Abstract
Scedosporium is an important pathogen in cystic fibrosis (CF) and post-transplant but rarely causes invasive infection. Treatment remains challenging, particularly due to inherent resistance to multiple antifungal agents. We present a young man with CF who developed invasive sternal and rib infection 10-months following lung transplant. The infection has been clinically and radiologically cured with extensive surgery and triazole therapy. This case highlights the importance of adjunctive surgery in addition to prolonged triazole treatment to manage invasive Scedosporium infections in immunosuppressed patients.
Keywords: Scedosporium, Cystic fibrosis, Transplant, Voriconazole, Posaconazole
1. Introduction
Scedosporium is a filamentous fungus that is ubiquitous in the environment [1]. There are two main species that cause human infection, Scedosporium apiospermum (S. apiospermum) and Scedosporium prolificans (S. prolificans) that have been shown to cause a range of infections in humans [2], [3]. It is the second most common fungal infection in patients with cystic fibrosis (CF), and represents approximately one quarter of non-Aspergillus filamentous fungal infections in solid organ transplant patients [4], [5], [6]. Although it is frequently isolated in organ transplant recipients it is only rarely associated with invasive disease [6]. Treatment of invasive disease remains challenging, particularly due to inherent resistance of Scedosporium to multiple antifungal agents.
2. Case
We describe a 21-year old male diagnosed with underlying CF who developed an invasive sternal and lower rib S. apiospermum infection post-bilateral sequential lung transplant (BSLT) that was cured with aggressive surgery and antifungal therapy. Time point references during the article are in comparison to Day 0. Day 0 is defined as the day of BSLT, 10.5 months before he developed symptoms of deep sternal infection.
After a perinatal diagnosis of CF, and a relatively medically stable childhood and early adolescence the patient began to develop multi-system manifestations of chronic CF during his teenage years. These included severe bronchiectasis with multi-drug resistant (MDR) Pseudomonas aeroginosa (P. aeroginosa) colonization, gastroesophageal reflux disease requiring Nissen fundoplication at age 15-years, pancreatic exocrine and endocrine insufficiency with resulting CF-related diabetes, malnutrition requiring percutaneous gastrostomy tube feeding and CF-liver disease complicated by cirrhosis, portal hypertension, mild uncomplicated esophageal varices and hypersplenism, with preserved synthetic function, treated with ursodeoxycholic acid and propranolol. Over a four-year period from age 17, he had a sub-acute but progressive decline in lung function due to recurrent lower respiratory tract infections. During this time he had six hospital admissions and first cultured positive for S. apiospermum in the sputum, identified by characteristic macroscopic and microscopic morphological features by a microbiologist experienced in fungal identification. He was treated with itraconazole for four months at age 17 and with voriconazole for four months at age 18 and nine months at age 20 due to persistent S. apiospermum sputum culture positivity. Despite this therapy he had persistent S. apiospermum sputum positivity during the peri-transplant period without evidence of invasive disease.
He underwent BSLT at age 20 years (Day 0). The procedure was uncomplicated with well size-matched donor lungs. Both donor and recipient were cytomegalovirus negative whilst the donor was Epstein-Barr Virus (EBV) negative and the recipient was positive. During the perioperative period he was treated with intravenous meropenem and tobramycin, triple immunosuppression with tacrolimus, mycophenolate and corticosteroids as well as basiliximab but no antifungal therapy. He received prophylaxis with ganciclovir for EBV and trimethoprim/sulfamethoxazole for Pneumocystis. His post-operative course was complicated by high intercostal catheter outputs and pleural effusions requiring high dose methylprednisolone for presumed acute rejection, one episode of hepatic encephalopathy, small bowel obstruction, pericardial effusion, atrial fibrillation and acute kidney injury.
Whilst the early post-transplant bronchoscopy was positive for MDR P. aeroginosa, he did not culture S. apiospermum initially. At Day 14 post-transplantation he did culture S. apiospermum in addition to P. aeroginosa on bronchoalveolar lavage (BAL) and voriconazole was commenced. On Day 56 following lung transplantation he was discharged home. He persistently cultured S. apiospermum on sputum and BAL despite treatment with continuous oral triazole therapy, firstly with voriconazole for 10 weeks with therapeutic levels and then posaconazole for the subsequent time with variable serum levels (some sub-therapeutic). On Day 126 post transplant his sputum and BAL cultures became persistently negative for S. apiospermum.
On Day 302 his posaconazole was stopped in the setting of deranged liver function tests, albumin 28 g/L, bilirubin 40 μmol/L, ALT 70 units/L, GGT 245 units/L, ALP 361 units/L, and concerns regarding risk of developing decompensated liver disease. At this point he had been S. apiospermum culture negative for 176 days.
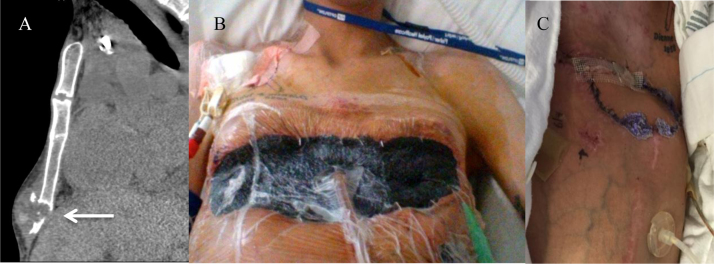
On Day 319 swelling and localized pain developed over the region of the lower sternum and ribs with dehiscence at the site of a previous intercostal catheter in the right anterior chest wall. Computerized tomography (CT) and ultrasound of the region revealed multiple collections over the bilateral costal margins and right anterior chest wall with a 3 cm collection adjacent to the lower sternum with displacement of the right 5–7th ribs and left 7th rib (Fig. 1A). Fungal culture of a wound swab of the area of dehiscence grew S. apiospermum and intravenous then oral voriconazole was restarted. Aggressive surgical debridement was undertaken with extensive resection of the lower sternum, bilateral lower ribs and associated chest wall tissue over five separate procedures with intervening V.A.C.® dressings (Fig. 1B). Fungal cultures from surgical specimens initially grew S. apiospermum and subsequently became negative on Day 334. Following conversion to culture negativity for S. apiospermum the patient underwent an extensive two-part reconstructive procedure with formation of an arterio-venous loop, a latissimus dorsi free flap to the sternum and a superficial skin graft (Fig. 1C). The recovery from these procedures was prolonged with an extensive period in the supine position due to concerns about the blood supply to the free flap; however, he was discharged home on Day 410 where he remains well more than two years following his transplant.
Fig. 1.
Computerized tomography images of the sternal area showing local sternal destruction. (A) Photograph of the patient's chest wall post-multiple extensive resections with V.A.C.® dressing in situ (B) and post-reconstructive surgery with a large free flap over previous resection sites (C).
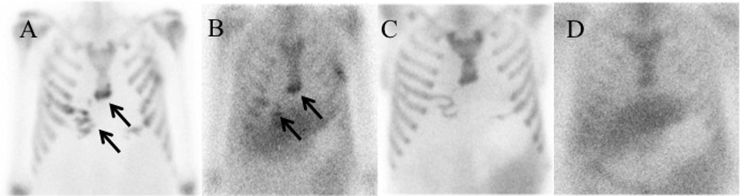
Follow up bone and Gallium-67 scans four months after surgery (Day 450) initially revealed uptake in the region of the anterolateral left 5th and right 5th and 6th ribs, inferior sternum, and right scapula extending into the adjacent soft tissues consistent with residual osteomyelitis and soft tissue infection (Fig. 2A and B). These findings completely resolved on repeat imaging 6 months later (Fig. 2C and D). The patient remains asymptomatic on voriconazole and we now believe him to be fully cured more than two years following his lung transplant.
Fig. 2.
Bone and Gallium-67 scan showing uptake in the region of the anterolateral left 5th and right 5th and 6th ribs, inferior sternum, and right scapula extending into the adjacent soft tissues consistent with osteomyelitis and soft tissue infection four months after starting treatment (A), (B). Bone and Gallium-67 scan showing no uptake in the anterolateral ribs or sternum consistent with complete resolution 10 months after starting treatment (C), (D).
3. Discussion
S. apiospermum is the second most common filamentous fungi isolated from the airways of patients with CF [5], [7]. The viscous secretions, impaired mucociliary clearance and structural lung changes create an ideal environment to establish both transient and chronic colonization. Whilst the treatment of invasive disease due to Scedosporium is more straightforward, the natural history of colonization in patients with CF is less clear and consequently, there are no consensus guidelines on whom or how to best treat these patients. Both CF and pre-transplant fungal colonization are independent risk factors for invasive fungal disease following transplant [8]. The most rational approach to managing patients with Scedosporium airway colonization in the pre- transplant setting still remains unclear but is in favor of aggressive antifungal therapy.
After transplantation, Scedosporium spp. infections tend to be disseminated and are associated with high mortality rates, highlighting the need for aggressive management [8]. In the setting of systemic immunosuppression the importance of adjunctive surgical debridement of Scedosporium spp. infection is increasingly recognized. Rodriguez-Tudela et al. in a review of 162 cases of S. prolificans infection found that surgical excision and reversal of the aplasias were independently associated with survival [9]. Whilst Husain et al. reviewed 80 cases of Scedosporium spp. infections in patients either post-stem cell transplant or solid organ transplant and demonstrated that adjunctive surgery was an independent predictor of survival [8].
Longitudinal studies of pre- and post-transplant patients are required to establish the true impact and consequence of colonization with Scedosporium species and direct best therapy. It is likely that the best time to aggressively manage Scedosporium would be in the pre-transplant setting when there is less immunosuppression and less potential for medication interactions.
As far as the Author's are aware this is the first case report outlining a cure from invasive S. apiospermum sternal infection following lung transplant. This young man's sternal, chest wall and rib infection has been clinically and radiologically eradicated with extensive resection of the infected tissue and prolonged triazole antifungal therapy. This case highlights the importance of peri-transplant treatment of Scedosporium colonization with the aim of eradication and adjunctive surgical therapy for management of disseminated infections in addition to the requirement for prolonged antifungal therapy if the infection recurs following transplant.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest
COM has been on the advisory board for, received investigator-initiated grants from and given lectures for Gilead Sciences, Merck Sharp and Dohme and Pfizer. All honoraria have been paid directly to Alfred Hospital.
No Conflict of interest declared for the other authors.
Acknowledgments
None.
References
- 1.Harun A., Gilgado F., Chen S.C., Meyer W. Abundance of Pseudallescheria/Scedosporium species in the Australian urban environment suggests a possible source for scedosporiosis including the colonization of airways in cystic fibrosis. Med. Mycol. 2010;48(Suppl 1):S70–S76. doi: 10.3109/13693786.2010.515254. [DOI] [PubMed] [Google Scholar]
- 2.Lamaris G.A., Chamilos G., Lewis R.E., Safdar A., Raad I.I., Kontoyiannis D.P. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin. Infect. Dis. 2006;43(12):1580–1584. doi: 10.1086/509579. [DOI] [PubMed] [Google Scholar]
- 3.Sole A. Scedosporium apiospermum disseminated infection in a single lung transplant recipient. Rev. Iberoam. Micol. 2011;28(3):139–142. doi: 10.1016/j.riam.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Nagano Y., Millar B.C., Goldsmith C.E., Elborn J.S., Rendall J., Moore J.E. Emergence of Scedosporium apiospermum in patients with cystic fibrosis. Arch. Dis. Child. 2007;92(7):607. doi: 10.1136/adc.2007.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimon B., Carrere J., Vinatier J.F., Chazalette J.P., Chabasse D., Bouchara J.P. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis.: Off. Publ. Eur. Soc. Clin. Microbiol. 2000;19(1):53–56. doi: 10.1007/s100960050011. [DOI] [PubMed] [Google Scholar]
- 6.Silveira F.P., Husain S. Fungal infections in solid organ transplantation. Med. Mycol. 2007;45(4):305–320. doi: 10.1080/13693780701200372. [DOI] [PubMed] [Google Scholar]
- 7.Paugam A., Baixench M.T., Demazes-Dufeu N., Burgel P.R., Sauter E., Kanaan R. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med. Mycol. 2010;48(Suppl 1):S32–S36. doi: 10.3109/13693786.2010.503665. [DOI] [PubMed] [Google Scholar]
- 8.Husain S., Munoz P., Forrest G., Alexander B.D., Somani J., Brennan K. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2005;40(1):89–99. doi: 10.1086/426445. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Tudela J.L., Berenguer J., Guarro J., Kantarcioglu A.S., Horre R., de Hoog G.S. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med. Mycol. 2009;47(4):359–370. doi: 10.1080/13693780802524506. [DOI] [PubMed] [Google Scholar]