Abstract
Intestinal radiation toxicity occurs during and after abdominopelvic radiotherapy. Endothelial cells play a significant role in modulating radiation-induced intestinal damage. We demonstrated that the endothelial cell surface receptor thrombomodulin (TM), a protein with anticoagulant, antiinflammatory and antioxidant properties, mitigates radiation-induced lethality in mice. The goal of this study was to determine whether recombinant TM (Solulin) can protect the intestine from toxicity in a clinically relevant rat model. A 4 cm loop of rat small bowel was exposed to fractionated 5 Gy X radiation for 9 consecutive days. The animals were randomly assigned to receive daily subcutaneous injections of vehicle or Solulin (3 mg/kg/day or 10 mg/kg/day) for 27 days starting 4 days before irradiation. Early intestinal injury was assessed two weeks after irradiation by quantitative histology, morphometry, immunohistochemistry and luminol bioluminescence imaging. Solulin treatment significantly ameliorated intestinal radiation injury, made evident by a decrease in myeloperoxidase (MPO) activity, transforming growth factor beta (TGF-β) immunoreactivity, collagen-I deposition, radiation injury score (RIS) and intestinal serosal thickening. These findings indicate the need for further development of Solulin as a prophylactic and/or therapeutic agent to mitigate radiation-induced intestinal damage.
Introduction
Intestinal radiation toxicity is a major dose-limiting factor after therapeutic abdominopelvic irradiation. It is generally assumed that radiation-induced injury to the intestinal epithelium is primarily responsible for early radiation enteropathy, whereas late radiation enteropathy is due to vascular damage and/or killing of slowly proliferating target cells. However, the pathogenesis of radiation enteropathy is not completely understood and is considered to be the result of the interaction among various cell types and soluble mediators (1). Also, it has been shown that early radiation enteropathy is associated with long-term complications (2, 3). Therefore, limiting early intestinal radiation damage would conceivably also lower the risk of delayed toxicity, thereby improving the quality of life for cancer survivors and reducing the global health and economic burden.
Radiation-induced damage to the vascular endothelium, a sheet of single-layer endothelial cells that line the inner wall of all blood and lymphatic vessels, has been shown to play important roles in the pathogenesis of both early and delayed radiation enteropathy (4). However, it is unclear how radiation-damaged endothelial cells contribute to the development of early radiation toxicity. Wang et al. proposed that radiation-induced endothelial dysfunction leads to inflammation, oxidative stress and enhanced TGF-β production, which in turn, suppress epithelial cell proliferation, causing depletion of epithelial cells, and finally resulting in breakdown of the epithelial barrier (4). Other experimental findings demonstrate that safeguarding endothelial cells from radiation injury confers protection of the intestinal epithelium (5, 6), indicating the potential role of endothelial cells in the pathogenesis of intestinal toxicity.
Endothelial thrombomodulin (TM), a multi-domain transmembrane receptor protein, has been shown to exert an array of beneficial biological effects on the vasculature because of its anti-inflammatory, cytoprotective, antifibrinolytic, antioxidant and anticoagulant properties (7). Our results from systemic administration of a soluble form of recombinant thrombomodulin, Solulin, or its downstream mediator, activated protein C (APC), support these findings. Solulin is comprised of the extracellular portion of TM (an N-terminal lectin-binding domain, 6 EGF-like repeats and a serine/threonine-rich domain), but lacks the transmembrane and intracellular domains and the chondroitin sulfate moiety. We also found wild-type mice to be less susceptible to radiation-induced lethality than mutant mice deficient in TM function (8), consistent with the notion that TM plays a critical role in modifying radiation response. Moreover, Solulin is effective in preventing a variety of other pathophysiological conditions including acute ischemic stroke (9), thrombin-mediated astrocyte activation (10) and middle cerebral artery occlusion (11).
The current study demonstrates that exogenous Solulin administration protects the intestine from structural and molecular radiation damage. A possible mechanism may be that Solulin forms a complex with the coagulation factor, thrombin, which activates protein C (APC). APC attenuates the intrinsic coagulation cascade by inhibiting factors Va and VIIIa, thereby limiting further thrombin generation (12). Studies from our laboratory demonstrate that thrombin inhibition by anticoagulant treatment suppresses radiation damage in rat intestine (13), and that recombinant APC administration provides radiation lethality protection in mice (8). In addition, the N-terminal lectin-like domain of Solulin and APC have antioxidant and anti-inflammatory properties (14–17), which are well known for their ability to counteract radiation injury. Solulin warrants further development as a protector against the adverse effects of clinical radiation therapy and as a mitigator of radiation toxicity from nuclear accidents and radiological terrorism.
Materials and Methods
Animals and Experimental Radiation Enteropathy Model
A total of 36 male Sprague-Dawley rats (200–250 g) were purchased from Envigo (Indianapolis, IN) and housed in conventional cages in a pathogen-free environment with controlled humidity, temperature and 12:12 h light-dark schedule with free access to tap drinking water and chow (cat. no. TD8640; Harlan Teklad, Madison, WI). The experimental protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee (IACUC).
The surgical model for localized small bowel irradiation was prepared as described previously (18). Briefly, rats were fasted overnight, anesthetized and orchiectomized. A loop of ileum was sutured to the inside of the scrotum. This model creates a “scrotal hernia” that contains a 4 cm loop of small intestine that can be irradiated locally without significant radiation exposure to other tissues, while the intestine remains functional and within the abdominal cavity. This model minimizes manipulation during irradiation and produces radiation-induced changes similar to those seen clinically. The surgical procedure itself does not cause structural, functional or cellular changes in the intestine.
After three weeks postoperative recovery, rats were anesthetized with isoflurane, and the transposed bowel segment within the “scrotal hernia” was sham irradiated or irradiated once daily with 5.0 Gy delivered in 9 daily fractions for 9 days using a Seifert Isovolt 320 X-ray machine (Seifert X-Ray Corporation, Fairview Village, PA), operated at 250 kVp and 15 mA, with 3 mm of added aluminum filtration. The resulting half-value layer was 0.85 mm copper, and the dose rate was 4.49 Gy/min. The radiation regimen was based on data from previous experiments and was designed to elicit moderate to severe radiation enteropathy (18).
Solulin
Solulin (soluble human recombinant TM), was provided by PAION Deutschland GmbH (Aachen, Germany). Solulin derives from the molecule originally described by Glaser et al. and is referred to as TMLEO (19), being distinguished by the following mutations: deletion of the first four amino acids of the amino terminus (Met388Leu, Arg456Gly, His457Gln, Ser474Ala) and deletion of the last seven amino acids of the carboxy terminus (20). A comparative diagrammatic representation of the amino acid sequences of thrombomodulin and Solulin are shown in Fig. 1. Solulin (4.6 mg/ml; lot no. 13PA201207) was provided as a sterile liquid solution for injection containing Solulin in 10 mM sodium phosphate, 2.7 mM potassium chloride, 137 mM sodium chloride and 5% mannitol at pH 7.0.
Fig. 1.
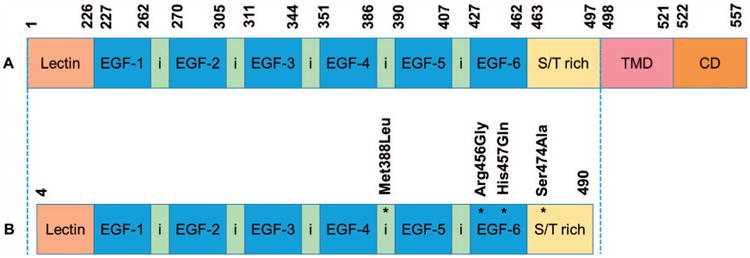
Comparative diagrammatic representation of the various domains of thrombomodulin (panel A) and Solulin (panel B) with the corresponding number of amino acids and their modifications. Lectin, N-terminal lectin-like domain; EGF = epidermal growth factor domain; S/T rich = serine-threonine rich domain; TMD = transmembrane domain; CD = cytoplasmic domain.
Administration of Solulin In Vivo
To test whether Solulin attenuates radiation-induced intestinal injury, the animals were randomly assigned to receive daily subcutaneous injections of 240 μl of vehicle (n = 15) or Solulin at 3 mg/kg/day (n = 12) or at 10 mg/kg/day (n = 9) for 27 days (starting from 4 days before, 9 days during and 14 days after irradiation). Previous pharmacokinetic results from a phase I human trial show that the time required to reach maximum plasma concentration (tmax) is approximately 96 h and the half-life (t1/2) of Solulin is 21.2–24.6 h when a daily dose of 1 or 10 mg Solulin was used for multiple days (21). In this study, we therefore, decided to start daily dosing of Solulin 4 days before irradiation. The stock solution of Solulin was diluted in 0.9% saline, according to the desired concentration and 0.9% saline was also used as vehicle. All animals were euthanized on day 28 (24 h after the last vehicle or Solulin dose) in accordance with the American Veterinary Medical Association Guidelines for the euthanasia of animals.
Assessment of Intestinal Radiation Response
After euthanasia, intestinal specimens were procured from the vehicle group (n = 15), 3 mg/kg Solulin group (n = 12) and 10 mg/kg Solulin group (n = 9), fixed in methanol-Carnoy's fixative and embedded in paraffin. We used 5 μm sections for histopathology, morphometry and immunohistochemistry. The observation time used in the study (two weeks) is representative of early radiation enteropathy in our model system.
Bioluminescence Imaging of Myeloperoxidase Activity In Vivo
Twelve days after irradiation, rats were anaesthetized (isoflurane inhalation), and luminol was administered by intraperitoneal injection (200 mg/kg body weight). Luminol (5-amino-2, 3-dihydro-1,4-phthalazinedione) is a redox-sensitive compound specific for MPO that emits blue luminescence (lambda max = 425 nm) when exposed to activated MPO (22). Five minutes after luminol injection, the irradiated area of each animal was imaged for MPO activity using the IVIS® 200 bioluminescence imaging system (Xenogen). Quantitative imaging was performed with Living Image Software (Caliper Life Sciences, Hopkinton, MA). Areas of luminescence were identified as regions of interest and quantified as photons emitted.
Quantitative Histopathology and Morphometry
Sections of intestine were stained with hematoxylin and eosin (H&E) and were used to determine radiation injury score (RIS), mucosal surface area and intestinal wall thickness, as described elsewhere (13, 23). The RIS provides a global measure of the severity of structural radiation injury in the intestine and is a composite histopathological scoring system that has been extensively used and validated in our laboratory (24). Briefly, seven histopathologic parameters of radiation injury (mucosal ulcerations, epithelial atypia, thickening of sub-serosa, vascular sclerosis, intestinal wall fibrosis, ileitis cystica profunda and lymph congestion) were assessed and graded from 0–3. The sum of the scores for the individual alterations constitutes the RIS. All specimens were evaluated in a blinded fashion by two separate researchers, and discrepancies in scores were resolved by consensus.
A radiation-induced decrease in the surface area of the intestinal mucosa is a sensitive parameter of small bowel radiation injury. Mucosal surface area was measured in vertical sections using a stereologic projection/cycloid method as described by Baddeley et al. and adapted by us to our model system (24, 25). The method does not require assumptions about the shape or orientation of the specimens and thus circumvents problems associated with most other procedures for surface area measurement.
Intestinal wall thickening is a measure of both reactive intestinal wall fibrosis and intestinal smooth muscle cell hyperplasia. In contrast, sub-serosal thickening reflects mainly reactive fibrosis. Intestinal wall thickness and sub-serosal thickness were measured with computer-assisted image analysis (Image-Pro® Plus, Media Cybernetics Inc., Silver Spring, MD). All measurements were made by utilizing a 10× objective lens. A total of 5 areas, 500 μm apart, were selected for measurement, with three measurements taken per area. The average of all 5 areas was used as a single value for statistical calculations.
Quantitative Immunohistochemistry and Image Analysis
Immunohistochemistry and computer-assisted image analysis (Image-Pro Plus) were used to assess the following established indicators of intestinal radiation injury: 1. neutrophil infiltration; 2. proliferation rate of intestinal smooth muscle cells, 3. deposition of collagen type I in the intestinal wall; and 4. expression of extracellular matrix-associated transforming growth factor-β (TGF-β), as described in detail and validated previously (26, 27).
Immunohistochemical staining was performed with a standard avidinbiotin complex (ABC) technique, diaminobenzidine (DAB) chromogen and hematoxylin counterstaining. Appropriate positive and negative controls were included. The primary antibodies, incubation times, dilutions, and sources were as follows: polyclonal anti-myeloperoxidase antibody (A0398, 2 h, 1:100; Dako Inc., Carpinteria, CA), monoclonal antibody against proliferating cell nuclear antigen (NA03, 2 h, 1:100; Calbiochem, Cambridge, MA), polyclonal antibodies against collagen types I (1310-01, 2 h, 1:100; Southern Biotechnology Associates, Birmingham, AL), and polyclonal antibody against TGF-β (AB-100-NA, 2 h, 1:300; R&D Systems, Minneapolis, MN).
Quantitative assessment of immunoreactivity was performed using computerized image analysis (Image–Pro Plus), as previously described and validated (28). Cells positive for MPO and proliferating cell nuclear antigen were determined by color threshold and were counted in twenty 40× fields per section, selected according to a predetermined grid pattern. Relative areas positive for TGF-β and collagen I were determined in 20 fields (40×), according to previously described procedures (29).
Statistical Analysis
Statistical analysis was performed using Prism software (GraphPad Software, La Jolla, CA). Mean values with standard errors, when applicable, were reported. Differences in end points between two groups were assessed with the Mann-Whitney U test, and P < 0.05 was considered statistically significant. All statistical tests were two sided with a 5% significance level.
Results
There were no obvious toxic effects of Solulin administration (3 mg/kg and 10 mg/kg) and no treatment-related mortality. Early structural alterations (two weeks after irradiation) consisted primarily of mucosal injury and ulcerations, reactive intestinal wall thickening and inflammatory cell infiltration.
In nonirradiated (shielded) intestine, administration of Solulin did not affect mucosal surface area, thickness of intestinal wall and serosa, neutrophil infiltration, TGF-β immunoreactivity, proliferation of intestinal smooth muscles and collagen deposit (data not shown).
Solulin Suppresses MPO Activity and Neutrophil Infiltration in Irradiated Rat Intestine
Neutrophils are the first responders to tissue injury during the early stages of inflammation. The azurophilic granules of neutrophils contain the MPO enzyme, and MPO's activity is important for bactericidal functions. Prior studies have established noninvasive imaging methods for monitoring neutrophil MPO activity in vivo by using bioluminescence imaging (22). To analyze whether luminol could be used in our model to monitor physiological MPO activity in vivo, we injected luminol intraperitoneally and the irradiated area of each animal was imaged (Fig. 2A, irradiated plus vehicle treated; Fig. 2B, irradiated plus 3 mg/kg Solulin treated; and Fig. 2C, irradiated plus 10 mg/kg Solulin treated). Compared to vehicle, Solulin-treated groups (both 3 and 10 mg/kg) showed a highly significant reduction in luminol bioluminescence at the irradiated site (Fig. 2D).
Fig. 2.
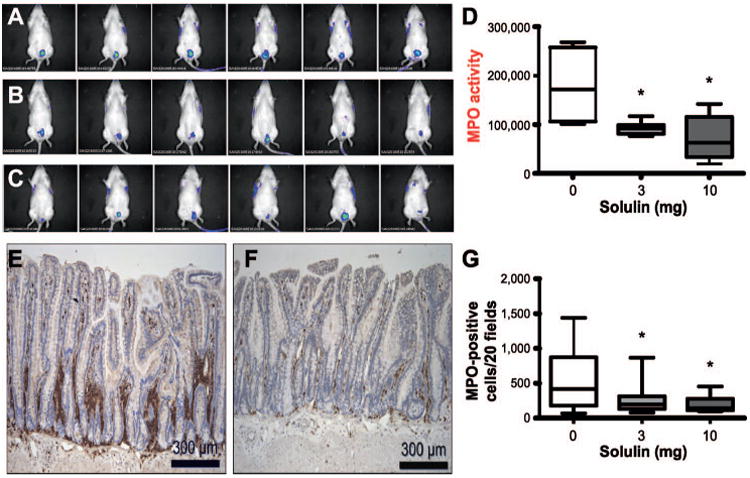
Panel A: Evidence of MPO activity in the intestine as detected by luminol bioluminescence in irradiated plus vehicle-treated group. Panel B: Irradiated plus 3 mg/kg Solulin-treated group. Panel C: Irradiated plus 10 mg/kg Solulin-treated group at two weeks. Panel D: MPO activity significantly decreased after 3 mg/kg (P = 0.015) as well as 10 mg/kg (P = 0.018) of Solulin treatment. Panels E and F: MPO-positive cells infiltrated the intestine in the irradiated plus vehicle-treated group (panel E) and irradiated plus Solulin (10 mg/kg/day)-treated group (panel F) at two weeks, as detected by immunohistochemical staining. Images were taken under 10× magnification. Panel G: Infiltration of MPO-positive cells was significantly reduced after 3 mg/kg (P = 0.009) as well as 10 mg/kg (P = 0.005) of Solulin treatment. Values are expressed as mean ± SE.
MPO activity is a marker of tissue neutrophil infiltration (30). We stained intestinal tissues with antibodies against MPO to detect neutrophil accumulation at the damaged site. Representative photomicrographs showed relatively high MPO immunostaining in irradiated vehicle-treated (Fig. 2E) compared to irradiated Solulin-treated (Fig. 2F) rat intestine at two weeks. As shown in Fig. 2G, Solulin treatment causes significant reduction in MPO-positive cell accumulation at the damaged intestine after fractionated radiation.
Solulin Prevents Radiation-Induced TGF-β Overexpression in Rat Intestine
TGF-β is considered a key growth factor in the development of radiation fibrosis (31), and is mechanistically involved in radiation enteropathy (27). Representative intestinal sections demonstrated higher TGF-β immunoreactivity in the irradiated vehicle-treated group (Fig. 3A) than in the irradiated Solulin-treated group (Fig. 3B) at two weeks. We found that irradiated intestine from Solulin-treated animals (at both doses) exhibited decreased TGF-β immunoreactivity compared to the vehicle-treated group (Fig. 3C).
Fig. 3.
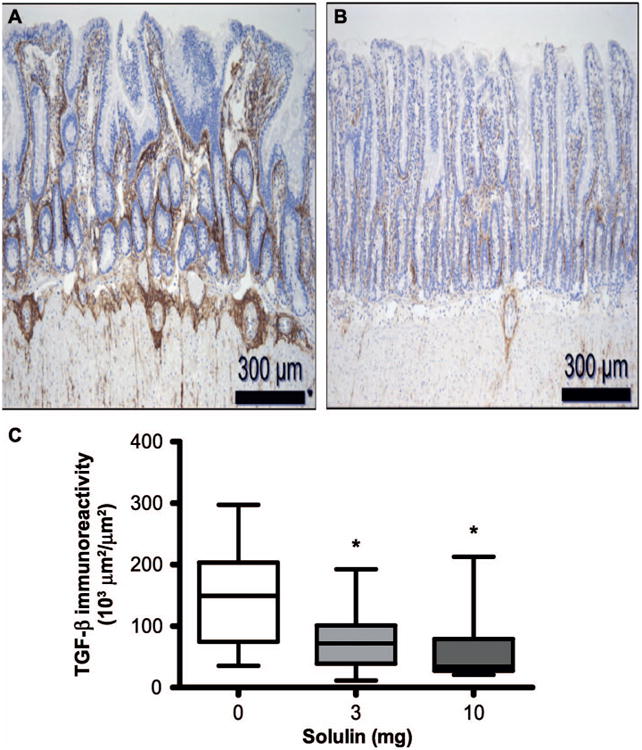
TGF-β immunoreactivity in the intestine as detected by immunohistochemical staining in (panel A) irradiated plus vehicle-treated group and (panel B) irradiated plus Solulin (10 mg/kg/day)-treated group at two weeks. Images were captured under 10× magnification. Panel C: TGF-β immunoreactivity significantly decreased after 3 mg/kg (P = 0.023) as well as 10 mg/kg (P = 0.016) Solulin treatment. Values are expressed as mean ± SE.
Solulin Limits Radiation-Induced Intestinal Wall Collagen Deposition
Collagen I accumulation in tissue is primarily a late end point of radiation toxicity. Representative images of the intestine showed considerably high collagen I deposition in the irradiated vehicle-treated group (Fig. 4A) compared to irradiated Solulin (Fig. 4B) treated group at two weeks postirradiation. Compared to vehicle, Solulin treatment was found to be effective in preventing collagen deposits in irradiated intestine (Fig. 4C).
Fig. 4.
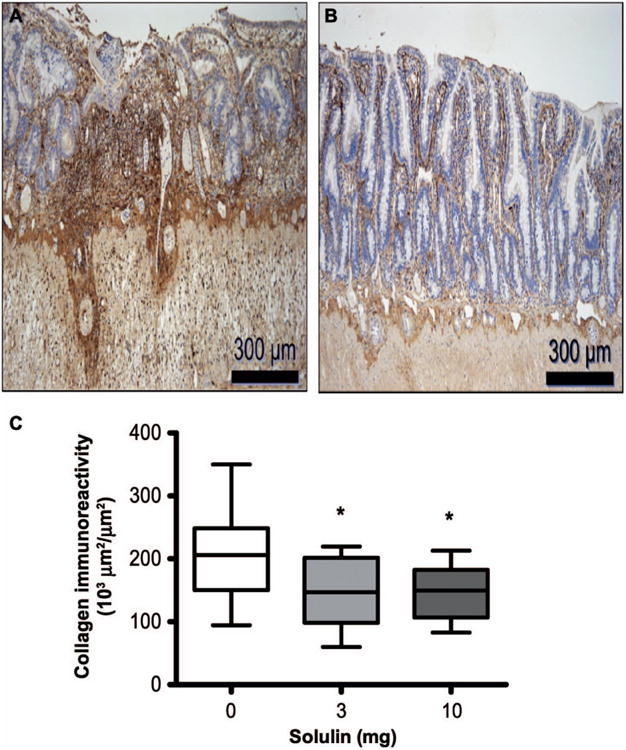
Collagen-I deposits in the intestine as detected by immunohistochemical staining in (panel A) irradiated plus vehicle-treated and (panel B) irradiated plus Solulin (10 mg/kg/day)-treated group at two weeks. Images were captured under 10× magnification. Panel C: Collagen-I deposits significantly decreased after 3 mg/kg (P = 0.016) as well as 10 mg/kg (P = 0.023) Solulin treatment. Values are expressed as mean ± SE.
Solulin Attenuates Radiation-Induced Early Histopathological Changes in Rat Intestine
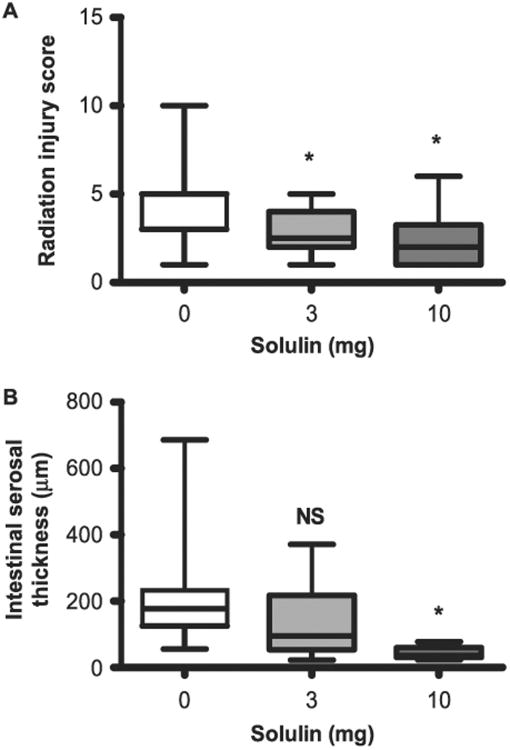
Histopathological assessment was performed to determine early adverse changes in the intestine two weeks after fractionated irradiation. Both doses of Solulin (3 mg/kg and 10 mg/kg) significantly reduced overall RIS (Fig. 5A) in the irradiated intestine. However, a decrease in serosal thickness (indicating so-called consequential injury) was only observed after the high (10 mg/kg) dose of Solulin but not after the 3 mg/kg Solulin dose (Fig. 5B). Overall, Solulin attenuated the intestinal radiation-induced histopathological alterations.
Fig. 5.
Panel A: Radiation injury score and serosal thickness in the intestine detected by H&E staining at two weeks. Radiation injury score significantly decreased after 3 mg/kg (P = 0.008) as well as 10 mg/kg (P = 0.008) Solulin treatment. Panel B: Serosal thickness significantly decreased after 10 mg/kg Solulin treatment (P = 0.015), but not after 3 mg/kg Solulin treatment (P = 0.127). Values are expressed as mean ± SE. NS = not statistically significant.
Discussion
The risk of radiation enteropathy is a major dose-limiting factor in abdominopelvic cancer treatment. Moreover, radiological and nuclear accidents or terrorist attacks may also cause acute intestinal toxicity of varying degrees depending on the absorbed dose and radiation quality. In both cases, the early intestinal radiation response is characterized by a breakdown of the epithelial barrier, mucosal inflammation and microvascular injuries. Therefore, strategies to prevent early intestinal injury are required both to improve the effectiveness and outcomes after therapeutic radiation and to mitigate radiation-induced acute gastrointestinal syndrome.
Studies from various groups, including ours, have shown that prevention of radiation-induced microvascular injury, particularly protection of endothelial cells, suppresses early intestinal damages. For example, prevention of endothelial apoptosis by inhibiting the enzyme acid sphingomyelinase and pro-apoptotic sphingolipid ceramide (5, 32), suppression of endothelial specific PAR-1 activation (a thrombin receptor and an essential mediator of vascular diseases) (6, 33) and inhibition of leucocyte attachment with endothelium by systemic administration of antioxidant enzyme superoxide dismutase (34) confer significant protection against radiation-induced intestinal epithelial cell apoptosis in mice. These studies suggest that maintaining normal endothelial functions in the intestinal microvasculature is crucial to limiting acute radiation enteropathy. Moreover, exposure to ionizing radiation may also increase intestinal microvascular permeability, resulting in passage of immune cells from the blood stream to the intestinal tissue, inducing inflammation. A recent study demonstrated that TM prevents vascular permeability in a mouse model of hemorrhagic shock by restricting the loss of tight junction proteins (35), thereby limiting inflammation.
Endothelial TM modifies a variety of pathological responses in many normal tissues, including the intestine. Although the mechanisms of TM-mediated intestinal radiation protection are not fully understood, studies by our group and others suggest that TM suppresses inflammation, an early postirradiation adverse event. For example, we found that statins, a potent TM inducer, reduce the expression of inflammatory and fibrotic markers in rat intestine exposed to fractionated radiation (36). Other studies have also revealed that TM and APC play a critical role in controlling intestinal inflammation by modulating inflammatory cytokines (37). Therefore, we reasoned that Solulin would prevent the emergence of early markers of radiation-induced intestinal inflammation, such as infiltration of neutrophils in the inflamed tissue represented by enhanced MPO activity. The current study did confirm that systemic administration of Solulin suppresses MPO activity in rat intestine after exposure to fractionated radiation.
Ionizing radiation induces the multifunctional fibrogenic cytokine TGF-β in various tissues, including intestine. In concordance with the current findings, we earlier reported on enhanced TGF-β immunoreactivity in rat intestine after two weeks of fractionated irradiation (38, 39). Increased TGF-β expression is a predictor of normal tissue injury, and was shown to cause endothelial damage (40), enhance endothelial barrier permeability (41), induce endothelial dysfunction (42) and suppress endothelial TM expression (43). Studies from our laboratory also suggest that there is a strong negative correlation between endothelial TM-positive vessels and TGF-β expression in small bowel resection specimens obtained from patients with radiation enteropathy (44). TM upregulation by statin treatment was shown to suppress the TGF-β signaling pathway by inhibiting ERK activation (45). TM is also known to deactivate HMGB1, a nuclear protein shown to activate the TGF-β signaling pathway (46), and inhibits thrombin-mediated activation of platelets (47), the major cellular source of TGF-β. Consistent with this notion, our current study showed that exogenous Solulin administration limited radiation-induced TGF-β overexpression in rat intestinal tissue.
Deposition of collagen is a major cause of delayed complications after radiation therapy. We have found collagen I and collagen III deposits two weeks after intestinal irradiation (36, 48). In the intestine, collagen formation is largely regulated by thrombin-mediated PAR-1 activation on smooth muscle cells and myofibroblasts. TM appears to play an important role in suppressing thrombin-mediated collagen deposition. For example, Wei et al. showed that mice bearing a wild-type TM extracellular domain exhibited suppressed PAR-1 activation through thrombin binding, compared to mice with mutant TM domains (49). In another study, recombinant TM treatment substantially inhibited thrombin-induced smooth muscle proliferation (50). Moreover, TM deficiency was shown to enhance collagen deposition (51), while conversely, collagen deposition was delayed in TM-overexpressing mice (52). Analogously, we found that statins, which potently induce endothelial TM, reduce collagen deposits in irradiated rat intestine (36). Finally, Sopel et al. reported that APC treatment reduced angiotensin-II infusion-induced collagen deposits in the murine heart (53).
Although the loss of normal intestinal structural architecture is multifactorial, studies by various groups suggest that postirradiation TGF-β activation and collagen deposition play important roles in the subsequent histopathological changes (54, 55). Since TM can inhibit excessive reactive oxygen species formation, TGF-β activation and collagen deposition, we hypothesized that Solulin treatment would ameliorate structural injury in the irradiated gut. We found that Solulin indeed reduced histopathological, pro-inflammatory and molecular changes in the irradiated rat intestine. The exact mechanism of Solulin-mediated protection of radiation damage to the intestine requires further investigation, particularly with regard to the contribution of APC. A number of studies demonstrate that APC administration reduces injury and improves functional activities in various animal models of intestinal toxicity (37, 56, 57). On the other hand, we did not observe reduction of intestinal injury in mice when APC was administered 24 h after acute total-body irradiation (8). This may be because there are differences between fractionated and single-dose irradiation and between localized and total-body irradiation, or that there are important biological differences between the rat and the mouse model. Another possibility is that intestinal radiation injury is largely independent of APC and more directly dependent on TM. For example, the lectin-like domain of TM (which is retained in Solulin) has the ability to scavenge free radicals, which clearly play an important role in radiation injury.
In conclusion, Solulin was found to be protective against early localized fractionated radiation-induced structural damage, inflammation, TGF-β production and collagen-I deposition in rat intestine. Although the mechanisms of intestinal radiation protection by Solulin require further investigation, Solulin's thrombin-inhibiting properties likely play a significant role in this regard, as we have shown previously that the thrombin inhibitor hirudin attenuates structural and molecular aspects of intestinal radiation injury (13). Moreover, thrombin inhibition is also known to suppress cancer cell growth (58); therefore, Solulin would be expected to reduce rather than enhance cancer growth, thereby increasing the therapeutic ratio of radiotherapy. A phase I trial of Solulin showed excellent overall tolerability (in dose ranges of 0.6–30 mg for single or multiple dose; 1–10 mg once daily for five days) with no bleeding risk, linear pharmacokinetics, dose-dependent thrombin inhibition and long plasma half-life (15–30 h) (21). Solulin should be tested clinically for the prevention of early intestinal radiation toxicity in patients undergoing abdominal or pelvic radiation therapy, and further studies to investigate the effects on delayed radiation enteropathy are warranted. Finally, Solulin should also be considered for protecting normal tissues from the adverse effects of nontherapeutic radiation exposure.
Acknowledgments
Assistance with performance of surgery and for tissue harvest by Ashwini Kulkarni is gratefully acknowledged. This study was supported by the National Institutes of Health (grant nos. P20 GM109005 and R37 CA71382), the U.S. Veterans Administration, Arkansas Space Grant Consortium through the National Aeronautics and Space Administration (grant no. NNX15AK32A) and the National Space Biomedical Research Institute (no. RE03701–NCC 9-58).
References
- 1.Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy–pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470–9. doi: 10.1038/nrgastro.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne RG, Kearsley JH, Grove WD, Roberts SJ. The relationship between early and late gastrointestinal complications of radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 1983;9:1445–50. doi: 10.1016/0360-3016(83)90316-4. [DOI] [PubMed] [Google Scholar]
- 3.Peach MS, Showalter TN, Ohri N. Systematic review of the relationship between acute and late gastrointestinal toxicity after radiotherapy for prostate cancer. Prostate Cancer. 2015;2015:624736. doi: 10.1155/2015/624736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–55. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 6.Rannou E, Francois A, Toullec A, Guipaud O, Buard V, Tarlet G, et al. In vivo evidence for an endothelium-dependent mechanism in radiation-induced normal tissue injury. Sci Rep. 2015;5:15738. doi: 10.1038/srep15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T, Maruyama I. Thrombomodulin: protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011;9(1):168–73. doi: 10.1111/j.1538-7836.2011.04319.x. [DOI] [PubMed] [Google Scholar]
- 8.Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–9. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su EJ, Geyer M, Wahl M, Mann K, Ginsburg D, Brohmann H, et al. The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J Thromb Haemost. 2011;9:1174–82. doi: 10.1111/j.1538-7836.2011.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niego B, Samson AL, Petersen KU, Medcalf RL. Thrombin-induced activation of astrocytes in mixed rat hippocampal cultures is inhibited by soluble thrombomodulin. Brain Res. 2011;1381:38–51. doi: 10.1016/j.brainres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Ryang YM, Dang J, Kipp M, Petersen KU, Fahlenkamp AV, Gempt J, et al. Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci. 2011;12:113. doi: 10.1186/1471-2202-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert JM, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost. 2004;2:2027–35. doi: 10.1111/j.1538-7836.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang SM, Ka SM, Wu HL, Yeh YC, Kuo CH, Hua KF, et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia. 2014;57:424–34. doi: 10.1007/s00125-013-3115-6. [DOI] [PubMed] [Google Scholar]
- 15.Yamaji K, Wang Y, Liu Y, Abeyama K, Hashiguchi T, Uchimura T, et al. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thromb Res. 2005;115:319–25. doi: 10.1016/j.thromres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Li YH, Kuo CH, Shi GY, Wu HL. The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci. 2012;19:34. doi: 10.1186/1423-0127-19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmon CT. Protein C anticoagulant system–anti-inflammatory effects. Semin Immunopathol. 2012;34:127–32. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauer-Jensen M, Poulakos L, Osborne JW. Effects of accelerated fractionation on radiation injury of the small intestine: a new rat model. Int J Radiat Oncol Biol Phys. 1988;14:1205–12. doi: 10.1016/0360-3016(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 19.Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I, et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J Clin Invest. 1992;90:2565–73. doi: 10.1172/JCI116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisel JW, Nagaswami C, Young TA, Light DR. The shape of thrombomodulin and interactions with thrombin as determined by electron microscopy. J Biol Chem. 1996;271:31485–90. doi: 10.1074/jbc.271.49.31485. [DOI] [PubMed] [Google Scholar]
- 21.van IT, Stroissnig H, Giesen P, Wemer J, Wilhelm-Ogunbiyi K. Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb Haemost. 2011;105:302–12. doi: 10.1160/TH10-05-0287. [DOI] [PubMed] [Google Scholar]
- 22.Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med. 2009;15:455–61. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zheng H, Kulkarni A, Ou X, Hauer-Jensen M. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. Int J Radiat Oncol Biol Phys. 2006;64:1528–36. doi: 10.1016/j.ijrobp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–7. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 25.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–76. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Halbreich A, Mager J. Proceedings: role of amino acids in the control of the synthesis of RNA and free nucleotides in yeasts. Isr J Med Sci. 1975;11:1201–2. [PubMed] [Google Scholar]
- 27.Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer-Jensen M. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology. 2000;119:1286–96. doi: 10.1053/gast.2000.19282. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531–40. doi: 10.1016/s0002-9440(10)65741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moschera J, Pigman W. The isolation and characterization of rat sublingual mucus-glycoprotein. Carbohydr Res. 1975;40:53–67. doi: 10.1016/s0008-6215(00)82668-3. [DOI] [PubMed] [Google Scholar]
- 30.Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115–23. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 31.Richter KK, Langberg CW, Sung CC, Hauer-Jensen M. Association of transforming growth factor beta (TGF-beta) immunoreactivity with specific histopathologic lesions in subacute and chronic experimental radiation enteropathy. Radiother Oncol. 1996;39:243–51. doi: 10.1016/0167-8140(95)01735-6. [DOI] [PubMed] [Google Scholar]
- 32.Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122:1786–90. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abderrahmani R, Francois A, Buard V, Tarlet G, Blirando K, Hneino M, et al. PAI-1-dependent endothelial cell death determines severity of radiation-induced intestinal injury. PLoS One. 2012;7:e35740. doi: 10.1371/journal.pone.0035740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molla M, Gironella M, Salas A, Closa D, Biete A, Gimeno M, et al. Protective effect of superoxide dismutase in radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2005;61:1159–66. doi: 10.1016/j.ijrobp.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Zhang WG, Sun J, Zhang Y, Lu JF, Han HB, et al. Protective effects of thrombomodulin on microvascular permeability after subarachnoid hemorrhage in mouse model. Neuroscience. 2015;299:18–27. doi: 10.1016/j.neuroscience.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Boerma M, Fu Q, Kulkarni A, Fink LM, Hauer-Jensen M. Simvastatin ameliorates radiation enteropathy development after localized, fractionated irradiation by a protein C-independent mechanism. Int J Radiat Oncol Biol Phys. 2007;68:1483–90. doi: 10.1016/j.ijrobp.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vetrano S, Ploplis VA, Sala E, Sandoval-Cooper M, Donahue DL, Correale C, et al. Unexpected role of anticoagulant protein C in controlling epithelial barrier integrity and intestinal inflammation. Proc Natl Acad Sci U S A. 2011;108:19830–5. doi: 10.1073/pnas.1107140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langberg CW, Hauer-Jensen M, Sung CC, Kane CJ. Expression of fibrogenic cytokines in rat small intestine after fractionated irradiation. Radiother Oncol. 1994;32:29–36. doi: 10.1016/0167-8140(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- 40.Kruse JJ, Floot BG, te Poele JA, Russell NS, Stewart FA. Radiation-induced activation of TGF-beta signaling pathways in relation to vascular damage in mouse kidneys. Radiat Res. 2009;171:188–97. doi: 10.1667/RR1526.1. [DOI] [PubMed] [Google Scholar]
- 41.Hurst V IV, Goldberg PL, Minnear FL, Heimark RL, Vincent PA. Rearrangement of adherens junctions by transforming growth factor-beta1: role of contraction. Am J Physiol. 1999;276:L582–L595. doi: 10.1152/ajplung.1999.276.4.L582. [DOI] [PubMed] [Google Scholar]
- 42.Feng W, Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-beta mediates endothelial dysfunction in rats during high salt intake. Am J Physiol Renal Physiol. 2015;309:F1018–25. doi: 10.1152/ajprenal.00328.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohji T, Urano H, Shirahata A, Yamagishi M, Higashi K, Gotoh S, et al. Transforming growth factor beta 1 and beta 2 induce down-modulation of thrombomodulin in human umbilical vein endothelial cells. Thromb Haemost. 1995;73:812–8. [PubMed] [Google Scholar]
- 44.Richter KK, Fink LM, Hughes BM, Sung CC, Hauer-Jensen M. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol. 1997;44:65–71. doi: 10.1016/s0167-8140(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 45.Shang L, Jia SS, Jiang HM, Wang H, Xu WH, Lv CJ. Simvastatin downregulates expression of TGF-betaRII and inhibits proliferation of A549 cells via ERK. Tumour Biol. 2015;36:4819–24. doi: 10.1007/s13277-015-3134-7. [DOI] [PubMed] [Google Scholar]
- 46.Pittet JF, Koh H, Fang X, Iles K, Christiaans S, Anjun N, et al. HMGB1 accelerates alveolar epithelial repair via an IL-1beta- and alphavbeta6 integrin-dependent activation of TGF-beta1. PLoS One. 2013;8:e63907. doi: 10.1371/journal.pone.0063907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esmon NL, Carroll RC, Esmon CT. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983;258:12238–42. [PubMed] [Google Scholar]
- 48.Wang J, Zheng J, Kulkarni A, Wang W, Garg S, Prather PL, et al. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Dig Dis Sci. 2014;59:2693–703. doi: 10.1007/s10620-014-3212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei HJ, Li YH, Shi GY, Liu SL, Chang PC, Kuo CH, et al. Thrombomodulin domains attenuate atherosclerosis by inhibiting thrombin-induced endothelial cell activation. Cardiovasc Res. 2011;92:317–27. doi: 10.1093/cvr/cvr220. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Garnette CS, Cahn M, Claytor RB, Rohrer MJ, Dobson JG, Jr, et al. Recombinant thrombomodulin inhibits arterial smooth muscle cell proliferation induced by thrombin. J Vasc Surg. 2000;32:804–13. doi: 10.1067/mva.2000.107992. [DOI] [PubMed] [Google Scholar]
- 51.Peterson JJ, Rayburn HB, Lager DJ, Raife TJ, Kealey GP, Rosenberg RD, et al. Expression of thrombomodulin and consequences of thrombomodulin deficiency during healing of cutaneous wounds. Am J Pathol. 1999;155:1569–75. doi: 10.1016/S0002-9440(10)65473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raife TJ, Lager DJ, Peterson JJ, Erger RA, Lentz SR. Keratinocyte-specific expression of human thrombomodulin in transgenic mice: effects on epidermal differentiation and cutaneous wound healing. J Investig Med. 1998;46:127–33. [PubMed] [Google Scholar]
- 53.Sopel MJ, Rosin NL, Falkenham AG, Bezuhly M, Esmon CT, Lee TD, et al. Treatment with activated protein C (aPC) is protective during the development of myocardial fibrosis: an angiotensin II infusion model in mice. PLoS One. 2012;7:e45663. doi: 10.1371/journal.pone.0045663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS One. 2013;8:e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graham MF, Diegelmann RF, Elson CO, Lindblad WJ, Gotschalk N, Gay S, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–65. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 56.Kumral A, Yesilirmak DC, Tugyan K, Baskin H, Tekman I, Duman N, et al. Activated protein C reduces intestinal injury in an experimental model of necrotizing enterocolitis. J Pediatr Surg. 2010;45:483–9. doi: 10.1016/j.jpedsurg.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann C, Meissner K, Knock A, Diedrich S, Pavlovic D, Grundling M, et al. Activated protein C improves intestinal microcirculation in experimental endotoxaemia in the rat. Crit Care. 2006;10:R157. doi: 10.1186/cc5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asanuma K, Wakabayashi H, Okamoto T, Asanuma Y, Akita N, Yoshikawa T, et al. The thrombin inhibitor, argatroban, inhibits breast cancer metastasis to bone. Breast Cancer. 2013;20:241–6. doi: 10.1007/s12282-012-0334-5. [DOI] [PubMed] [Google Scholar]