Data from the 1990′s suggested a 3.2% incidence of sepsis after splenectomy over 6.9 years of follow-up [9], though rates have dropped since universal institution of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000 for young children, replaced by 13-valent pneumococcal conjugate vaccine (PCV13) in 2010 [3]. Some authors have advocated asplenic patients have a “pill-in-pocket”, referring to an anti-pneumococcal oral antimicrobial rapidly available to take at home at first onset of fever [2]. In our patient’s case, she had not received appropriate post-splenectomy vaccinations, and she did not have any antimicrobials available at home. Given the severity of her condition upon presentation to medical attention, these likely were the only interventions that would have improved her outcome.
Post-mortem, she was found to have bilateral adrenal hemorrhage, consistent with the Waterhouse-Friderichsen syndrome. Given her random cortisol level was 2.5ug/dL, she was treated with stress-dose steroids, but adrenal crisis may have contributed to her fulminant course. A cortisol level greater than 20 ug/dL excludes the diagnosis, while a cortisol level <5 ug/dL during a state of shock supports the diagnosis [10]. Waterhouse-Friderichsen syndrome is typically caused by Neisseria meningitidis, but has been reported in sepsis from Streptococcus pneumoniae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, Escherichia coli, Haemophilus influenzae, and Staphylococcus aureus [11]. Waterhouse-Friderichsen syndrome is seen most frequently in patients with asplenia, and cases have even been reported in asplenic patients who received the appropriate vaccines. Treatment is with corticosteroids and appropriate antimicrobials. Waterhouse-Friderichsen syndrome typically presents in patients who additionally have abdominal or flank pain and disseminated intravascular coagulopathy with associated purpura fulminans [12], as was seen in our patient.
The role of therapeutic protein C in the management of patients with purpura fulminans is a matter of controversy. The 2001 large, randomized-controlled PROWESS trial [13] suggested that giving activated protein C (tradename Xigris) to patients with sepsis significantly improved 30-day mortality. However, in 2012 a very similar trial, PROWESS-SHOCK [14], failed to show any significant mortality benefit, and a 2012 Cochrane Review [15] similarly showed no benefit, prompting the removal of activated Protein C (Xigris) from the market. However, purpura fulminans represents the extreme of the pro-thrombotic spectrum of sepsis, and several small case-series and case-control studies have suggested benefit of protein C administration in these patients [5], [16], [17], [18]. For instance, White et al. looked at 36 patients with purpura fulminans given protein C concentrate (tradename Ceprotin) and saw improvement compared to outcomes from other purpura fulminans patients not given protein C concentrate [5]. While activated protein C is no longer available on the US market, protein C concentrate (Ceprotin) still is and often is used in cases of congenital purpura fulminans (from congenital protein C deficiency). Given the suggestion of benefit in patients with sepsis-related purpura fulminans, we attempted to give our patient protein C concentrate but were not able to do so before she expired.
Rapid access to on-hand antimicrobials at the first sign of fever, a so called “pill-in-pocket” strategy, is an established part of care in asplenic adult patients [2]. However, data supporting this approach is sparse. While some studies have shown benefit to daily penicillin prophylaxis in asplenic children [19], this benefit seems to wane after 5 years of age [20], and antimicrobial prophylaxis in adults is based on expert opinion [21]. Regardless, it is recommended that febrile asplenic adults immediately take a high-dose oral beta-lactam with encapsulated organism activity such as amoxicillin-clavulanate 875 mg/125 mg, or extended-spectrum fluoroquinolone such as levofloxacin 750 mg. Such patients should then undergo prompt medical evaluation. In our case, the patient had never been prescribed prophylactic antimicrobials, though this intervention may have changed her outcome. Unfortunately, this is a common occurrence among asplenic patients [4]. Improving access for asplenic patients to “pill-in-pocket” prophylactic antimicrobials, and increasing provider awareness, is necessary.
Streptococcus pneumoniae remains the most important cause of morbidity and mortality in asplenic patients. Appropriate post-splenectomy vaccinations and “pill-in-pocket” antimicrobials can improve outcomes in this population. Purpura fulminans and Waterhouse-Friderichsen syndrome represent life-threatening complications for which optimal management is unclear.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Informed consent
Written informed consent was unobtainable because the patient is deceased and has no next of kin of legal age.
Acknowledgements
None.
Contributor Information
Andrew J. Hale, Email: ahale@bidmc.harvard.edu.
Mary LaSalvia, Email: mlasalvi@bidmc.harvard.edu.
James E. Kirby, Email: jekirby@bidmc.harvard.edu.
Allison Kimball, Email: askimbal@bidmc.harvard.edu.
Rachel Baden, Email: rbaden@alamedahealthsystem.org.
References
- 1.Bisno A.L., Freeman J.C. The syndrome of asplenia, pneumococcal sepsis, and disseminated intravascular coagulation. Ann Intern Med. 1970;72:389–393. doi: 10.7326/0003-4819-72-3-389. PMID 5415422. [DOI] [PubMed] [Google Scholar]
- 2.Rubin L.G., Schaffner W. Care of the asplenic patient. N Engl J Med. 2014;371:349. doi: 10.1056/NEJMcp1314291. PMID 25054718. [DOI] [PubMed] [Google Scholar]
- 3.Pilishvili T., Lexau C., Farley M.M. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. PMID 19947881. [DOI] [PubMed] [Google Scholar]
- 4.Waghorn D.J., Mayon-White R.T. A study of 42 episodes of overwhelming post-splenectomy infection: is current guidance for asplenic individuals being followed? J Infect. 1997;35(November (3)):289–294. doi: 10.1016/s0163-4453(97)93232-1. [DOI] [PubMed] [Google Scholar]
- 5.White B., Livingstone W., Murphy C., Hodgson A., Rafferty M., Smith O.P. An open-label study of the role of adjuvant hemostatic support with protein C replacement therapy in purpura fulminans-associated meningococcemia. Blood. 2000;96(December (12)):3719–3724. PMID 11090052. [PubMed] [Google Scholar]
- 6.Waterhouse R. A case of suprarenal apoplexy. Lancet. 1911;1:577–578. [Google Scholar]
- 7.Krause P.J., Gewurz B.E., Hill D., Marty F.M., Vannier E., Foppa I.M. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46(3):370. doi: 10.1086/525852. PMID 18181735. [DOI] [PubMed] [Google Scholar]
- 8.Wald K., Martinez A., Moll S. Capnocytophaga canimorsus infection with fulminant sepsis in an asplenic patient: diagnosis by review of peripheral blood smear. Am J Hematol. 2008;83(November (11)):879. doi: 10.1002/ajh.21028. PMID 18098127. [DOI] [PubMed] [Google Scholar]
- 9.Bisharat N., Omari H., Lavi I., Raz R. Risk of infection and death among postsplenectomy patients. J Infect. 2001;43:182–186. doi: 10.1053/jinf.2001.0904. PMID 11798256. [DOI] [PubMed] [Google Scholar]
- 10.Puar T., Stikkelbroeck N., Smans L., Zelissen P., Hermus A. Adrenal crisis: still a deadly event in the 21 st century. Am J Med. 2015;129(March (3)):339. doi: 10.1016/j.amjmed.2015.08.021. e1-339. e9. PMID 26363354. [DOI] [PubMed] [Google Scholar]
- 11.Margaretten W., Nakai H., Landing B. Septicemic adrenal hemorrhage. Am J Dis Child. 1963;105:346–351. doi: 10.1001/archpedi.1963.02080040348004. PMID 139322989. [DOI] [PubMed] [Google Scholar]
- 12.Vincentelli C., Molna E.G., Robinson M.J. Fatal pneumococcal Waterhouse-Friderichsen syndrome in a vaccinated adult with congenital asplenia. Am J Emerg Med. 2009;27(July (6)):751. doi: 10.1016/j.ajem.2008.09.042. e3-5. PMID 19751638. [DOI] [PubMed] [Google Scholar]
- 13.Bernard G., Vincent J.L., Laterre P.F., LaRosa S., Dhainaut J., Lopez-Rodriguez A. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(March (8) 2001):699–709. doi: 10.1056/NEJM200103083441001. PMID 11236773. [DOI] [PubMed] [Google Scholar]
- 14.Ranieri V., Thompson B., Barie P., Dhainaut J.F., Douglas I., Finfer S. Drotrecogin alfa (Activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. PMID 22616830. [DOI] [PubMed] [Google Scholar]
- 15.Martí-Carvajal A.J., Solà I., Gluud C., Lathyris D., Cardona A.F. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12(December) doi: 10.1002/14651858.CD004388.pub6. PMID 23235609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith O.P., White B., Vaughan D., Rafferty M., Claffey L., Lyons B. Use of protein-C concentrate, heparin, and haemodiafiltration in meningococcus-induced purpura fulminans. Lancet. 1997;350(November (9091)):1590–1593. doi: 10.1016/s0140-6736(97)06356-3. PMID 9393338. [DOI] [PubMed] [Google Scholar]
- 17.Veldman A., Fischer D., Wong F.Y., Kreuz W., Sasse M., Eberspächer B. Human protein C concentrate in the treatment of purpura fulminans: a retrospective analysis of safety and outcome in 94 pediatric patients. Crit Care. 2010;14(4):R156. doi: 10.1186/cc9226. Epub 2010 Aug 19, PMID 20723255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kleijn E.D., de Groot R., Hack C.E., Mulder P.G., Engl W., Moritz B. Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: a randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med. 2003;31(June (6)):1839–1847. doi: 10.1097/01.CCM.0000072121.61120.D8. PMID12794428. [DOI] [PubMed] [Google Scholar]
- 19.Gaston M.H., Verter J.I., Woods G., Pegelow C., Kelleher J., Presbury G. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(June (25)):1593–1599. doi: 10.1056/NEJM198606193142501. PMID3086721. [DOI] [PubMed] [Google Scholar]
- 20.Falletta J.M., Woods G.M., Verter J.I., Buchanan G.R., Pegelow C.H., Iyer R.V. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. J Pediatr. 1995;127(November (5)):685–690. doi: 10.1016/s0022-3476(95)70154-0. PMID 7472817. [DOI] [PubMed] [Google Scholar]
- 21.Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. BMJ. 1996;312(February (7028)):430–434. doi: 10.1136/bmj.312.7028.430. PMID 8601117. [DOI] [PMC free article] [PubMed] [Google Scholar]