Abstract
Cladophialophora bantiana is a neurotropic dematiaceous fungus which rarely causes disseminated disease. We report a case of proven C. bantiana osteomyelitis in a renal transplant recipient, complicated with probable cerebral disease. Stable disease was reached after combined antifungal therapies, immune enhancement and amputation of the infected lower limb.
Keywords: Cladophialophora bantiana, Osteomyelitis, Phaeohyphomycosis, Immunocompromised, Transplantation
1. Introduction
Cladophialophora bantiana is a black fungus with worldwide distribution, but a general preference for warm climates with high humidity [1]. It is a strongly neurotropic fungus which mostly causes life-threatening cerebral phaeohyphomycosis [1]. A recent review of 124 C. bantiana brain abscess cases showed an equal distribution in immunocompetent and immunocompromised hosts with a significantly higher mortality in the latter group [2]. Complete excision of the brain lesions in immunocompetent hosts led to significant better survival. Non-cerebral C. bantiana infections are extremely uncommon and include mainly cutaneous and pulmonary manifestations [1]. In this case report, we describe a C. bantiana osteomyelitis in a renal transplant patient. C. bantiana, as well as other dematiaceous fungi, only rarely cause osteomyelitis or arthritis [3]. One recent case of a young immunocompetent man with disseminated C. bantiana infection reported biopsy proven vertebral osteomyelitis [4]. Another fatal case of C. bantiana septic arthritis of the knee was described by Lim et al. [5]. C. bantiana bone or joint infection with concomitant cerebral infection is a life-threatening disease. In contrast to the two previous reported cases, stable disease was reached after combined antifungal therapies, immune enhancement and amputation of the infected lower limb.
2. Case
A 34-year-old woman of North African origin, who had undergone kidney transplantation 17 months before, presented at day 0 with progressive pain in the left lower leg since 3 weeks. One year before, in her homeland, she had suffered a traumatic wound of the same leg, which had resolved spontaneously and without sequelae.
Upon presentation, immunosuppressive treatment consisted of tacrolimus, mycophenolate mofetil (MMF) and low dose methylprednisolone. She had been treated with a course of high dose steroids for biopsy-proven allograft rejection 5 months earlier. Other relevant medical history included new-onset diabetes after transplantation and splenectomy following thrombosis of the vena lienalis.
She presented with a C-reactive protein (CRP) of 61.4 mg/L (reference <5 mg/L) and a normal neutrophil count. Graft function was stable with a serum creatinine level of 1.49 mg/dL (reference 0.51–0.95 mg/dL). Magnetic Resonance Imaging (MRI) of the left lower limb showed subacute osteomyelitis of the left proximal tibia with bifocal Brodie abscesses in the proximal diaphysis.
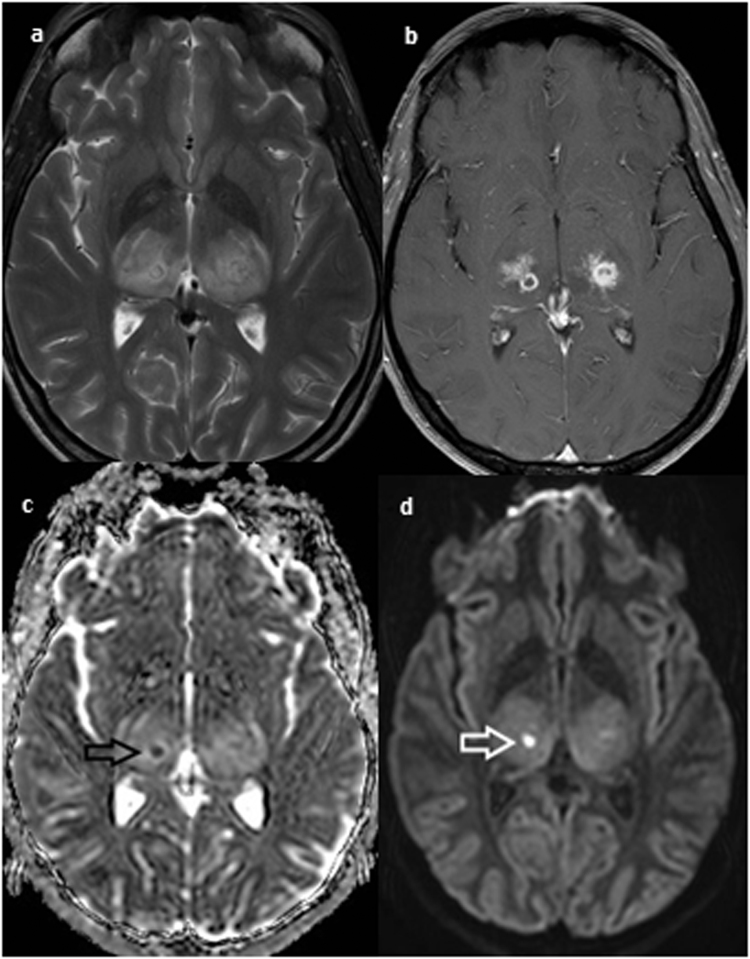
Surgical debridement was performed at day +6 with sampling for bacterial, fungal and mycobacterial culture and panbacterial and panfungal PCR (UMD-Universal, Molzym, Germany). Initial bacterial culture results revealed growth of three organisms (Staphylococcus capitis, Corynebacterium amycolatum and Staphylococcus epidermidis) and anatomopathologically no inflammation was found. Intravenous amoxicillin-clavulanic acid (2 g BID) was started with careful follow-up. At day +9, panfungal PCR on the perioperative sample tested positive. Additional deeper bone biopsy sections showed a chronic osteomyelitis with presence of hyphae. Fungal cultures showed a black fungus, which was identified by internal transcribed spacer (ITS) sequencing and morphological characteristics as C. bantiana on day +29. ITS sequencing was performed in both sequencing directions with primer pairs ITS1/ITS4 and ITS86/ITS4. The aligned sequence had closest hit in GenBank with C. bantiana (accession number KP131826.1) indicating 98% sequence identity for ITS1 and ITS2 region and showed 100% sequence identity with the ITS2 C. bantiana barcode identifier (accession number EU103989) described by Heinrichs et al. [6]. In the meantime, the patient had developed neurological symptoms, with progressive right-sided weakness and loss of sensibility. Cerebrospinal fluid (CSF) analysis showed normal white blood cell count (2.4/µL), normal protein and glucose level. Bacterial and fungal cultures and molecular diagnostics for viruses on CSF were negative. Brain MRI revealed acute diffuse encephalomyelitis with bilateral thalamic involvement, compatible with cerebral infection (Fig. 1).
Fig. 1.
T2 weighted brain magnetic resonance imaging shows bilateral diffuse thalamic abnormalities with edema in the internal capsules (a). Multiple confluent micronodules with small cystic components are seen on a gadolinium enhanced T1 weighted image (b). An area of diffusion restriction, indicated by arrows, is noted in the right thalamus on diffusion weighted imaging, reflecting the presence of pus (c,d).
Given the location of the lesions, neurosurgical intervention was considered hazardous and no tissue biopsy was performed to confirm the diagnosis of cerebral phaeohyphomycosis. Antifungal treatment with oral voriconazole was started on day +30 and amoxicillin-clavulanic acid was stopped. In addition, MMF was stopped and lower tacrolimus trough levels were targeted (6 µg/L). Despite improvement of inflammatory parameters and having adequate voriconazole trough levels of 3.2–6.8 mg/L, new neurological symptoms of left-sided paresthesia and stiffness developed. Follow-up brain MRI showed progressive disease. Liposomal amphotericin B (3 mg/kg QD) was added to voriconazole on day +59.
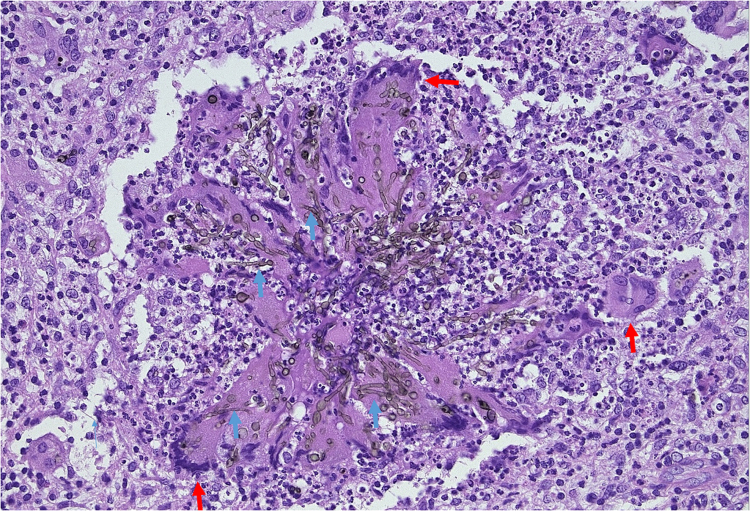
Worsening of the osteomyelitis urged for a second surgical debridement on day +71. All perioperative samples again showed the abundant presence of C. bantiana (Fig. 2), triggering a complete withdrawal of all immunosuppressive drugs and transplantectomy. Hemodialysis was started on day +92. Unfortunately, clinical assessment and novel imaging showed further disease progression in bone and brain.
Fig. 2.
Hematoxylin-eosin stain of tibia bone biopsy (day +71). Granuloma with central hyphae and giant cells, surrounded by mononuclear inflammatory cells. Hyphae (blue arrows) are mainly present in the cytoplasm of the multinucleated giant cells (red arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Susceptibility testing of the isolate was performed by both Clinical and Laboratory Standards institute (CLSI) and European Committee on Antimicrobial Susceptibility testing (EUCAST) broth dilution method for voriconazole (minimal inhibitory concentration (MIC) 0.5 mg/L), amphotericin B (MIC <0.5 mg/L), isavuconazole (MIC 0.25 mg/L (CLSI), 4 mg/L (EUCAST)), posaconazole (MIC 0.016 mg/L (CLSI), 0.5 mg/L (EUCAST)) and anidulafungin (MIC 0.031 mg/L). Given the unfavorable evolution, liposomal amphotericin B - voriconazole therapy was switched to liposomal amphotericin B - isavuconazole (200 mg TID on first and second day, then 200 mg QD) combination therapy, reaching therapeutic serum isavuconazole through levels (range: 1.6–2.3 mg/mL).
Unfortunately, the disease progressed despite adaptation of the antifungal regimen. Local progression in the bone, with incipient involvement of the knee joint, urged for above-the-knee amputation of the left leg on day +129. Hereafter, isavuconazole was stopped. Oral delayed-release tablets of posaconazole (loading dose of 300 mg BID on first day; then 300 mg QD) and intravenous flucytosine (25 mg/kg QD) were added to the liposomal amphotericin B therapy, now at a higher dose of 10 mg/kg QD.
Following surgery there was no recurrence of osteomyelitis, but cerebral lesions further progressed. The first serum through level of posaconazole was 1.0 mg/L. The dose of posaconazole was doubled (300 mg BID) on day +156, which resulted in posaconazole trough levels ranging from 1.7 to 4.1 mg/L. Because of increased intracranial pressure, methylprednisolone (40 mg TID, intravenously) was associated on day +172. Following tapering and stopping steroids on day +224, intracranial overpressure relapsed and steroids were restarted.
Combination antifungal therapy with posaconazole, flucytosine, and liposomal amphotericin B and low dose steroids has finally resulted in clinical and radiological stabilization of the cerebral disease, without recurrence of osteomyelitis. Anti-fungal treatment was eventually downgraded to monotherapy with oral posaconazole and the patient started an intensive rehabilitation program while obviously continuing thrice weekly hemodialysis. At present (day +539), the patient has regained her ability to walk using a leg prosthesis.
3. Discussion
We report a case of C. bantiana osteomyelitis in a transplant recipient, with probable cerebral involvement. Trauma is the main cause of the rarely described cases of osteomyelitis with melanized fungi [1]. Our patient suffered a minor trauma 1 year before presentation. The often indolent characteristics of fungal osteomyelitis can explain the absence of symptoms until 3 weeks before hospitalization. In cerebral phaeohyphomycosis, C. bantiana is assumed to primarily colonize the lung, followed by hematogenous spread to the central nervous system (CNS) [7]. The surgical debridement may have theoretically induced hematogenous spread from bone to brain. However, since initially no brain imaging was performed and given the indolent nature of these infections, cerebral involvement may have already been present on day 0. Therefore, in non-cerebral C. bantiana infections, cerebral imaging at presentation should be considered.
C. bantiana infection is difficult to treat with a high mortality rate of approximately 70% [8]. Timely diagnosis of the fungal infection is important, but the challenging identification of C. bantiana may delay start of treatment. Complete surgical excision, combined antifungal therapy and immune enhancement seem to be the best treatment option for cerebral phaeohyphomycosis and fungal osteomyelitis [3], [7], [8].
In our patient, surgical bone debridement and finally amputation of the leg resulted in resolution of the osteomyelitis. In contrast, treatment of the cerebral lesions relied solely on antifungals since neurosurgical resection could not be performed due to the high risk of collateral neurological damage.
Experience with antifungal treatment is limited to isolated cases or small series, which mostly involved amphotericin B, a triazole (itraconazole, voriconazole or posaconazole) and/or flucytosine [3], [7], [8], [9].
Data concerning in vitro susceptibility of C. bantiana isolates and animal studies are sparse, but our in vitro susceptibility results of C. bantiana are in accordance with previously reported MICs [9]. Posaconazole, isavuconazole and amphotericin B showed the highest in vitro activity, with slightly higher MICs for voriconazole. However, voriconazole penetrates good in brain tissue and abscess material [10]. In cerebral C. bantiana infections, both successes and clinical failures have been reported with voriconazole [1].
Amphotericin B in mono- or combination therapy showed mixed results [8]. Furthermore, animal models showed less activity of amphotericin B compared to itraconazole and posaconazole [11]. In our case, association of liposomal amphotericin B could not stop progression.
Posaconazole has good in vitro susceptibility concerning C. bantiana, but the in vivo activity in CNS infections is not well documented. Only a limited number of clinical cases are available [10]. In murine models, brain tissue concentrations of posaconazole were 50–80% of those in serum [12], [13]. Moreover, animal studies reported better survival outcomes and better reduction of the brains fungal burden with posaconazole compared to itraconazole and amphotericin B [11].
Isavuconazole has low MICs for C. bantiana [9]. Furthermore, isavuconazole penetrates in the CNS of animal models with invasive candidiasis and few cases of successful treatment of cerebral mucormycosis are described [14], [15]. More animal and clinical data are needed to investigate the role of isavuconazole in treating C. bantiana infections.
Finally flucytosine, also with an excellent CNS penetration, was added to the therapy with posaconazole and liposomal amphotericin B [10]. In murine models with disseminated C. bantiana infections synergism and prolonged survival were seen when combining posaconazole, flucytosine and micafungin [16]. Furthermore, case series of C. bantiana infection reported a better survival with the combination of amphotericin B, flucytosine and an azole [7], [8].
In our patient, steroids had an important role in controlling cerebral edema. It remains unclear whether this edema was mainly caused by disease progression or by CNS-immune reconstitution syndrome (IRIS) after immune enhancement. IRIS, as seen in human immunodeficiency virus (HIV)-infected patients, has also been observed in solid organ transplant (SOT) patients with fungal (mainly Cryptococcosis) infections. SOT recipients developed IRIS typically 4–6 weeks (but until up to 9 months) after initiating antifungal therapy and IRIS preferentially manifested in the CNS. No cases of C. bantiana infection related IRIS were described. The optimal management of IRIS in SOT recipients remains unknown, but based on case reports and series corticosteroids have been suggested [17].
Stabilization of the cerebral infection was finally achieved with a combined use of liposomal amphotericin B, posaconazole and flucytosine as well as low dose methylprednisolone.
Conflict of interest
Katrien Lagrou has received research grants, travel support and lecture honoraria from Gilead, MSD and Pfizer. Inge Derdelinckx received travel support van Gilead sciences, MSD, ViiV, Boehringer-Ingelheim, Janssen-Cilag. Johan Maertens has served as consultant to Schering-Plough, Gilead Sciences, Merck, Sharp & Dohme, Pfizer Inc., Bio-Rad, Fujisawa healthcare Inc., Astellas, Nextar, Zeneus (Cephalon), Viropharma, and Boehringer-Ingelheim; has received research funding from Bio-Rad, Merck, Sharp & Dohme, and Pfizer Inc., and is on the speaker's bureau for Schering-Plough, Gilead Sciences, Merck, Sharp & Dohme, Pfizer Inc., Bio-Rad, Fujisawa healthcare, Inc, Astellas and Zeneus (Cephalon).
Acknowledgments
We thank Walsh Thomas J. (Infectious Diseases Section, National Cancer Institute, Bethesda, Maryland, USA), Revankar Sanjay G. (Division of Infectious Diseases, Wayne State University, Detroit, Michigan, USA), Marr Kieren A. (Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, USA), Avery Robin (Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, USA), Kontoyiannis Dimitrios (Department of Infectious Diseases, Infection Control and Employee Health, University of Texas MD Anderson Cancer Center, Houston, USA) and Temesgen Zelalem (Division of Clinical Microbiology, Mayo Clinic and Foundation, Rochester, Minnesota, USA) for their availability to remotely discuss the case and the treatment steps on multiple occasions. We thank Meis Jacques (Canisius-Wilhelmina Ziekenhuis, Department of Medical Microbiology and Infectious Diseases, Nijmegen, Netherlands) for performing the susceptibility testing.
Basilea Pharmaceutica International Ltd and Astellas Pharma Inc. are acknowledged for providing isavuconazole through their compassionate use program.
References
- 1.Revankar S.G., Sutton D.A. Melanized fungi in human disease. Clin. Micro. Rev. 2010;23:884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti A., Kaur H., Rudramurthy S.M., Appannanavar S.B., Patel A., Mukherjee K.K. Brain abscess due to Cladophialophora bantiana: a review of 124 cases. Med. Mycol. 2016;54:111–119. doi: 10.1093/mmy/myv091. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhary A., Meis J.F., Guarro J., de Hoog G.S., Kathuria S., Arendrup M.C. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin. Microbiol. Infect. 2014;20:47–75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 4.Mansour A., Jordan K. Disseminated Cladophialophora bantiana disease in a patient with prediabetes. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-206426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim A., Speers D., Inderjeet C. Cladophialophora (Xylohypha) bantiana – an unusual cause of septic arthritis. Rheumatology. 2013;52:958–959. doi: 10.1093/rheumatology/kes317. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs G., de Hoog G.S., Haase G. Barcode identifiers as a practical tool for reliable species assignment of medically important black yeast species. J. Clin. Microbiol. 2012;50:3023–3030. doi: 10.1128/JCM.00574-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D.M., de Hoog G.S. Cerebral phaeohyphomycosis – a cure at what lengths? Lancet Infect. Dis. 2009;9:376–383. doi: 10.1016/S1473-3099(09)70131-8. [DOI] [PubMed] [Google Scholar]
- 8.Revankar S.G., Sutton D.A., Rinaldi M.G. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin. Infect. Dis. 2004;38:206–216. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 9.Badali H., de Hoog G.S., Curfs-Breuker I., Klaassen C.H., Meis J.F. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J. Clin. Microbiol. 2010;48:2350–2356. doi: 10.1128/JCM.00653-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felton T., Troke P.F., Hope W.W. Tissue penetration of antifungal agents. Clin. Micro. Rev. 2014;27:68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Abdely H.M., Najvar L.K., Bocanegra R., Graybill J.R. Antifungal therapy of experimental cerebral phaeohyphomycosis due to Cladophialophora bantiana. Antimicrob. Agents Chemother. 2005;49:1701–1707. doi: 10.1128/AAC.49.5.1701-1707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvo E., Pastor F.J., Rodriguez M.M., Pujol I., Guarro J. Antifungal therapy in a murine model of disseminated infection by Cryptococcus gattii. Antimicrob. Agents Chemother. 2010;54:4074–4077. doi: 10.1128/AAC.00172-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo E., Pastor F.J., Rodriguez M.M., Mayayo E., Salas V., Guarro J. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 2010;54:919–923. doi: 10.1128/AAC.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majithiya J., Sharp A., Parmar A., Denning D.W., Warn P.A. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J. Antimicrob. Chemother. 2009;63:161–166. doi: 10.1093/jac/dkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ervens J., Ghannoum M., Graf B., Schwartz S. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014;42:429–432. doi: 10.1007/s15010-013-0552-6. [DOI] [PubMed] [Google Scholar]
- 16.Marine M., Pastor F.J., Guarro J. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 2009;47:45–49. doi: 10.1080/13693780802526840. [DOI] [PubMed] [Google Scholar]
- 17.Sun H.Y., Singh N. Opportunistic infection-associated immune reconstitution syndrome in transplant recipients. Clin. Infect. Dis. 2011;53:168–176. doi: 10.1093/cid/cir276. [DOI] [PubMed] [Google Scholar]