Abstract
Background
For treatment of patients diagnosed with schizophrenia, comparative long-term effectiveness of antipsychotic drugs to reduce relapses when minimising adverse effects is of clinical interest, hence prompting this review.
Aims
To evaluate the comparative long-term effectiveness of antipsychotic drugs.
Method
We systematically searched electronic databases for reports of randomised controlled trials (RCTs) of antipsychotic monotherapy aimed at reducing relapse risks in schizophrenia. We conducted network meta-analysis of 18 antipsychotics and placebo.
Results
Studies of 10 177 patients in 56 reports were included; treatment duration averaged 48 weeks (range 4–156). Olanzapine was significantly more effective than chlorpromazine (odds ratio (OR) 0.35, 95% CI 0.14–0.88) or haloperidol (OR=0.50, 95% CI 0.30–0.82); and fluphenazine decanoate was more effective than chlorpromazine (OR=0.31, 95% CI 0.11–0.88) in relapse reduction. Fluphenazine decanoate, haloperidol, haloperidol decanoate and trifluoperazine produced more extrapyramidal adverse effects than olanzapine or quetiapine; and olanzapine was associated with more weight gain than other agents.
Conclusions
Except for apparent superiority of olanzapine and fluphenazine decanoate over chlorpromazine, most agents showed intermediate efficacy for relapse prevention and differences among them were minor. Typical antipsychotics yielded adverse neurological effects, and olanzapine was associated with weight gain. The findings may contribute to evidence-based treatment selection for patients with chronic psychotic disorders.
Declaration of interest
R.J.B. received grants from the Bruce J. Anderson Foundation and the McLean Private Donors Psychopharmacology Research Fund.
Copyright and usage
© The Royal College of Psychiatrists 2016. This is an open access article distributed under the terms of the Creative Commons Non-Commercial, No Derivatives (CC BY-NC-ND) licence.
Chronic psychotic disorders diagnosed as schizophrenia are severe, idiopathic conditions affecting 26 million people worldwide and resulting in substantial disability in a majority of cases.1 Because of their early onset, chronic course and debilitating effects, schizophrenia ranks among the top 20 causes of years lived with disability.2 The course of the illness varies, but most patients have a chronic course with erratic exacerbations or relapses with repeated hospital admissions, decreased quality of life and a high economic burden. Successive relapses also are associated with progressively declining outcomes. Therefore, relapse prevention is critical for adequate clinical management of this devastating illness.3,4
Long-term maintenance treatment with antipsychotic medication has become the standard for the treatment of patients diagnosed with schizophrenia, with the aim of limiting symptomatic relapses and disability. Although many effective antipsychotic drugs have been developed since the 1950s, all are limited in effectiveness and are associated with a range of potentially serious adverse effects including neurological, metabolic and cardiovascular problems which complicate their long-term use.5,6 Generally, ‘second-generation’ or ‘atypical’ antipsychotics are better tolerated than older antipsychotic agents, at least with regard to some extrapyramidal neurological symptoms, but sometimes present high risks of adverse effects associated with weight gain, including metabolic syndrome.7 An additional, major limitation to all long-term maintenance treatments is lack of sustained adherence to them.8–10 Long-acting, injectable antipsychotic agents promise to improve treatment adherence, but evidence of superior clinical outcomes with such drugs compared with oral agents is inconsistent.11,12
Given the pressing need for effective long-term treatments for schizophrenia and a growing number of available antipsychotic drugs, evidence of the relative merits of individual agents is of great interest. Available reviews of evidence of efficacy and tolerability of antipsychotic agents generally indicate minor and variable differences between specific drugs, with the notable exception of clozapine.6,7,11–14 Such comparisons also are severely limited by the paucity of direct, head-to-head comparisons of specific agents. Recent developments in methods of meta-analysis promise to improve this situation, even without direct comparisons of specific treatments, based on application of network meta-analysis.15 In contrast to traditional pairwise meta-analyses, network methods allow indirect comparisons between treatments carried out in different trials, under presumably, if not demonstrably, highly comparable conditions.16 We now report on results of a network meta-analysis to evaluate a total of 18 orally administered and long-acting injectable (LAI) antipsychotic drug preparations.
Method
Literature search
We first performed a PubMed search to identify recent systematic reviews (2009–2015) evaluating the use of antipsychotics for relapse prevention in patients diagnosed with schizophrenia.17 Then, individual reports of randomised controlled trials (RCTs) identified in the reviews were added to those identified in literature searches of the PubMed/Medline and Cochrane Library databases. Searching was based on applying combinations of the following search terms: schizophrenia, antipsychotics, amisulpride, aripiprazole, chlorpromazine, flupenthixol decanoate, fluphenazine decanoate, haloperidol and its decanoate, olanzapine, paliperidone, long-acting injectable paliperidone, pipothiazine palmitate, quetiapine, risperidone, long-acting injectable risperidone, sulpiride, trifluoperazine, ziprasidone and zuclopenthixol decanoate (see the search term list in the data supplement).
Study selection
Two reviewers (Y.J.Z. and L.L.) screened titles and abstracts against pre-defined study inclusion criteria and promising articles were reviewed as full texts. The degree of agreement as evaluated by interrater reliability was 0.91. Any disagreements about reports selected or data extracted were resolved by consensus with a third, senior co-investigator (K.S.). Trials were eligible if they involved the use of antipsychotic monotherapy for relapse prevention among initially, clinically stable patients diagnosed with schizophrenia. We excluded trials conducted in patients with predominant negative symptoms or known treatment resistance and trials for acute illness. We included head-to-head and placebo-controlled RCTs that were single or double-blinded. We did not restrict the minimum trial length.
Data collection and quality assessment
Data regarding patient characteristics, study design, duration of test treatments, outcomes (relapse rates, drop-out rates, adverse effects) and funding sources were extracted independently by two reviewers (Y.J.Z. and L.L.) with disagreement resolved through consensus with the same third senior co-investigator (K.S.; Tables DS1 and DS2 in the data supplement). The degree of agreement as evaluated by interrater reliability was 0.90. Quality assessment of included studies was performed using the Cochrane Risk of Bias Tool, considering the following six domains of bias: random sequence generation, allocation concealment, masking of participants and personnel, masking of outcome assessors, incomplete data and selective reporting.18
Data synthesis and analysis
We carried out a random effects network meta-analysis using the mvmeta routine in STATA 13 statistical software (StataCorp. College Station, Texas, USA).19,20 Evidence from both direct (head-to-head trials) and indirect comparisons (between apparently similar trials using one or more common comparators) were combined in the analysis. The primary outcome of interest was relapse as defined in each study at its longest follow-up; and when relapse was not reported, we used clinically assessed or rating scale-based exacerbation of psychotic symptoms considered in the reports to be clinically significant, or readmission, and verified that outcome measures were sufficiently comparable between trials compared. Additional outcomes considered included study withdrawal for any reason as a proxy measure of adherence to treatment, study withdrawal because of inefficacy, study withdrawal because of adverse events and adverse events including extrapyramidal symptoms (EPS), substantial weight gain, abnormally elevated serum glucose concentration, hyperprolactinaemia, death and suicide attempts. Relative effect size was based on categorical outcomes, expressed as odds ratio (OR) with its 95% confidence interval (CI). We also used Surface Under the Cumulative RAnking curve (SUCRA) values to provide a hierarchy of efficacy and tolerability of the treatments considered.21 SUCRA is a transformation of the mean rank representing the probability that a treatment was among the n best treatments, where n ranges from 1 to the total number of treatments (here 1–18) in the data network. SUCRA of 1 indicates a treatment is the most effective and 0 the least effective.
As the network meta-analysis combined both direct and indirect comparisons to synthesise treatment effects, we assessed agreement between these two sources of evidence with tests of inconsistency.22 A loop-specific test evaluates inconsistency in all closed loops formed by three or four treatments within each network, by comparing direct with indirect estimates of a specific treatment effect. A design × treatment interaction model evaluates whether the network as a whole demonstrates inconsistency, by evaluating the agreement of evidence generated by trials with different treatment designs.
We investigated potential sources of heterogeneity among trials with meta-regression modelling and sensitivity analysis by taking account of study level characteristics. For meta-regression, we analysed the impact of follow-up duration (<6, 6–11 or ≥12 months), trial setting (in-patient, out-patient or both), trial quality (presence or absence of high risk of bias in any of the Cochrane risk of bias domains defined above), funding source (with or without industry sponsorship) and publication year (published before or after year 2000). For sensitivity analysis, we evaluated the impact of excluding trials with shorter follow-up duration (<6 months), high doses (equivalent of >10 mg/day of haloperidol) and published before 1980.23 Publication bias was assessed using comparison-adjusted funnel plot. In the absence of publication bias, the comparison-adjusted funnel plot is symmetric around zero line. This study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates for comparisons between antipsychotic treatments and placebo.
Results
Description of included studies
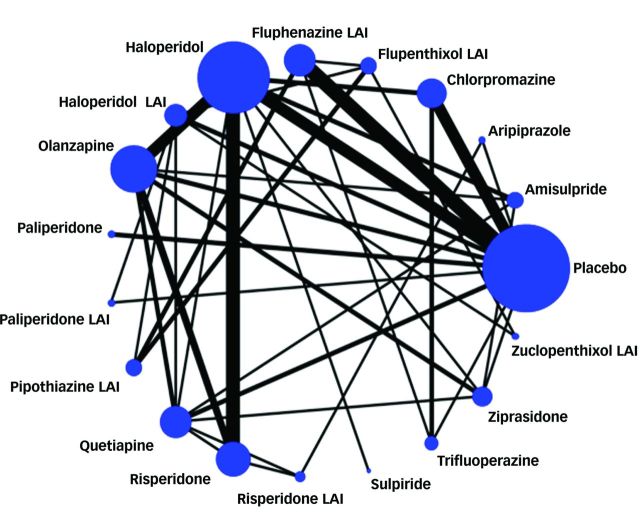
Preliminary searching of 32 systematic reviews yielded a total of 186 potentially usable citations of reports of RCTs; another 2054 reports (total of 2477) were identified by direct searching of electronic databases (Fig. 1). Based on preliminary screening of abstracts and detailed review of full reports, 56 reports met study inclusion criteria and were included for analysis, as indicated in the study network of direct and indirect comparisons (Fig. 2, reference list in the data supplement). Included trials involved 18 antipsychotic drug products: amisulpride, aripiprazole, chlorpromazine, flupenthixol decanoate (flupenthixol LAI), fluphenazine decanoate (fluphenazine LAI), haloperidol, haloperidol decanoate (haloperidol LAI), olanzapine, paliperidone, paliperidone palmitate (paliperidone LAI), pipothiazine palmitate (pipothiazine LAI), quetiapine, risperidone, risperidone LAI, sulpiride, trifluoperazine, ziprasidone and zuclopenthixol decanoate (zuclopenthixol LAI). A total of 10 177 adult participants were represented in the 56 included trials reported between 1960 and 2014; mean age (range) was 39 (15–82) years, and 64% were men. Mean, nominal study duration was 48 weeks (range 4–156). Most trials were conducted in Europe and American continents; 7% involved Asians. Out-patients were represented in 29 (52%) trials; in-patients in 10 (18%), both in 10 (18%), and setting was not reported in another 7 (12%) trials. Financing was based on pharmaceutical industry support in 23 trials (41%), public sponsors (11%), no support (25%), and unreported sponsorship (23%). Definitions of ‘relapse’ varied: rating scale-based criteria (23%), hospital admission (15%), combination of rating scale and hospital admission (23%), clinical worsening of symptoms (29%), need for a change of medication (5%) and undefined methods (5%).
Fig. 1. Flowchart of selection of reports for inclusion in study-based PRISMA recommendations (www.prisma-statement.org/statement.htm) to yield 56 study reports included in analyses.

Fig. 2. Network of all direct and indirect comparisons between antipsychotics. LAI, long-acting injection.
Assessment of potential bias
Most trials did not provide details about randomisation procedures and allocation concealment (Fig. DS1). One study (2% of all reports) had high risk of bias in random sequence generation, 10 (18%) appear to have had adequate sequence generation and 4 (7%) had apparently adequate allocation concealment. Most studies (47/56, 84%) were nominally double-blind and 21 (38%) tested and confirmed the success of blinding. In most trials, relapse represented an end-point leading to discontinuation from the study; however, drop-out rates were balanced across interventions in most studies (mean (range): 34% (0–86%)). No evidence of selective reporting was found in 28 (50%) trials included and the bias on selective report was judged to be unclear in 20 (36%) trials because of the unavailability of study protocol. Most selective reporting (5/8, 63%) was of adverse events, which some investigators reported only when at least 5–10% of participants were affected.
Consistency between direct and indirect evidence
Loop-specific tests revealed no significant inconsistency in 17 available loops (formed by three or four treatments) within the data network for relapse prevention (based on the 95% CI crossing a null value of 1.00). However, the CI was wide for four of the loops: placebo–fluphenazine LAI–trifluoperazine, placebo–chlorpromazine–trifluoperazine, flupenthixol LAI–haloperidol LAI–zuclopenthixol LAI and olanzapine–quetiapine–risperidone–risperidone LAI. Data extraction was checked and there were no apparent variables that differed across these comparisons. Based on a design × treatment interaction model, no significant inconsistency between direct and indirect evidence was identified within the evidence network as a whole (P=0.14).
Efficacy
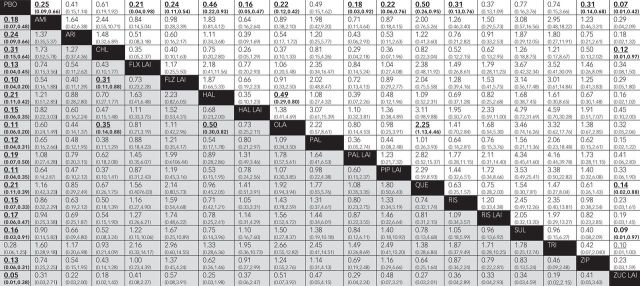
All antipsychotic treatments were significantly better than placebo for relapse prevention, with the single and surprising exception of trifluoperazine. However, most CIs overlapped between treatments so that few comparisons were different in statistical significance (Figs. 3 and 4). Olanzapine was significantly more effective than chlorpromazine (OR=0.35, 95% CI 0.14–0.88) and haloperidol (OR=0.50, 95% CI 0.30–0.82) in reducing relapses, and fluphenazine LAI was superior to chlorpromazine (OR=0.31, 95% CI 0.11–0.88; Fig. 4). Differences between other pairs of drugs were minor and not significant. We also evaluated hierarchies of efficacy on the basis of SUCRA rankings for relapse rate. The top five drugs ranked by their SUCRA values were zuclopenthixol LAI (0.85), fluphenazine LAI (0.78), olanzapine (0.76), pipothiazine LAI (0.68) and paliperidone (0.67). Findings for relapse prevention were consistent when we analysed oral and long-acting agents separately (Fig. DS2).
Fig. 3. Forest plot for relapse rate of antipsychotics compared with placebo. LAI, long-acting injection; OR, odds ratio.
Fig. 4. Relapse rate and discontinuation because of all reasons for all antipsychotics.
AMI, amisulpride; ARI, aripiprazole; CHL, chlorpromazine; FLX, flupenthixol decanoate; FLZ, fluphenazine decanoate; HAL, haloperidol; LAI, long-acting injection; OLA, olanzapine; PAL, paliperidone; PBO, placebo; PIP, pipothiazine palmitate; QUE, quetiapine; RIS, risperidone; SUL, sulpiride; TRI, trifluoperazine; ZIP, ziprasidone; ZUC, zuclopenthixol decanoate.
Fourteen trials (2886 participants) were included for the network meta-analysis of hospital admissions. Oral agents including amisulpride, haloperidol, olanzapine, quetiapine and ziprasidone were significantly more effective in reducing readmissions than placebo, but aripiprazole, chlorpromazine, paliperidone, trifluoperazine did not differ from placebo (Fig. DS3). There was a lack of data on hospital admission rates with long-acting agents except for fluphenazine LAI and risperidone LAI, which both reduced readmission risk more than placebo. In reducing readmission, olanzapine and ziprasidone were significantly more effective than haloperidol or quetiapine; amisulpride, fluphenazine LAI, haloperidol, olanzapine, risperidone LAI and ziprasidone all were superior to aripiprazole, and ziprasidone alone outperformed chlorpromazine and trifluoperazine.
Safety and tolerability
Most drugs were less associated with all-cause discontinuation than placebo, except for aripiprazole, chlorpromazine, paliperidone, risperidone LAI, sulpiride and trifluoperazine. In general, long-acting agents tended to be better tolerated than oral agents, but not statistically significant (Fig. 4). Olanzapine was associated with less all-cause discontinuation than quetiapine (OR=0.44, 95% CI 0.22–0.88) or haloperidol (OR=0.49, 95% CI 0.29–0.80), whereas zuclopenthixol LAI yielded less all-cause discontinuation than chlorpromazine (OR=0.12 (0.01–0.97)), quetiapine (OR=0.14 (0.02–0.88)) or sulpiride (OR=0.09 (0.01–0.97)). In separate analyses of discontinuation because of lack of efficacy or adverse events, most antipsychotics demonstrated lower discontinuation rates because of lack of efficacy compared with placebo, whereas no drug demonstrated a significant difference in discontinuation rates because of adverse events in comparison with placebo (Fig. DS4).
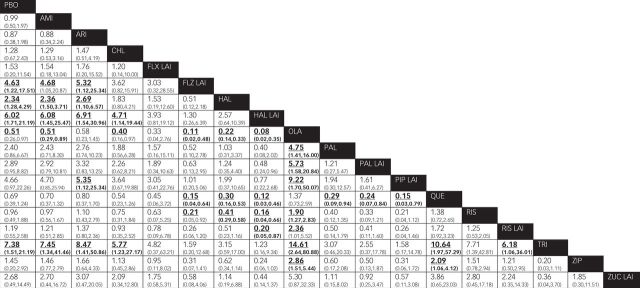
Thirty trials (7381 participants) were included for the analysis of EPS. Only fluphenazine LAI, haloperidol, haloperidol LAI and trifluoperazine, but not other antipsychotics, were associated with more reported EPS than placebo (Fig. 5). Olanzapine was associated with less risk of EPS than other agents except aripiprazole, flupenthixol LAI, quetiapine and zuclopenthixol LAI. As expected, quetiapine had less reported EPS than fluphenazine LAI, haloperidol, haloperidol LAI, paliperidone, paliperidone LAI, pipothiazine LAI, trifluoperazine and ziprasidone. Fluphenazine LAI, haloperidol, haloperidol LAI and trifluoperazine were associated with significantly more EPS than several other agents.
Fig. 5. Extrapyramidal symptoms in the context of antipsychotic use.
AMI, amisulpride; ARI, aripiprazole; CHL, chlorpromazine; FLX, flupenthixol decanoate; FLZ, fluphenazine decanoate; HAL, haloperidol; LAI, long-acting injection; OLA, olanzapine; PAL, paliperidone; PBO, placebo; PIP, pipothiazine palmitate; QUE, quetiapine; RIS, risperidone; SUL, sulpiride; TRI, trifluoperazine; ZIP, ziprasidone; ZUC, zuclopenthixol decanoate.
Only 15 trials (5147 participants) were synthesised for weight gain. Olanzapine produced significantly more weight gain than amisulpride, haloperidol, quetiapine, risperidone, ziprasidone and placebo. Ziprasidone was associated with less weight gain than amisulpride, quetiapine and risperidone (Fig. DS5).
Amisulpride, haloperidol, olanzapine, quetiapine, ziprasidone and paliperidone LAI were not associated with higher rate of glucose intolerance than placebo or as compared with each other. Aripiprazole, paliperidone and paliperidone LAI did not lead to more hyperprolactinaemia than placebo, whereas amisulpride, risperidone and risperidone LAI produced hyperprolactinaemia more often than haloperidol, olanzapine, quetiapine or ziprasidone (Fig. DS6). Additionally, we found no differences between antipsychotics and placebo or among antipsychotics in terms of death or suicide attempt (Fig. DS7).
Meta-regression analysis and sensitivity analysis
Treatment effects in reducing relapse rate did not differ significantly (P>0.05) with length of follow-up, trial setting, quality of the trials, funding source and publication year as assessed in network meta-regressions. When analysing the association between relapse rate and trial duration regardless of the treatment administered, we found that relapse rate was strongly related to nominal trial duration (t=6.00, P<0.0001). Accordingly, we carried out a sensitivity analysis that excluded trials with nominal trial follow-up for <6 months, the difference in relapse prevention between fluphenazine LAI and chlorpromazine became insignificant (OR= 0.39, 95% CI 0.12–1.21). Other treatments except sulpiride excluded from the analysis as its two trials involved brief exposures demonstrated a consistent trend in reducing relapse rate compared with the primary analysis (Fig. DS8a). By excluding trials with relatively high doses of haloperidol (>10 mg/day), the treatment effects in relapse prevention remained largely unchanged (Fig. DS8b). By excluding trials published before 1980, sulpiride and trifluoperazine were excluded from the analysis and other treatments demonstrated a consistent trend in preventing relapse compared with the primary analysis (Fig. DS8c). There was no evidence of publication bias given that comparison-adjusted funnel plot was symmetric around the zero line (Fig. DS9).
Discussion
This study compared effectiveness of 18 antipsychotics in preventing relapses among initially stable patients with schizophrenia and provided evidence-based hierarchies using network meta-analysis. This method can extend existing data derived from traditional pairwise meta-analyses and help to overcome limited availability of head-to-head comparisons of most psychotropic treatments.15 The present findings support the conclusion that maintenance treatment with most clinically available antipsychotics reduced relapse rate in patients with schizophrenia over time, averaging 1 year. Olanzapine was significantly more effective than chlorpromazine or haloperidol, and fluphenazine decanoate yielded significant lower relapse rate than chlorpromazine. Of the top five drugs, ranked by their SUCRA values for relapse prevention, three were LAI preparations: fluphenazine decanoate, olanzapine, paliperidone, pipothiazine palmitate and zuclopenthixol decanoate. These findings corroborate the results of earlier reviews which found olanzapine to be significantly superior to older, typical antipsychotic agents in relapse prevention within schizophrenia,7 and found fluphenazine decanoate to be more effective than several oral antipsychotics.11 Although zuclopenthixol decanoate was associated with apparently particularly favourable effects on relapse prevention, it did not differ statistically from other agents, based on findings of only two trials involving fewer than 40 participants per trial.24,25 Additionally, the apparent superiority of sulpiride and inferiority of trifluoperazine compared with placebo need to be interpreted with caution because of relatively wide CIs associated with their treatment effects.
With respect to readmission rates, olanzapine was also found to be significantly more effective than aripiprazole, haloperidol or quetiapine. Also, ziprasidone was associated with lower readmission rates than aripiprazole, chlorpromazine, haloperidol, quetiapine or trifluoperazine, which were consistent with other evidence of superiority of ziprasidone to some first-generation antipsychotics, at least, in preventing readmission.7
LAI agents tended generally to be less likely to be discontinued prematurely for all causes (intolerability or inefficacy) than oral antipsychotics, but most of these comparisons were not statistically significant. However, zuclopenthixol decanoate was significantly less associated with all-cause discontinuation than quetiapine or trifluoperazine, but it is not clear whether these differences reflect superior benefit or better tolerability. Similarly, olanzapine had significantly lower all-cause discontinuation rates than other agents including haloperidol and quetiapine. As expected, most drugs were associated with less discontinuation because of lack of efficacy than placebo but such differences were not sustained for adverse events.
In general, as expected, older, typical antipsychotics were associated with more EPS than modern, atypical agents or placebo.7 However, this association was driven by only some antipsychotics: fluphenazine decanoate, haloperidol, haloperidol decanoate and trifluoperazine, whereas others, including chlorpromazine, flupenthixol decanoate, pipothiazine palmitate and zuclopenthixol decanoate, were not associated with more EPS than placebo. Among atypical antipsychotics, olanzapine and quetiapine had less risk of EPS than most other modern agents evaluated. However, atypical agents, on average, and especially olanzapine, produced more weight gain than placebo. Olanzapine also produced significantly more weight gain than amisulpride, haloperidol, quetiapine, risperidone and ziprasidone, whereas ziprasidone was associated with less weight gain than amisulpride, quetiapine and risperidone. These findings accord with previous research indicating that olanzapine and quetiapine have low risks of EPS, but are more likely to lead to weight gain and metabolic syndrome,26 whereas ziprasidone had relatively low risks of both EPS and weight gain.27
Amisulpride, aripiprazole, haloperidol, paliperidone, paliperidone palmitate, quetiapine and ziprasidone were associated with similarly low rates of hyperglycaemia as with placebo. Risk of hyperprolactinaemia was highest with amisulpride, risperidone and risperidone LAI, consistent with previous reviews.28,29 Also, there were no significant differences among drugs or between antipsychotics and placebo in rates of suicidal behaviours or death from all causes, although studies with such information were few and their duration averaged less than a year. Finally, treatment discontinuation, in turn, represents a major contribution to risk of relapse, readmission and poor clinical outcomes in psychotic disorders.8–10
This is the first study using network meta-analysis to compare the comparative efficacy and tolerability of antipsychotic monotherapy aimed at reducing rates of relapse or readmission in patients diagnosed with schizophrenia. The method of network meta-analysis can overcome the lack of head-to-head clinical trials in the search for evidence-based hierarchies of comparative treatment effects, provided that the underlying assumptions (notably very close similarity of compared trials) of the method are met.16 We considered 18 antipsychotic drug preparations that are commonly used internationally for maintenance treatment of patients with schizophrenia, and considered several outcome measures related to relapse, including risk of psychiatric hospital admission, which is one of the main contributors to the cost of care in schizophrenia. We also compared drugs for their risks of early discontinuation because of various causes, as well as for adverse events severe enough to produce treatment discontinuation and which tend to counterbalance the benefits of treatment.
The results of this study should be interpreted in the context of its limitations. It is difficult with any data network investigating indirect drug–drug comparisons by network meta-analytic methods to consistently compare perfectly matched trials. In the present analyses, notably the range of treatment exposure time ranged from 1 month to 2 years, criteria for ‘relapse’ varied greatly among trials and were not always matched in the comparisons reported. In addition, 44% (8/18) of the agents were represented in only 2–3 trials and placebo controls were available for only 45% (25/56) of trials. Less certain is how broadly the present findings might pertain to different types of typically heterogeneous patients with chronic psychotic disorders that meet standard diagnostic criteria for schizophrenia. That is, it was not possible with the available data to consider potential effects of such clinical factors as age at onset or current age, years of illness, rates of previous hospital admission, diagnostic subtypes, prominence of particular symptoms, cognitive and social impairment or other functional measures, or even to compare changes in standardised symptom rating scales. Individuals also tend to be more adherent in clinical trials than real-world setting that may diminish the difference between oral and long-acting antipsychotics. Therefore, caution needs to be taken when we interpret the relative effectiveness between oral and long-acting antipsychotics. Most adverse effects reported appear to have arisen from typically passive and incidental reports rather than deliberate, systematic and protocol-guided assessments. Moreover, the findings on adverse events such as glucose intolerance, hyperprolactinaemia and death or suicide attempt need to be interpreted with caution given the limited amount of evidence available. Ideally, a larger and more detailed data-set would allow better examination of their relative effects. It must be pointed out, however, that most of these limitations are found in similar reviews and meta-analyses, be they pairwise or network meta-analyses.
In conclusion, relatively minor differences in relapse prevention were observed among most antipsychotics, although olanzapine and fluphenazine decanoate were associated with particularly lower relapse rates. These relative apparent benefits need to be weighed against the risks of adverse effects of all antipsychotic drugs, notably of weight gain and metabolic syndrome with olanzapine, and EPS with fluphenazine decanoate.
References
- 1.Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The burden of mental disorders. Epidemiol Rev 2008; 30: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen NC. Symptoms, signs, and diagnosis of schizophrenia. Lancet 1995; 346: 477–81. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17: 325–51. [DOI] [PubMed] [Google Scholar]
- 5.Rettenbacher MA, Hofer A, Eder U, et al. Compliance in schizophrenia: psychopathology, side effects, and patients’ attitudes toward the illness and medication. J Clin Psychiatry 2004; 65: 1211–18. [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev 2012; 5: CD008016. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto T, Agarwal V, Kishi T, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry 2013; 18: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002; 63: 892–909. [DOI] [PubMed] [Google Scholar]
- 9.Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv 2007; 58: 1187–92. [DOI] [PubMed] [Google Scholar]
- 10.Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl 2009; 52: S63–7. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull 2014; 40: 192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med 2012; 157: 498–511. [DOI] [PubMed] [Google Scholar]
- 13.Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev 2009; 1: CD000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asenjo Lobos C, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010; 11: CD006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005; 331: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildiz A, Vieta E, Correll CU, Nikodem M, Baldessarini RJ. Critical issues on the use of network meta-analysis in psychiatry. Harv Rev Psychiatry 2014; 22: 367–72. [DOI] [PubMed] [Google Scholar]
- 17.Ziakas PD, Kourbeti IS, Mylonakis E. Systemic antifungal prophylaxis after hematopoietic stem cell transplantation: a meta-analysis. Clin Ther 2014; 36: 292–306 e1. [DOI] [PubMed] [Google Scholar]
- 18.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials 2005; 2: 209–17. [DOI] [PubMed] [Google Scholar]
- 19.Mavridis D, Salanti G. A practical introduction to multivariate meta-analysis. Stat Methods Med Res 2013; 22: 133–58. [DOI] [PubMed] [Google Scholar]
- 20.Jackson D, White IR, Riley RD. A matrix-based method of moments for fitting the multivariate random effects model for meta-analysis and meta-regression. Biom J 2013; 55: 231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Cuzick J, Mesher D, Richmond B, Howell A. Effect of baseline serum vitamin D levels on aromatase inhibitors induced musculoskeletal symptoms: results from the IBIS-II, chemoprevention study using anastrozole. Breast Cancer Res Treat 2012; 132: 625–9. [DOI] [PubMed] [Google Scholar]
- 23.Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry 2010; 167: 686–93. [DOI] [PubMed] [Google Scholar]
- 24.Dencker SJ, Lepp M, Malm U. Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. I. A one year double-blind study of clopenthixol decanoate and flupenthixol palmitate. Acta Psychiatr Scand Suppl 1980; 279: 10–28. [DOI] [PubMed] [Google Scholar]
- 25.Wistedt B, Koskinen T, Thelander S, Nerdrum T, Pedersen V, Molbjerg C. Zuclopenthixol decanoate and haloperidol decanoate in chronic schizophrenia: a double-blind multicentre study. Acta Psychiatr Scand 1991; 84: 14–21. [DOI] [PubMed] [Google Scholar]
- 26.Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Reviews 2010; 3: CD006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komossa K, Rummel-Kluge C, Hunger H, et al. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2009; 4: CD006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010; 123: 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2011; 1: CD006626. [DOI] [PMC free article] [PubMed] [Google Scholar]