Abstract
Background
Low birth weight has been inconsistently associated with risk of developing affective disorders, including major depressive disorder (MDD). To date, studies investigating possible associations between birth weight and bipolar disorder (BD), or personality traits known to predispose to affective disorders such as neuroticism, have not been conducted in large cohorts.
Aims
To assess whether very low birth weight (<1500 g) and low birth weight (1500–2490 g) were associated with higher neuroticism scores assessed in middle age, and lifetime history of either MDD or BD. We controlled for possible confounding factors.
Method
Retrospective cohort study using baseline data on the 83 545 UK Biobank participants with detailed mental health and birth weight data. Main outcomes were prevalent MDD and BD, and neuroticism assessed using the Eysenck Personality Inventory Neuroticism scale - Revised (EPIN-R)
Results
Referent to normal birth weight, very low/low birth weight were associated with higher neuroticism scores, increased MDD and BD. The associations between birth weight category and MDD were partially mediated by higher neuroticism.
Conclusions
These findings suggest that intrauterine programming may play a role in lifetime vulnerability to affective disorders.
Declaration of interest
None.
Copyright and usage
© The Royal College of Psychiatrists 2016. This is an open access article distributed under the terms of the Creative Commons Non-Commercial, No Derivatives (CC BY-NC-ND) licence.
Previous studies have reported associations between low birth weight (under 2500 g) and risk of cardiometabolic disease1 and some psychiatric disorders.2 Associations between low birth weight and bipolar disorder (BD) have not been reported, and associations with personality traits such as neuroticism have only been investigated in small studies that focused on very low birth weight,3 extremely low birth weight4 or specifically preterm participants5 (e.g. range n=71–158).3–5 The hypothesis of a possible causal association with cardiometabolic disease and psychiatric disorders has been variously termed the ‘Barker hypothesis’, the ‘foetal origins hypothesis’ or the ‘developmental origins of adult health and disease hypothesis’.6 This hypothesis suggests that as a result of fetal plasticity, the fetus is able to undergo physiological adaptations in response to an adverse intrauterine environment; for example, poor early nutrition may adjust glucose–insulin metabolism to maximise possible fitness. Whereas this may potentially protect against early mortality, it may have negative sequelae in later life, including susceptibility to some chronic diseases.7
Low birth weight and MDD
A recent meta-analysis which pooled assessments of the association between low birth weight and risk of major depressive disorder (MDD) derived in 18 separate studies, comprising a total of 59 442 participants, did not find evidence for an association once publication bias was taken into account.2 The authors noted significant heterogeneity between studies, including wide age ranges of participants, different outcome measures, inadequate follow-up rates and low power. Studies also often failed to control for important potential confounders such as maternal smoking or maternal MDD, socioeconomic status, gestational age, gender and family history of MDD, and some studies included extremely low birth weight (<1000 g) participants from specialist hospital environments.2 We aimed to add to the literature in this area by testing for an association between low/very low birth weight and risk of MDD (in adulthood) using the data collected at recruitment of a large population cohort (UK Biobank). We were also able to assess the associations between birth weight and neuroticism scores, and risk of BD in adulthood. Neuroticism is recognised as a risk factor for MDD and for psychopathology in general,8 with individuals who score highly on neuroticism characterised as having a tendency to be less emotionally stable, more anxious and more reactive to negative events.9
Current study
UK Biobank recruited 502 649 participants aged around 40–70 years from the general population between 2006 and 2010.10 Participants completed a touchscreen questionnaire that included demographic information, questions on physical health, recalled birth weight, and a battery of cognitive and mental health inventories.
We investigated the association between birth weight and each of: neuroticism, MDD and BD. The advantages of using the UK Biobank cohort for this assessment include the large sample size, extensive information on possible covariates/confounders (e.g. maternal smoking) and mediators (e.g. cardiovascular disease), and consistent measurement in terms of outcome variables (compared with meta-analysis, which pools studies with different approaches).
The objectives of this study were: to test for an association between birth weight category and each outcome; to determine whether there was evidence of a dose-effect across very low (<1500 g), low (1500–2490 g) and normal (≥2500 g) birth weight; to determine whether any associations persisted after adjustment for potential confounding variables; and to explore whether physical health and neuroticism had possible mediating roles in the associations with MDD and BD, using formal tests of mediation for the latter.11,12
Method
All participants attended 1 of 22 assessment centres. Participants were asked to report their birth weight (either in kilograms directly, or in pounds and ounces and converted to kilograms to two decimal points) and whether they had any physical disorders, including hypertension, heart/cardiac problems, stroke, peripheral vascular disease, type 2 diabetes, chronic obstructive pulmonary disease (COPD) and osteoporosis. Participants self-reported whether their mothers had ever had MDD (at any time), and whether their mothers smoked around the time of pregnancy. We coded ‘White’ ethnicity for participants who reported themselves at assessment as ‘White’, ‘White-British’, ‘White-Irish’ or ‘Any other White background’,13 and Black and minority ethnic for the remainder. Townsend scores were obtained from postcode of residence. They are an area-based index of socioeconomic deprivation derived from census data on car ownership, household overcrowding, owner-occupation and unemployment.14 Higher Townsend scores equate to greater socioeconomic deprivation.
Neuroticism was assessed with 12 questions from the Eysenck Personality Inventory Neuroticism scale - Revised (EPIN-R15,16) and the 172 751 participants recruited in the past 2 years were asked to provide more detailed information on lifetime experience of mood disorder features and were grouped into probable MDD or probable BD (or not) based on a structured classification which we have previously described in detail.16 Briefly, current and past depressive features were assessed by items relating to lifetime experience of minor/major depression, items from the Patient Health Questionnaire (PHQ) and items related to mental health help-seeking.16,17 Probable history of BD was based on questions in the baseline self-report assessment which were analogous to questions within the Structured Clinical Interview for DSM-IV Disorders (SCID-118). If participants had probable BD and also probable MDD, they were classified as BD only.
This study was conducted under generic approval from the NHS National Research Ethics Service (approval letter ref 11/NW/0382).
Statistical analyses
For the analyses of birth weight and neuroticism, we used linear regression. Neuroticism scores were positively skewed, and log/square root transformations did not improve the distribution; we report linear regression statistics because the final results were not meaningfully different from Spearman non-parametric correlations (not shown) but are easier to interpret, and assumptions of normality are to an extent eased in very large data-sets.19 Because the MDD and BD groups had significantly higher neuroticism scores, as would be expected (both P<0.001), we removed participants with either of these disorders from the neuroticism analyses.
We used Poisson regression to estimate relative risk (RR) ratios for the MDD and BD analyses in relation to birth weight. RR ratios are preferable to odds ratios when the outcome is not rare (i.e. >10%) in the population, as odds ratios are poor estimates of RRs in such circumstances, and are harder to interpret.20 P-values less than 0.05 were considered statistically significant. All analyses were conducted with IBM SPSS V.22, except for Fig. 1 which was made by using STATA SE v.13.
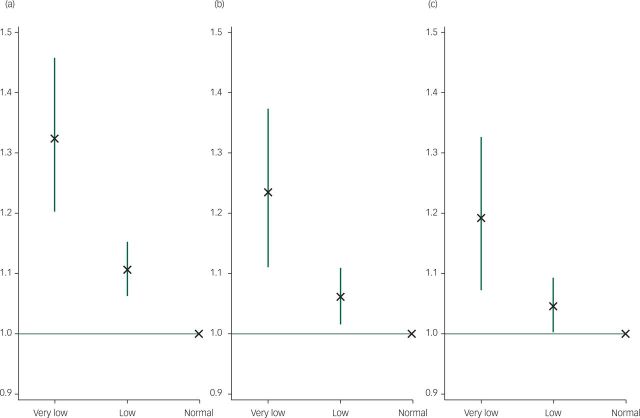
Fig. 1. Relative risk ratios and 95% confidence intervals for very low/low birth weight groups (v. normal weight) and probable major depressive disorder. Normal birth weight group = referent y-axis line.
(a) Unadjusted. (b) Adjusted for year of birth, Townsend deprivation score, ethnicity, gender, maternal smoking and maternal depression (i.e. covariates). (c) Additionally adjusted for hypertension, heart/cardiac problems, stroke, perivascular disease, type-2 diabetes, chronic obstructive pulmonary disease and osteoporosis (i.e. physical disease mediators).
We found a positive association between year of birth and birth weight in kilograms, controlling for maternal smoking and maternal depression (r=0.01, P<0.001) suggesting a possible birth cohort effect. We tested each association unadjusted for covariates, and then adjusted for possible confounding variables: year of birth (i.e. controlling for possible age and birth cohort effects), Townsend deprivation score, White v. Black and minority ethnic,gender, maternal smoking and maternal depression. Finally, because physical disorders can be risk factors for psychiatric disorders21 and are, therefore, potential mediators, for the MDD and BD analyses we added the covariates of hypertension, cardiac disease, stroke, peripheral vascular disease, type 2 diabetes, COPD and osteoporosis. For each model, we first tested for an overall deleterious very low>low>normal birth weight dose effect, and then for an effect of birth weight group (very low/low) v. normal weight as the referent category.
We formally tested whether significant birth weight and mood disorder associations were mediated by trait neuroticism scores, using the PROCESS macro.12 Briefly, the effect of a predictor variable (e.g. birth weight group) on an outcome variable (e.g. MDD) can be either direct or indirect via a mediator (e.g. neuroticism). In a mediation context, a three-way association has two products: ‘Path A’: the association between the predictor and mediator (e.g. birth weight and neuroticism), and ‘Path B’: the mediator and outcome association adjusted for the predictor (e.g. association for neuroticism and MDD adjusted for birth weight). The indirect effect is the combined product of these two paths.11,12 We used the PROCESS macro ‘Model 4’, which allows for dichotomous independent variables/outcomes in mediation models (bootstrap n=1000, bias-corrected).
We excluded all participants who had requested withdrawal from the UK Biobank as of March 2015. As a check, we tested for differential recall of birth weight (i.e. data present v. not reported) by disease status: i.e. whether participants with probable MDD/BD or higher neuroticism were more likely to recall their birth weight; a potential source of bias.
Results
We limited analysis to the participants with mood disorder data (n=172 751) who also had birth weight data available (final n=83 545). Table 1 shows descriptive statistics (including prevalence rates) stratified by birth weight category. Overall, 931 (1.1%) participants had very low birth weight, 7320 (8.8%) low birth weight and 75 294 (90.1%) had normal birth weight. The normal birth weight category included 4113 participants with high birth weight (4500 g) which can be a risk factor for cardiovascular diseases in later life.22 Exclusion of these participants did not alter the findings. Therefore, they were included in the normal birth weight category in all analyses. We additionally tested for a linear effect of birth weight in normal range participants (2500–4500 g), and report these associations in Tables 2 and 3; however, it is worth noting that this model assumes a linear association which may not be the case with these phenotypes.
Table 1. Clinical and demographic characteristicsa.
| Total N | Very low birth weight (<1500 g) n=931 | Low birth weight (1500–2490 g) n=7320 | Normal birth weight (≥2500 g) n=75 294 | P | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 83 545 | 56.70 (8.07) | 56.56 (8.00) | 55.33 (8.24) | <0.001 |
| Male, n (%) | 83 545 | 327 (35.1) | 2103 (28.7) | 31 090 (41.3) | <0.001 |
| Black and minority ethnic, n (%) | 83 324 | 48 (5.2) | 427 (5.9) | 3278 (4.4) | <0.001 |
| Neuroticism score, mean (s.d.) | 71 006 | 4.47 (3.26) | 4.18 (3.23) | 3.94 (3.18) | <0.001 |
| Townsend deprivation score, mean (s.d.) | 83 408 | −0.67 (3.16) | −1.18 (2.91) | −1.38 (2.81) | <0.001 |
| Depression, n (%) | 83 545 | 288 (31.2) | 1897 (26.1) | 17 650 (23.6) | <0.001 |
| Bipolar disorder, n (%) | 83 545 | 16 (1.7) | 87 (1.2) | 791 (1.1) | 0.085 |
| Maternal smoking, n (%) | 74 966 | 326 (40.5) | 2317 (35.8) | 18 687 (27.6) | <0.001 |
| Maternal depression, n (%) | 80 197 | 74 (8.6) | 498 (7.1) | 4578 (6.3) | 0.001 |
| Hypertension, n (%) | 83 545 | 327 (35.1) | 2115 (28.9) | 16 984 (22.6) | <0.001 |
| Cardiac disease, n (%) | 83 545 | 6 (0.6) | 22 (0.3) | 205 (0.3) | 0.095 |
| Stroke, n (%) | 83 545 | 25 (2.7) | 89 (1.2) | 732 (1.0) | <0.001 |
| Peripheral vascular disease, n (%) | 83 545 | 1 (0.1) | 6 (0.1) | 40 (0.1) | 0.490 |
| Type 2 diabetes, n (%) | 83 545 | 18 (1.9) | 60 (0.8) | 397 (0.5) | <0.001 |
| COPD history, n (%) | 83 545 | 1 (0.1) | 27 (0.4) | 202 (0.3) | 0.180 |
| Osteoporosis, n (%) | 83 545 | 26 (2.8) | 168 (2.3) | 1120 (1.5) | <0.001 |
COPD, chronic obstructive pulmonary disease.
Note that total N values vary due to missing data in some instances.
Table 2. Association between birth weight and neuroticism in normal range participants (2500–4500 g).
| Birth weight, g | ||
|---|---|---|
| b (95% CI) | P | |
| Neuroticism | ||
| Unadjusted (n=46 110) | –0.02 (–0.22 to –0.09) | <0.001 |
| Adjusted for potential confoundersa (n=41 800) | –0.01 (–0.10 to 0.03) | 0.328 |
Adjusted for year of birth, Townsend deprivation score, ethnicity, gender, maternal smoking and maternal depression.
Table 3. Features of depression and bipolar disorder in normal range participants (2500–4500 g).
| Relative risk ratios (95% CI) | P | |
|---|---|---|
| Depression | ||
| Unadjusted (n=83 172) | 0.93 (0.91–0.95) | <0.001 |
| Adjusted for potential confoundersa (n=71 591) | 0.98 (0.96–1.00) | 0.074 |
| Adjusted for potential physical disease mediatorsb (n=71 591) | 0.99 (0.97–1.01) | 0.387 |
| Bipolar disorder | ||
| Unadjusted (n=83 545) | 0.93 (0.84–1.03) | 0.187 |
| Adjusted for potential confoundersa (n=71 953) | 0.91 (0.82–1.02) | 0.091 |
| Adjusted for potential physical disease mediatorsb (n=71 953) | 0.93 (0.83–1.03) | 0.160 |
Adjusted for year of birth, Townsend deprivation score, ethnicity, gender, maternal smoking and maternal depression.
Additionally adjusted for hypertension, heart/cardiac problems, stroke, perivascular disease, type-2 diabetes, chronic obstructive pulmonary disease and osteoporosis.
Neuroticism
As shown in Table 4, there was a significant dose effect (very low>low>normal) across the birth weight categories in terms of neuroticism scores, for both the unadjusted (P<0.001) and adjusted models (P=0.002). Compared with the normal birth weight group, there was a significant association between low birth weight and higher neuroticism scores, in the unadjusted model (unstandardised b=0.21, 95% CI 0.11–0.30, P<0.001). This association persisted after adjustment for possible confounding variables (b=0.23, 95% CI 0.13–0.33, P<0.001). The magnitudes of the associations were greater for very low birth weight in the unadjusted (b=0.27, 95% CI 0.11–0.52, P=0.041) and adjusted models (b=0.31, 95% CI 0.03–0.60, P=0.031).
Table 4. Association between low and very low birth weight and neuroticism.
| Low birth weight | Very low birth weight | ||||
|---|---|---|---|---|---|
| b (95% CI) | P | b (95% CI) | P | Overall dose P | |
| Neuroticism | |||||
| Unadjusted (n = 48 835) | 0.21 (0.11–0.30) | <0.001 | 0.27 (0.11–0.52) | 0.041 | <0.001 |
| Adjusted for potential confoundersa (n = 46 961) | 0.23 (0.13–0.33) | <0.001 | 0.31 (0.03–0.60) | 0.031 | 0.002 |
Adjusted for year of birth, Townsend deprivation score, ethnicity, gender, maternal smoking and maternal depression.
Probable MDD
There was a significant overall birth weight dose effect for MDD rates in the unadjusted and confounder-adjusted models (P<0.001) Table 5. Compared with the normal birth weight group there was a significant association between low birth weight and MDD in the unadjusted model (RR=1.11, 95% CI 1.06–1.15, P<0.001). This was attenuated but remained significant after adjusting for potential confounders (RR=1.06, 95% CI 1.02–1.11, P=0.007). Adjustment for potential mediating physical diseases produced little attenuation (RR=1.05, 95% CI 1.00–1.09, P=0.041). There was a stronger association with very low birth weight in both the unadjusted (RR=1.32, 95% CI 1.20–1.46, P<0.001) and adjusted models (RR=1.24, 95% CI 1.11–1.37, P<0.001). Adjustment for potential physical disease mediators produced greater attenuation than for low birth weight (RR=1.19, 95% CI 1.07–1.33, P=0.001). All RRs are shown in Fig. 1.
Table 5. Association between low and very low birth weight and features of depression and bipolar disordera.
| Low birth weight | Very low birth weight | ||||
|---|---|---|---|---|---|
| Relative risk ratios (95% CI) | P | Relative risk ratios (95% CI) | P | Overall dose P | |
| Depression | |||||
| Unadjusted (n=83 127) | 1.11 (1.06–1.15) | <0.001 | 1.32 (1.20–1.46) | <0.001 | <0.001 |
| Adjusted for potential confoundersb (n=71 591) | 1.06 (1.02–1.11) | 0.007 | 1.24 (1.11–1.37) | <0.001 | <0.001 |
| Adjusted for potential physical disease mediatorsc(n=71 591) | 1.05 (1.00–1.09) | 0.041 | 1.19 (1.07–1.33) | 0.001 | 0.001 |
| Bipolar disorder | |||||
| Unadjusted (n=83 545) | 1.13 (0.91–1.41) | 0.272 | 1.64 (1.00–2.67) | 0.049 | 0.048 |
| Adjusted for potential confoundersb (n=71 935) | 1.13 (0.89–1.44) | 0.332 | 1.74 (1.05–2.87) | 0.032 | 0.047 |
| Adjusted for potential physical disease mediatorsc(n=71 935) | 1.10 (0.86–1.40) | 0.436 | 1.63 (0.98–2.69) | 0.058 | 0.089 |
All risk ratio statistics are relative to the normal birth weight group, except for the overall dose P-value which refers to an ordinal dose effect (i.e. very low>low>normal birth weight).
Adjusted for year of birth, Townsend deprivation score, ethnicity, gender, maternal smoking and maternal depression.
Additonally adjusted for hypertension, heart/cardiac problems, stroke, perivascular disease, type 2 diabetes, chronic obstructive pulmonary disease and osteoporosis.
In Fig. 2, we show the associations (in terms of beta coefficients and RRs) between birth weight group and mood disorder, before and after adjustment for neuroticism scores. This figure shows that the associations between low and very low birth weight and probable depression, expressed in RRs respectively attenuate by 44% (unadjusted RR=1.09, to adjusted RR=1.05) and by 31% (RR=1.36, to 1.25) when adjusted for neuroticism, although both remained significant. (Note that the RRs are very slightly different in the mediation model because the macro includes participants with complete birth weight/neuroticism/mood data.) Formal tests of mediation showed neuroticism significantly mediated the birth weight/MDD association with both low birth weight (v. normal; indirect coefficient=0.05, 95% CI 0.04–0.07) and very low birth weight (v. normal; indirect coefficient=0.06, 95% CI 0.03–0.09). Raw mediation model statistics are provided in Table 6.11,23
Fig. 2. Three-way associations between low/very low birth weight (v. normal weight), neuroticism and probable major depressive disorder.
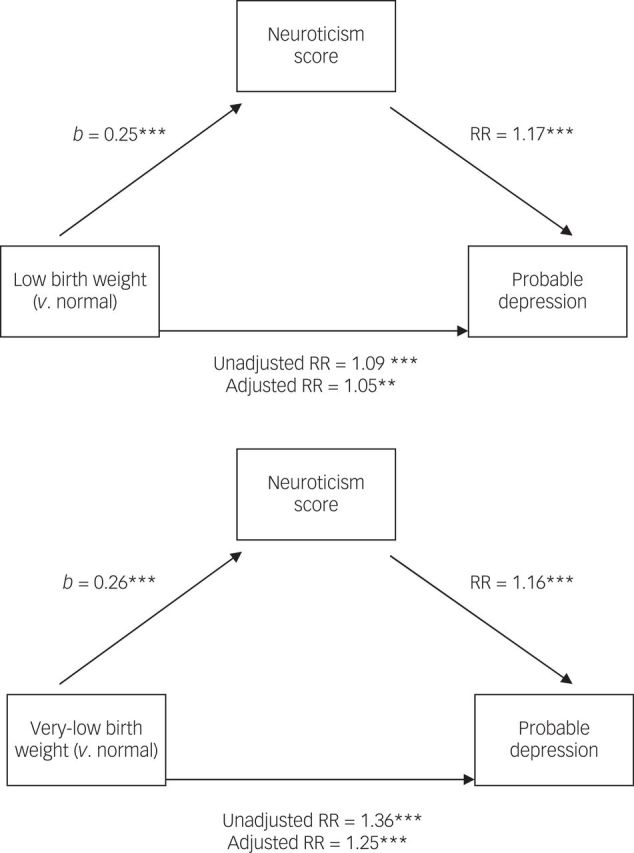
Values in brackets are relative risk (RR) ratio statistics, before controlling for neuroticism scores. ***P<0.001, **P<0.01. b = unstandardised beta coefficient. Adjusted RR = neuroticism scores included the model. A full description of the mediation process is provided by Preacher & Hayes.11
Table 6. Raw mediation statistics.
| Estimate (b) | s.e. | P-value | 95% CI (lower) | 95% CI (upper) | |
|---|---|---|---|---|---|
| Low v. normal birth weight | |||||
| Model without mediator | |||||
| Intercept | −1.18 | 0.01 | <0.001 | −1.20 | −1.16 |
| Low birth weight → MDD (Path c) | 0.11 | 0.03 | <0.001 | 0.05 | 0.17 |
| Model with mediator | |||||
| Intercept | −2.17 | 0.02 | <0.001 | −2.21 | −2.14 |
| Low birth weight → neuroticism (Path a) | 0.25 | 0.04 | <0.001 | 0.16 | 0.33 |
| Neuroticism → MDD (Path b) | 0.22 | 0.00 | <0.001 | 0.22 | 0.23 |
| Low birth weight → MDD (Path c’) | 0.07 | 0.03 | 0.040 | 0.00 | 0.13 |
| Indirect effect (Paths a * b) | 0.05 | 0.01 | – | 0.04 | 0.07 |
| Very low v. normal birth weight | |||||
| Model without mediator | |||||
| Intercept | −1.18 | 0.01 | <0.001 | −1.20 | −1.16 |
| Very low birth weight → MDD (path c) | 0.21 | 0.04 | <0.001 | 0.14 | 0.29 |
| Model with mediator | |||||
| Intercept | −2.18 | 0.02 | <0.001 | −2.12 | −2.14 |
| Very low birth weight → neuroticism (Path a) | 0.26 | 0.06 | <0.001 | 0.15 | 0.38 |
| Neuroticism → MDD (Path b) | 0.22 | 0.00 | <0.001 | 0.22 | 0.23 |
| Very low birth weight → MDD (Path c’) | 0.18 | 0.04 | <0.001 | 0.10 | 0.26 |
| Indirect effect (Paths a * b) | 0.06 | 0.01 | – | 0.03 | 0.09 |
Path a: association between predictor and mediator (i.e., low/very low birth weight → neuroticism).
Path b: the mediator→ outcome association (neuroticism → MDD) adjusted for the predictor (birth weight).
Path c: the ‘total effect’ of the predictor → outcome (low/very low birth weight → MDD).
Indirect effect: the product of Path a * Path b.
Probable BD
There was a significant overall dose effect of lower birth weight in the unadjusted (P=0.048) and confounder-adjusted model (P=0.047), which attenuated when adjusted for potential mediators (P=0.089). There was no evidence of association between low v. normal birth weight group and BD rates, in any of the unadjusted models based on P-values. For the very low v. normal birth weight analyses, there was a significant association in the unadjusted model (RR=1.64, 95% CI 1.00–2.67, P=0.049), which remained significant in the confounder-adjusted model (RR=1.74, 95% CI 1.05–2.87, P=0.032) (Table 5). Following inclusion of the potential mediators as covariates, the association was attenuated and no longer statistically significant (P>0.05). Because of this, we did not investigate neuroticism as a mediator of the very low birth weight/BD association.
Birth weight recall bias
To assess whether there was differential ability to recall birth weight – i.e. a reporting/selection bias – we tested for association between likelihood of reporting birth weight v. not and higher rates of MDD/BD/neuroticism scores. Participants who provided birth weight data were significantly more likely to have probable MDD (RR=1.11, 95% CI 1.09–1.13, P<0.001) but not BD (P>0.05). In healthy participants (i.e. no MDD or BD), those who provided birth weight data had significantly lower neuroticism (b=−0.16, 95% CI −0.20 to −0.13, P<0.001).
Discussion
Main findings
Birth weight was significantly associated with neuroticism, MDD and BD in 83 545 adults recruited from the general population. There were clear trends whereby the risk of all three increased with reducing birth weight, from normal to very low. Tests of mediation showed that the association between birth weight and MDD was partially mediated by its association with neuroticism.
We found a significant association between low birth weight and higher neuroticism, which survived adjustment for relevant covariates. There was a significant dose effect, such that lower birth weight was associated with higher neuroticism in adulthood (i.e. very low>low>normal). The birth weight/neuroticism associations were characterised by relatively small effect sizes: around a 0.20–0.30 increase in neuroticism scores (out of 12) for low/very low v. normal birth weight. Given the large sample size, statistical significance may not be clinically meaningful, in terms of being a risk factor for MDD/BD.
We found a significant association between low/very low birth weight and increased risk of MDD. This survived adjustment for potential confounding variables and physical disorder history. There appeared to be dose effect, with lower birth weight being associated with higher MDD rates. We found a similar dose effect association for BD. When we separately contrasted very low and low birth weight categories with normal birth weight, we found a significant association between very low birth weight and risk of BD in the unadjusted and confounder- but not mediator-adjusted model (i.e. additionally corrected for physical disorders). Note that sample sizes for the BD analyses were relatively small, and this may contribute to non-significant P-values where the relevant effect sizes are similar or even stronger than in the MDD results.
Interpretation
Publication bias was considered previously to have contributed to over-representation of associations between lower birth weight and MDD/depressive symptoms. Wojcik et al 2 reported a relatively weak association between lower birth weight and risk of MDD or ‘psychological distress’, which did not survive adjustment for possible publication bias (OR=1.08, P>0.05). The current report has several strengths relative to Wojcik et al's meta-analysis: a large sample size, greater than the combined total in the studies summarised by Wojcik et al, a consistent classification method for probable MDD/BD and detailed information of possible confounders. It therefore contributes a large amount of high-quality data to the literature. The fact that the associations reported here between MDD and birth weight survived adjustment for a history of physical disorder known to be associated with low birth weight6,7 suggests that the association is not entirely mediated through low birth weight leading to increased risk of physical disorders, with subsequent depression as a complication.
Our results in terms of neuroticism are relatively novel in that they represent the first demonstration of association between lower birth weight and higher neuroticism in a large sample, several orders of magnitude greater than previous reports,3–5 although the effect size is small. It will be important to replicate these findings in large independent cohorts. Note that low birth weight was only moderately associated with MDD, BD and higher neuroticism, which might be because of measurement error (i.e. from errors in birth weight recall), and the fact that low birth weight is only a blunt indication of possible problems during fetal development.24 We had no information on pregnancy complications or gestational age at birth, which may also contribute to the association between birth weight and deleterious outcomes;2 we therefore cannot identify low birth weight because of preterm in this report.
Limitations and future research
The birth weight data in UK Biobank were obtained by asking the participants to recall it in late adulthood. Whereas actual recorded data would be preferable as recall will be subject to inherent noise/error – Inskip et al 24 showed that Bland-Altman plots revealed reasonable agreement between the recalled weight (at assessment) and recorded (hospital record-based) birth weights in 1729 women (Spearman rank r=0.87), although this was in young women. This nonetheless suggests that recalled birth weight is reasonably accurate; when people can recall their birth weight, their recall is quite good, but many, of course, were excluded from these analyses as their birth weight was unknown. It is possible that certain recall biases influence our final results; for example, perhaps participants with chronic illnesses are more likely to recall information such as maternal depression or smoking. There was a significant association between likelihood of reporting birth weight (v. not) and probable MDD/BD; however, this was modest.
The present study corrects for several limitations compared with the recent meta-analysis. However, there are additional variables that were not accounted for, including gestational age, maternal socioeconomic status and pregnancy-related complications such as preterm birth. The classification of probable MDD/BD was based on self-report data which may be subject to under-reporting. The current study does not take into account genetic factors that may contribute to low birth weight or mood disorder, potentially important variables such as parent's socioeconomic status, or whether parent's attitudes or behaviours are different for children with low birth weight. We did not control for multiple births which may contribute to the prevalence of low birth weight in the sample; however, we are not aware of multiple pregnancies being a risk factor for MDD/neuroticism independent of any effect of low birth weight, and it therefore might not act as a confounder.
Participants with lifetime histories of depression may have been less likely to participate in UK Biobank research, and this may interact with other variables such as lower socioeconomic status; the sample may not be entirely representative of psychiatric disease prevalence in a range of different backgrounds. Additionally, developmental problems (such as low birth weight) may result in a bias where the most impaired participants do not reach older age or participate in research. We found a small but significant bias where participants who reported birth weight data had higher prevalence of MDD. This may reflect a degree of selection bias where the participants that did not report birth weight data may have a weaker weight/MDD association, which may partly attenuate the relatively modest associations reported here.
Final summary
We have found significant associations between low/very low birth weight and higher neuroticism and increased risk of MDD and BD. In terms of MDD, our findings contribute significantly to the literature, supporting the association reported in a recent meta-analysis,2 which may have been somewhat weakened by heterogeneity between the 18 studies that were included. Our study however did not control for pregnancy-related issues such as preterm births (which may account for a degree of the association) and future studies should attempt to take account of this. Our findings support the hypothesis that fetal and early life factors may have long-term effects on health across a broad range of outcomes, including mental health outcomes, and lend support to initiatives which target improved maternal health as a means for improving the future health of offspring.25,26
Acknowledgements
This research has been conducted using the UK Biobank resource; we are grateful to UK Biobank participants. UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. The funders had no role in study design, data collection or management, analyses or interpretation of the data, nor preparation, review or approval of the manuscript.
References
- 1.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008; 300: 2886–97. [DOI] [PubMed] [Google Scholar]
- 2.Wojcik W, Lee W, Colman I, Hardy R, Hotopf M. Foetal origins of depression? A systematic review and meta-analysis of low birth weight and later depression. Psychol Med 2012; 43: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesonen AK, Räikkönen K, Heinonen K, Andersson S, Hovi P, Järvenpää AL, et al. Personality of young adults born prematurely: the Helsinki study of very low birth weight adults. J Child Psychol Psychiatry Allied Discip 2008; 49: 609–17. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt LA, Miskovic V, Boyle MH, Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics 2008; 122: e181–7. [DOI] [PubMed] [Google Scholar]
- 5.Allin M, Rooney M, Cuddy M, Wyatt J, Walshe M, Rifkin L, et al. Personality in young adults who are born preterm. Pediatrics 2006; 117: 309–16. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJP. The origins of the developmental origins theory. J Int Med 2007; 261: 412–7. [DOI] [PubMed] [Google Scholar]
- 7.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008; 105: 17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke D. Neuroticism: moderator or mediator in the relation between locus of control and depression? Pers Individ Dif 2004; 37: 245–58. [Google Scholar]
- 9.Matthews G, Deary IJ, Whiteman MC. Personality Traits. Cambridge University Press, 2009. [Google Scholar]
- 10.Collins R. What makes UK Biobank special? Lancet 2012; 379: 1173–4. [DOI] [PubMed] [Google Scholar]
- 11.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods 2008; 40: 879–91. [DOI] [PubMed] [Google Scholar]
- 12.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol 2013; 67: 457–70. [DOI] [PubMed] [Google Scholar]
- 13.Tyrrell JS, Yaghootkar H, Freathy RM, Hattersley AT, Frayling TM. Parental diabetes and birthweight in 236 030 individuals in the UK Biobank study. Int J Epidemiol 2013; 42: 1714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend P. Townsend deprivation index. J Soc Policy 1987; 16: 125–46. [Google Scholar]
- 15.Eysenck HJ, Eysenck SBG. Manual for the Eysenck Personality Questionnaire. (EPQ-R Adult). Hodder & Stoughton, 1994. [Google Scholar]
- 16.Smith DJ, Nicholl BI, Cullen B, Martin D, Ul-Haq Z, Evans J, et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK Biobank: cross-sectional study of 172,751 participants. PLoS One 2013; 8: e75362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer RL, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA 1999; 282: 1737–44. [DOI] [PubMed] [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). American Psychiatric Press, 1996. [Google Scholar]
- 19.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Ann Rev Publ Health 2002; 23: 151–69. [DOI] [PubMed] [Google Scholar]
- 20.Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med 2009; 163: 438–45. [DOI] [PubMed] [Google Scholar]
- 21.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J 2013; 35: 1365–72. [DOI] [PubMed] [Google Scholar]
- 22.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ 1994; 308: 942–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods 2011; 16: 93–115. [DOI] [PubMed] [Google Scholar]
- 24.Inskip HM, Dunn N, Godfrey KM, Cooper C, Kendrick T. Is birth weight associated with risk of depressive symptoms in young women? Evidence from the Southampton Women's Survey. Am J Epidemiol 2008; 167: 164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. J Health Econ 2005; 24: 365–89. [DOI] [PubMed] [Google Scholar]
- 26.Campbell F, Conti G, Heckman JJ, Moon SH, Pinto R, Pungello E, et al. Early childhood investments substantially boost adult health. Science 2014; 343: 1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]