Abstract
The generation and shedding of extracellular vesicles (EVs), including exosomes and microvesicles (MVs), by cells has emerged as a form of intercellular communication with important roles in several physiological processes and diseases such as cancer. These membrane-enclosed packets can transfer specific proteins, RNA transcripts, microRNAs, and even DNA to target cells, thereby altering their function. Despite the exponential growth of the EV field, a great deal remains unclear about the mechanisms that regulate exosome and MV biogenesis, as well as about how to isolate different classes of EVs and how to best take advantage of them for clinical applications.
Overview
A growing list of exciting discoveries has emerged that demonstrate new ways by which cells communicate with their neighboring cells through the secretion of non-classical secretory vesicles referred to as extracellular shed vesicles (EVs) (Lo Cicero et al., 2015; Raposo and Stoorvogel, 2013; Février and Raposo, 2004; Cocucci et al., 2009; Al-Nedawi et al., 2009a, 2009b; Ratajczak et al., 2006a; Mathivanan et al., 2010; Muralidharan-Chari et al., 2010; D’Souza-Schorey and Clancy, 2012; Denzer et al., 2000; Thery et al., 2009; Valadi et al., 2007). The existence of EVs was initially viewed with some skepticism, as they were thought to represent artifacts of cell and membrane isolation procedures that lacked physiological relevance (Cocucci et al., 2009). However, as will be expanded upon below, there now exists substantial and compelling evidence that highlights the importance of EVs in various biological processes, with two in particular being cancer progression and stem cell biology.
At present, EVs are typically divided into two general classes, as distinguished by the underlying mechanisms responsible for their biogenesis. One of these classes of EVs, which has the potential to be as large as 0.2–1 µm in diameter, are referred to by a variety of names, including ectosomes, microparticles, and microvesicles (MVs), and, when discussed in the context of cancer, as tumor-derived MVs (TMVs) or oncosomes (Lo Cicero et al., 2015; Raposo and Stoorvogel, 2013; Cocucci et al., 2009; Ratajczak et al., 2006a; Muralidharan-Chari et al., 2010; Cocucci and Meldolesi, 2011). Throughout this review, we refer to them as MVs. Given their ability to reach relatively large sizes, MVs can be detected by electron microscopy and immunofluorescence, in the latter case by staining for known MV-associated cargo proteins or through the use of lipid-binding dyes (Antonyak et al., 2011; Al-Nedawi et al., 2008; Di Vizio et al., 2012; Muralidharan-Chari et al., 2009; Tian et al., 2010; Scott, 2012). The second most widely characterized class of EVs, known as exosomes, are typically much smaller than MVs, ranging in size from 0.04 to 0.1 µm in diameter (Ge et al., 2012; Teis et al., 2009; Hanson and Cashikar, 2012). These two classes of EVs are formed through distinct cellular mechanisms (Figure 1, left side). MVs are plasma membrane-derived vesicles that are shed as an outcome of the budding and fission of the plasma membrane. MV budding has been suggested to occur at specific membrane sites or “microdomains” (referred to as lipid rafts), such that the lipid-raft protein, flotillin, is often used as a marker for MVs (Gangalum et al., 2011; Lopez et al., 2005; Mairhofer et al., 2002; Del Conde et al., 2005; Liu et al., 2012). In cancer cells, MVs were shown to “mature” at the cell surface through RhoA-dependent signals that activate the Rho-associated coiled-coil-containing protein kinase (Rho kinase) and the LIM kinase (Li et al., 2012). Unlike MVs, exosomes do not initially form at the plasma membrane. Instead, they are produced through the re-routing of multi-vesicular bodies that at least in some cases are formed in an ESCRT (endosomal sorting complex required for transport)-dependent manner, to the cell surface where they then fuse with the plasma membrane and undergo exocytosis.
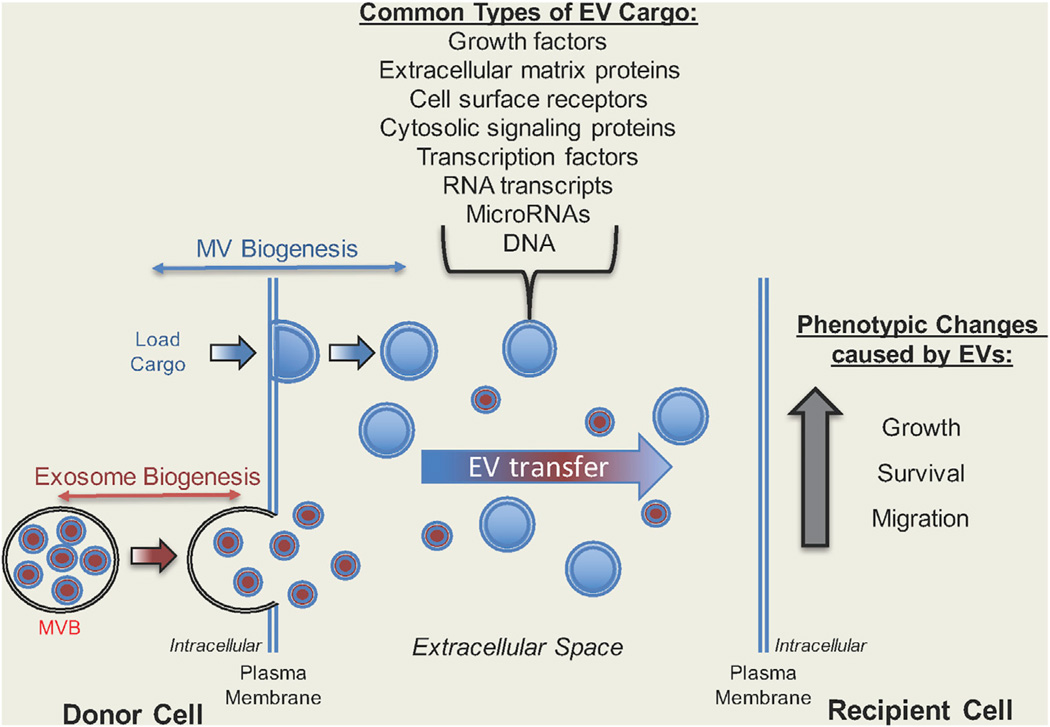
Figure 1. Diagram Highlighting How EVs Function as a Novel Form of Intercellular Communication.
(Left) Most cell types generate two distinct types of EVs, exosomes and microvesicles (MVs). Exosomes (in red) are formed as a result of directing multi-vesicular bodies (MVBs) containing endosomes to the surface of a cell, where the MVBs fuse with the plasma membrane and release their contents (exosomes) into the extracellular space. In contrast, MVs (in blue) directly bud from the surface of a cell, are loaded with various cargo, and then are released or shed from the cell. (Right) Both exosomes and EVs are transferred to recipient cells, an outcome that often changes their phenotype. Some of the most common types of EV cargo are also listed.
Both MVs and exosomes have been reported to contain specific protein cargo, as well as RNA transcripts, microRNAs (miRNAs), and even DNA (see Figure 1, list of EV cargo; also Muralidharan-Chari et al., 2010; Melo et al., 2015; Skog et al., 2008; Hosseini-Beheshti et al., 2012; Balaj et al., 2011; Gallo et al., 2012; Zhuang et al., 2012; Hao et al., 2006; Hessvik et al., 2012; Chiba et al., 2012; Zhang et al., 2015; Tominaga et al., 2015; Kanada et al., 2015). Among the major questions in the field is how specific proteins and nucleic acids are selectively targeted for incorporation into the different classes of EVs. There have been some reports suggesting that specific post-translational modifications are required for the trafficking of protein cargo into MVs; in particular, glycosylphosphatidylinositol anchors that are attached to the C terminus of various plasma membrane-associated proteins (Fujita and Kinoshita, 2012; Muller et al., 2011). Others have shown that the addition of acyl, myristoyl, and palmitoyl tails to proteins can facilitate their recruitment into EVs (Shen et al., 2011). However, protein cargo lacking these types of post-translational modifications can still be recruited to EVs, suggesting that additional mechanisms that selectively target proteins to MVs or exosomes exist. As is the case for proteins, both RNA and miRNAs exhibit selectivity in their ability to be incorporated into EVs. While the mechanisms that regulate this process are still poorly understood, there are some indications that the recruitment of at least a set of RNA species to EVs may be mediated through their non-coding regions (Bolukbasi et al., 2012).
MVs and exosomes are often thought to be functionally identical; in fact, these names are sometimes used interchangeably in the literature. While the two major classes of EVs appear to share some common cargo, there have been clear demonstrations of cargo specificity, i.e., proteins that are exclusively found in one or the other of these classes of vesicles (Antonyak and Cerione, 2014). Given that the biogenesis of MVs and exosomes appears to be an outcome of distinct cellular mechanisms, as well as contain distinct cargo, it seems highly likely that these two classes of EVs have specific biological functions. However, determining the specific functional roles played by these two populations of EVs has been somewhat hampered due to a lack of a strict definition of what constitutes an exosome and an MV (this is why their names are used interchangeably), as well as approaches that can reliably separate these two classes of EVs (Lo Cicero et al., 2015; Raposo and Stoorvogel, 2013). Thus, there is now a good deal of effort in the field aimed at resolving these key issues.
Cancer progression represents one of the major biological contexts in which both MVs and exosomes have been heavily implicated (Antonyak et al., 2011; Al-Nedawi et al., 2008; Melo et al., 2015; Zhang et al., 2015; Al-Nedawi et al., 2009a, 2009b; van der Vos et al., 2011; Wysoczynski and Ratajczak, 2009; Shao et al., 2012; Costa-Silva et al., 2015; Fong et al., 2015; Zomer et al., 2015; Boelens et al., 2014). MVs shed from aggressive cancer cells have been shown to be capable of activating fibroblasts and altering the tumor microenvironment, as well as to provide a unique mechanism for stimulating tumor angiogenesis (Antonyak et al., 2011; Hosseini-Beheshti et al., 2012). Likewise, exosomes have been heavily implicated in human cancers, in particular through their roles in mediating the communication between stromal cells and surrounding cancer cells, and in the education of bone marrow-derived cells, which in turn affects the development of the pre-metastatic niche (Costa-Silva et al., 2015; Boelens et al., 2014).
In this Perspective, we highlight some of the important discoveries that have demonstrated roles for EVs in different stages of tumorigenesis and metastasis. However, there are a number of other biological contexts in which EVs have either recently been implicated and/or are likely to have important functions. One, in particular, is in development and stem cell biology. Indeed, we also describe recent data that now highlight a unique mechanism by which EVs shed by adult and embryonic stem cells play crucial roles in promoting tissue regeneration and the maintenance of stemness.
EVs and Cancer Progression
Effects of EVs on Primary Tumor Growth
It is now generally believed that nearly all cell types are capable of generating EVs to some extent. However, the mechanisms that regulate MV and exosome biogenesis in cancer cells, especially highly malignant forms, often appear to be deregulated such that they generate significantly more EVs than lower grade cancer cells or normal cells. Moreover, the EVs generated by some cancer cells are now known to contain unique signaling proteins, such as the small guanosine triphosphatase Ras and the cell-surface receptor tyrosine kinase epidermal growth factor (EGF) receptor (Al-Nedawi et al., 2008; Antonyak et al., 2011), and different RNA species that regulate the expression of proteins that stimulate cell growth and survival (Skog et al., 2008). Thus, when cancer cells that constitute a tumor release MVs and exosomes into their local environment, it is tempting to speculate about how they might potentially function in a paracrine manner and influence the behavior of nearby cells to stimulate tumor growth. One of the more straightforward ways in which EVs affect this outcome is through their transfer to other cancer cells. This was perhaps best demonstrated in two critical early studies that in many ways set the stage for much of the current interest in EV biology. In one of these studies it was shown that cultures of primary cancer cells derived from patients diagnosed with glioblastoma, a highly aggressive form of cancer with a poor patient prognosis, shed large amounts of MVs enriched with RNA transcripts that encoded for proteins linked to the promotion of cell growth (Skog et al., 2008). When MVs isolated from these primary cultures were added to the established U87 glioblastoma cell line they were taken up by the cells, and evidence was provided that suggested the RNA transcripts contained in the MVs were capable of being translated into functional proteins by the recipient cells. As a result of the MV-mediated horizontal transfer of these RNA transcripts, the U87 cells grew at a faster rate compared with the untreated controls.
The second study that reinforced the idea that EVs are transferred between cancer cells came from another group studying glioblastoma. Specifically, they showed that U373 glioma cells ectopically expressing a highly oncogenic form of the EGF receptor, EGFRvIII, generated MVs that when added to cultures of parental (non-EGFRvIII expressing) U373 cells, could stimulate the transforming signaling activities of AKT and ERK, as well as promote the anchorage-independent growth of the cells (Al-Nedawi et al., 2008). The authors then went on to make the rather remarkable discovery that the component in the MVs derived from the U373 glioma cells expressing EGFRvIII, which was responsible for their growth-promoting actions, was in fact the EGFRvIII itself. Collectively, these findings highlighted for the first time that EVs from cancer cells contain proteins and RNA transcripts that could be transferred to other cancers cells as a mechanism to potentiate cell growth. When considered in the context of primary tumors, which are often characterized by a heterogeneous population of cancer cells (Marusyk and Polyak, 2010), they also raise the interesting possibility that EVs could serve as an important form of intercellular communication that propagates the oncogenic phenotype among the various cancer cell types.
Effects of EVs on the Tumor Microenvironment
The tumor microenvironment is not simply composed of cancer cells but also consists of normal cell types, referred to as stroma, that surround the tumor and help sustain its growth (Quail and Joyce, 2013). In addition to mediating the transfer of information between different cancer cells, EVs have also been shown to have a significant impact on the function and behavior of non-cancerous cells within the tumor microenvironment. Indeed, it was shown that breast cancer cell-derived EVs can transfer their protein cargo to both normal mammary epithelial cells and to fibroblasts, with the result being that the “normal” recipient cells acquired some of the transformed features of the original cancer cells that shed the EVs, including an enhanced proliferative capability, increased survival, and anchorage-independent growth (see Figure 1, right side; also Antonyak et al., 2011). Moreover, the introduction of NIH 3T3 fibroblasts, together with mitotically arrested breast cancer cells that were still capable of continuously generating and shedding EVs, into immune- deficient mice resulted in tumors of fibroblast origin. The ability of these non-cancerous cells to respond to the cancer cell-derived EVs and exhibit transformed features required the continuous exposure of the cells to the EVs, i.e., the observed cellular changes were not the outcome of permanent and stable effects on the genetic composition of the cells. Interestingly, the EV-driven changes in the recipient cells were dependent on fibronectin that was situated along the outer surfaces of the EVs, where it was covalently crosslinked by the acyl transferase, transglutaminase-2.
Still another set of interesting discoveries suggests that the EV-mediated transfer of information between cancer cells and non-cancerous cells in the microenvironment is reciprocal. For example, within the context of breast cancer, stromal cells have been shown to release EVs that in turn contribute to chemoresistance and tumor re-initiation (Boelens et al., 2014). Specifically, RNA cargo in EVs released by stromal cells was responsible for activating interferon-dependent genes, such as STAT1, in breast cancer cells. EV-dependent activation of STAT1, together with the expression of NOTCH3 on the surfaces of stromal cells and the resulting activation of JAG1 in the breast cancer cells, then gave rise to increased numbers of tumor-initiating cells, which are resistant to many standard types of cancer therapy.
Effects of EVs on Tumor Angiogenesis
As a tumor grows and increases in size, it needs to develop mechanisms to recruit new blood vessels (i.e., angiogenesis) to ensure a sufficient supply of oxygen and nutrients. This process requires that the tumor establishes a pathway through its microenvironment to provide the necessary space for new blood vessel formation, and sends the appropriate signals to trigger the recruitment and growth of endothelial cells to generate blood vessels (Weis and Cheresh, 2011). EVs have been implicated in each of these processes. Specifically, the stress of hypoxia or serum starvation has been shown to increase the release of EVs from cancer cells (King et al., 2012; Sun et al., 2014). Cancer cell-derived MVs have also been shown to contain matrix metalloproteinases (Dolo et al., 1999; Graves et al., 2004; Janowska-Wieczorek et al., 2006; 2005), which can contribute to the degradation of the extracellular matrix and help to provide a path for blood vessel formation. In addition, cancer cell-derived EVs can directly attract and activate endothelial cells through some of their cargo, which includes vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and platelet-derived growth factor (Baj-Krzyworzeka et al., 2006; Choi et al., 2007; Graner et al., 2009; Martins et al., 2013; Mause and Weber, 2010; Svensson et al., 2011). Some studies have also implicated the mRNA and miRNA cargo of EVs in angiogenesis (Al-Nedawi et al., 2009a; Chen and Gorski, 2007; Grange et al., 2011; Hong et al., 2009; Kosaka et al., 2013; Skog et al., 2008; Umezu et al., 2014; Yang et al., 2011). Moreover, an intriguing mechanism was proposed whereby activated EGF receptors associated with EVs, when transferred to endothelial cells, triggered a signaling pathway that led to the upregulated expression of VEGF in the recipient cells. This in turn was suggested to lead to the secretion of VEGF and the activation of endothelial cell VEGF receptors through an autocrine mechanism (Al-Nedawi et al., 2009b).
Effects of EVs in Cancer Metastasis
An area receiving a great deal of research attention involves the roles played by EVs in the metastatic process. In one interesting study, EVs containing the CD81 protein, when released from fibroblasts, were subsequently endocytosed by breast cancer cells (Luga et al., 2012). The endocytosed EVs were then loaded with the Wnt11 signaling factor and recycled back to the extracellular space, at which point the EV-tethered Wnt11 gave rise to an autocrine stimulation of the breast cancer cells, activating their core planar cell polarity components, which are distributed asymmetrically in individual cancer cells to help stimulate directional cell motility.
EVs have also been heavily implicated in the creation of what is referred to as the pre-metastatic niche. For example, Peinado et al. (2012) showed that highly metastatic melanoma cells release EVs, which increased vascular leakiness and enhanced the metastasis of melanoma cells that were orthotopically injected into mice. On the other hand, EVs isolated from poorly metastatic cells exhibited little ability for enhancing metastasis. In a subsequent study, the same group reported that the formation of the pre-metastatic niche by pancreatic ductal carcinoma cells in the liver was mediated by EVs (Costa-Silva et al., 2015). Specifically, EVs from these cancer cells, when injected into the bloodstream of mice, increased the ability of the pancreatic cancer cells to metastasize to the liver. Kupffer cells in the liver were shown to preferentially take up the circulating EVs, causing them to respond to the macrophage migration inhibitory factor in the EVs by secreting transforming growth factor β. This, in turn, prompted hepatic stellate cells to secrete fibronectin and to recruit bone marrow-derived macrophages, which together created a fibrotic environment ideal for metastasis.
In addition to triggering changes that favor metastasis, EVs have also been linked to the specificity exhibited by cancer cells with regard to the organs where they form secondary sites of tumor colonization. Thus, Hoshino et al. (2015) showed that priming a mouse with EVs derived from a specific cancer cell line that normally metastasizes to the lung can redirect a bone-metastasizing cancer cell to the lungs. Mass spectrometry analysis of EVs, isolated from liver, lung, and brain metastatic cancer cells, suggested that integrins were responsible for the specificity of where cancer cells metastasize (i.e., organotropism). Integrin α6 was linked to lung metastasis, whereas integrins αv and β5 were implicated in liver metastasis and integrin β3 was connected to brain metastasis. Moreover, integrins α2 and β1 were found to be present in all EVs shed by metastatic cancer cells, thus suggesting that these integrins might serve as metastatic markers.
EVs in Stem Cell Biology
EVs and Adult Stem Cells
Most of what is known regarding EV biogenesis and function has come from studies involving cancer cells. However, since it is now recognized that most types of non-cancerous cells are also capable of generating EVs, more attention is being paid to the roles played by EVs in various physiological settings. This is perhaps best exemplified by some of the recent findings in the fields of regenerative medicine and stem cell biology.
Mesenchymal stem cells (MSCs) are one type of adult stem cell that is most often derived from bone marrow, but can also be isolated from placental and adipose tissue (Dominici et al., 2006). They grow well in culture, are capable of undergoing self-renewal, and can be induced to differentiate into multiple cell lineages, making MSCs particularly attractive for use in many cell-based regenerative applications. Indeed, MSCs have been suggested to have potential therapeutic benefits against a wide range of disease conditions and injuries, ranging from renal failure and neurodegeneration to skin burns (Wei et al., 2013). For example, the transplantation of MSCs into damaged or diseased hearts as a means to stimulate its repair and regeneration has been aggressively pursued in both animal models and clinical trials (Choi et al., 2011; Pittenger and Martin, 2004). MSCs injected into rodent models of ischemic heart disease, such as myocardial infarction, have been shown to significantly improve heart function (Amado et al., 2005; Kawamoto et al., 2006; Tomita et al., 1999). Some of this outcome could be attributed to the MSCs directly differentiating into cardiomyocytes to generate new cardiac tissue (Toma et al., 2002). However, the main benefit of transplanting MSCs into ischemic hearts appears to involve their ability to secrete factors into the local environment that help to maintain the viability of damaged cardiac cells until they have the opportunity to undergo repair, as well as promote the expansion of healthy populations of cells (Ranganath et al., 2012; Timmers et al., 2011). In fact, it was shown that treating cultures of cardiomyocytes exposed to hypoxic conditions with the conditioned medium taken from MSCs suppressed the activation of the apoptotic-inducing protein caspase-3 in the cardiomyocytes and promoted their survival (Xiang et al., 2009). Consistent with this finding, injecting MSC-conditioned medium into ischemic hearts nearly completely recapitulated the actions of injecting the MSCs, further suggesting that paracrine factors released by MSCs likely played an important role in mediating their therapeutic potential (Chen et al., 2008; Gnecchi et al., 2006; Timmers et al., 2011; 2008).
Efforts to determine the components of MSC secretomes that were responsible for their protective and proliferative effects led to the discovery that MSCs derived from several tissue sources generated EVs. Surprisingly, when EVs were isolated from MSC-conditioned medium and injected into animal models of ischemia/reperfusion injury, they were shown to be sufficient to elicit cardioprotection and decrease the damage caused by the ischemic conditions (Lai et al., 2010). These outcomes appear to be, at least in part, due to the ability of the EVs to activate the major survival-promoting signaling proteins phosphoinositide 3-kinase and AKT, as well as increase metabolic activities, in the targeted cardiomyocytes (see Figure 2, also Arslan et al., 2013). However, the injected EVs were also able to influence the function of another cell type found in the local environment, namely endothelial cells. In this case, the uptake of EVs from MSCs by endothelial cells stimulated their recruitment to the damaged heart tissue, resulting in the formation of new blood vasculature that further contributed to the repair/regeneration process (see Figure 2, also Kawamoto et al., 2006; Sahoo et al., 2011).
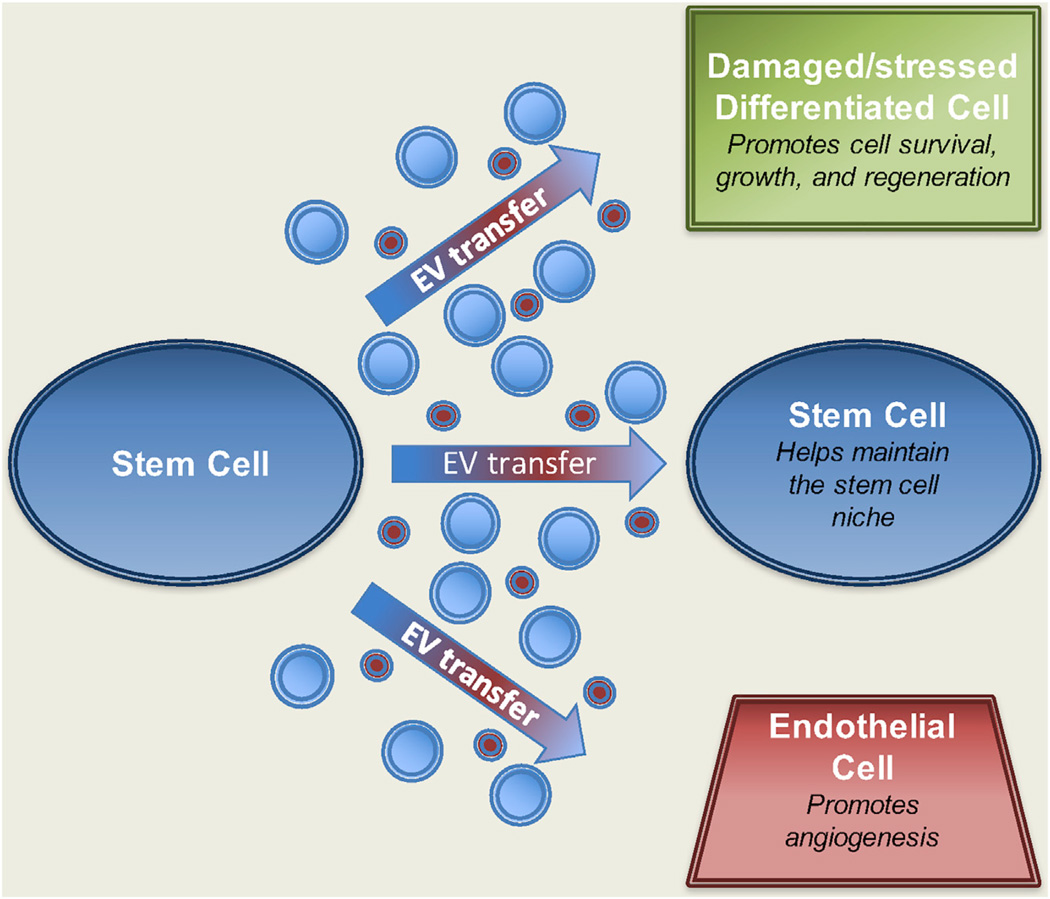
Figure 2. Stem Cells Generate EVs that Can Affect Their Environment.
Both adult stem cells and ESCs (blue cell on left) have been shown to release exosomes (red EVs) and MVs (blue EVs) into their surroundings. These EVs can be transferred to other stem cells (blue cell on right) to help maintain the stem cell niche by promoting cell growth (i.e., self-renewal). However, EVs from stem cells are also capable of being transferred to differentiated cell types (green cell on right) located within diseased or damaged tissue as a means to stimulate tissue regeneration (by promoting the growth and survival of the differentiated cell type). Moreover, EVs from stem cells have been shown to activate endothelial cells (red cell on right) found nearby diseased/damaged tissue to form new blood vessels (i.e., angiogenesis) as a mechanism to further stimulate regenerative processes.
The exact identity of the cargo in MSC-derived EVs that is responsible for mediating their biological functions still remains largely undetermined. However, proteomic and RNA-sequencing analyses revealed that they contain many of the same classes of cargo as EVs derived from cancer cells, including proteins, RNA transcripts, and miRNAs that regulate cell growth, survival, and migration (Feng et al., 2014; Lai et al., 2012; Rani et al., 2015; Yu et al., 2013). Given that EVs from MSCs and cancer cells are able to induce similar phenotypic changes in recipient cells, it will be especially interesting to ultimately see to what extent the cargo in the EVs derived from these different cell types is conserved.
EVs and Embryonic Stem Cells
While EVs from MSCs are currently in the spotlight due to their potential therapeutic benefits for acute tissue injury, additional lines of evidence have emerged suggesting that embryonic stem cells (ESCs) are also capable of shedding EVs with potentially unique functional capabilities (Katsman et al., 2012; Khan et al., 2015; Ratajczak et al., 2006b; Yuan et al., 2009). ESCs are derived from the inner cell mass of blastocyst-stage embryos and can self-renew indefinitely, as well as differentiate into virtually any cell type in the body (Smith, 2001). Some studies have shown that ESC EVs contain many of the proteins and/or RNA transcripts known to be important for maintaining “stemness” including Oct3/4, Sox2, Wnt-3, Nanog, and Rex-1 (Katsman et al., 2012; Ratajczak et al., 2006b). These findings suggest that ESCs may generate EVs to communicate with other ESCs as a mechanism to help maintain the pluripotent phenotype (see Figure 2). However, what makes these findings particularly intriguing is that ectopically expressing several of these stemness-promoting proteins in fully differentiated cell types (e.g., fibroblasts) can reprogram the cells to dedifferentiate into a stem cell-like state (Yu et al., 2007). This process, referred to as induced pluripotency, provides opportunities for modeling diseases, as well as offering new strategies for drug development, and holds exciting promise as an approach for treating a wide range of diseases and neurodegenerative disorders (Nishikawa et al., 2008; Robinton and Daley, 2012). However, inducing pluripotency in adult cells has also proved to be extremely tedious and inefficient. Because the current approaches involve the genetic manipulation of the target cells, they cannot be used for therapeutic applications in humans (Nishikawa et al., 2008; Robinton and Daley, 2012). Thus, more efficient approaches to induce pluripotency are needed; especially strategies that do not require genetically altering the genome of a cell. This is why we are becoming increasingly attracted to the idea that EVs derived from ESCs, which contain the very proteins required for inducing pluripotency, could potentially be used as an alternative way to achieve this outcome. In fact, it is tempting to speculate that the EV-mediated transfer of these pluripotent proteins to differentiated cells could be sufficient to induce a transient stem cell-like phenotype. Although we are only at the earliest stages of these experiments, recent studies showing that treating hematopoietic progenitor cells with EVs isolated from ESCs can promote their survival and stimulate their expansion in vitro (Ratajczak et al., 2006b) are extremely encouraging.
Future Directions
Because of the important roles played by EVs in cancer progression and stem cell biology, as outlined in the preceding sections, there are a number of key questions that will likely receive a great deal of research attention in the future. For example, we need to learn much more about the underlying mechanisms that enable EVs to be loaded with specific protein, RNA, and DNA cargo. In light of the indications that at least some of the specific cargo contained by cancer cell-derived MVs contributes to malignant transformation, it is reasonable to speculate that new therapeutic strategies will be developed that are based on a better understanding of how EV cargo is being recruited into these vesicles, thereby making it possible to block the recruitment of these essential components. Yet another exciting possibility regarding how EVs may eventually be used in the clinics is as a therapy delivery system. In this case, efforts to load isolated EVs with specific therapeutic cargo, such as drugs, RNA transcripts, or even DNA, and then use them to efficiently transfer the therapy to diseased or damaged target cells are being aggressively pursued (Vader et al., 2016). At present, we also know very little about the mechanisms by which MVs mature and bud from the plasma membranes of cells. In particular, what distinguishes MVs from membrane blebs, such that MVs are shed from the surface of the plasma membrane, rather than being retracted back into the cell interior? Moreover, what roles do the actin cytoskeleton and microtubules play in the formation and maturation of MVs along the surfaces of cancer cells? What substitutes for these roles in stem cells, such that signals to the RhoA-Rho kinase-Lim kinase pathway, which are essential for MV formation in aggressive cancer cell lines (Li et al., 2012), do not seem to be required for MV formation in ESCs?
It seems likely that another important area of future study will involve establishing the potential for MVs as biomarkers of disease, especially in cancer. Given the reports that MVs can be identified in different biological fluids, together with the indications that MVs contain specific cargo based on their cells of origin, there exist exciting possibilities regarding the identification of EVs in the blood and serum samples from patients as indicators of disease progression. In fact, there already has been a recent report in which the detection of glypican-1-enriched exosomes, in the serum of pancreatic cancer patients, has been proposed to represent an early non-invasive diagnostic indicator of this disease (Melo et al., 2015). However, to fully capitalize on this exciting potential we will need to develop the necessary technology to ensure the reliable isolation of disease- specific EVs from serum and tissue samples, in a manner that rigorously distinguishes these vesicles from those generated by normal (non-diseased) cells. Also required will be the establishment of the necessary methodology for the high-sensitivity detection of specific cargo proteins or RNA/miRNA. One especially intriguing possibility will be to take advantage of specific RNA transcripts that are present in cancer cell-derived MVs and use the available technology to amplify these messages as a highly sensitive method for detection.
Finally, we can anticipate that the roles of EVs in other important biological contexts, aside from those that have been elaborated upon in this review, will emerge in the coming years. There are good reasons to suspect that EVs will be important contributors in immune surveillance and the response of our immune system to invading infectious agents. However, EVs are also likely to play important roles in the actions of infectious agents themselves (both in bacteria and viruses). Moreover, it is reasonable to predict important functions for EVs in various developmental processes including the brain and nervous system. Clearly, there is much to look forward to in the coming years as the field of EVs continues to blossom and attract researchers from a wide range of biological areas, and with an ever expanding skill set of technologies that can be brought to bear in studying these interesting and unique “satellites of information transfer.”
REFERENCES
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009a;8:2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc. Natl. Acad. Sci. USA. 2009b;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie J-S, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak MA, Cerione RA. Microvesicles as mediators of intercellular communication in cancer. Methods Mol. Biol. 2014;1165:147–173. doi: 10.1007/978-1-4939-0856-1_11. [DOI] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc. Natl. Acad. Sci. USA. 2011;108:4852–4857. doi: 10.1073/pnas.1017667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Szatanek R, Węglarczyk K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immuno. Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Weimann BZ, Ishwaran H, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, Fan JB, Breakefield XO, Saydam O. miR-1289 and “Zipcode”-like sequence enrich mRNAs in microvesicles. Mol. Ther. Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gorski DH. Regulation of angiogenesis through a micro-RNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2007;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol. Rep. 2012;28:1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D-S, Lee J-M, Park GW, Lim H-W, Bang JY, Kim Y-K, Kwon K-H, Kwon HJ, Kim KP, Gho YS. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J. Proteome Res. 2007;6:4646–4655. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- Choi Y-H, Kurtz A, Stamm C. Mesenchymal stem cells for cardiac cell therapy. Hum. Gene Ther. 2011;22:3–17. doi: 10.1089/hum.2010.211. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. Ectosomes. Curr. Biol. 2011;21:R940–R941. doi: 10.1016/j.cub.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin. Exp. Metastasis. 1999;17:131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor ST, Li S, Chin AR, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Kinoshita T. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim. Biophys. Acta. 2012;1821:1050–1058. doi: 10.1016/j.bbalip.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J. Biol. Chem. 2011;286:3261–3269. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Tan E, Sharghi-Namini S, Asada HH. Exosomes in cancer microenvironment and beyond: have we overlooked these extracellular messengers? Cancer Microenviron. 2012;5:323–332. doi: 10.1007/s12307-012-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–7049. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, Xiang J. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp. Oncol. 2006;28:126–131. [PubMed] [Google Scholar]
- Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim. Biophys. Acta. 2012;1819:1154–1163. doi: 10.1016/j.bbagrm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Hong BS, Cho J-H, Kim H, Choi E-J, Rho S, Kim J, Kim JH, Choi D-S, Kim Y-K, Hwang D, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:1–13. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol. Cell. Proteomics. 2012;11:863–885. doi: 10.1074/mcp.M111.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Marquez Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–1209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl. Acad. Sci. USA. 2015;112:E1433–E1442. doi: 10.1073/pnas.1418401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsman D, Stackpole EJ, Domin DR, Farber DB. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One. 2012;7:e50417. doi: 10.1371/journal.pone.0050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:1–10. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic MicroRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int. J. Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31:4740–4749. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler. Thromb. Vasc. Biol. 2012;32:2113–2121. doi: 10.1161/ATVBAHA.112.255471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. J. Thromb. Haemost. 2005;3:1737–1744. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Mairhofer M, Steiner M, Mosgoeller W, Prohaska R, Salzer U. Stomatin is a major lipid-raft component of platelet alpha granules. Blood. 2002;100:897–904. doi: 10.1182/blood.v100.3.897. [DOI] [PubMed] [Google Scholar]
- Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013;25:66–75. doi: 10.1097/CCO.0b013e32835b7c81. [DOI] [PubMed] [Google Scholar]
- Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta. 2010;1805:105. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J. Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Mause SF, Weber C. Microparticles protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Schneider M, Biemer-Daub G, Wied S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011;23:1207–1223. doi: 10.1016/j.cellsig.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S-I, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006a;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006b;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. Demonstration of melanosome transfer by a shedding microvesicle mechanism. J. Invest. Dermatol. 2012;132:1073–1074. doi: 10.1038/jid.2012.20. [DOI] [PubMed] [Google Scholar]
- Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012;18:1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J. Biol. Chem. 2011;286:14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- Sun L, Wang H-X, Zhu X-J, Wu P-H, Chen W-Q, Zou P, Li Q-B, Chen Z-C. Serum deprivation elevates the levels of microvesicles with different size distributions and selectively enriched proteins in human myeloma cells in vitro. Acta Pharmacol. Sin. 2014;35:381–393. doi: 10.1038/aps.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson KJ, Kucharzewska P, Christianson HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis D, Saksena S, Emr SD. SnapShot: the ESCRT machinery. Cell. 2009;137:182–182.e1. doi: 10.1016/j.cell.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell Biochem. 2010;111:488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2008;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lötvall J, Nakagama H, Ochiva T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Li R-K, Weisel RD, Mickle DAG, Kim E-J, Sakai T, Jia Z-Q. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016 doi: 10.1016/j.addr.2016.02.006. http://dx.doi.org/10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van der Vos KE, Balaj L, Skog J, Breakefield XO. Brain tumor microvesicles: insights into intercellular communication in the nervous system. Cell. Mol. Neurobiol. 2011;31:949–959. doi: 10.1007/s10571-011-9697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Yang X, Han Z-P, Qu F-F, Shao L, Shi Y-F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer. 2009;125:1595–1603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M-X, He A-N, Wang J-A, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J. Zhejiang Univ. Sci. B. 2009;10:619–624. doi: 10.1631/jzus.B0920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:1–13. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, Ashraf M, Xu M. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells Is partially mediated by translocation of miR-221 in microvesicles. PLoS One. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, Farber DB. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527:100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SI, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]