Abstract
Communication between the 5′ and 3′ ends of a eukaryotic messenger RNA (mRNA) or viral genomic RNA is a ubiquitous and important strategy used to regulate gene expression. Although the canonical interaction between initiation factor proteins at the 5′ end of an mRNA and proteins bound to the polyadenylate tail at the 3′ end is well known, in fact there are many other strategies used in diverse ways. These strategies can involve “non-canonical” proteins, RNA structures, and direct RNA-RNA base-pairing between distal elements to achieve 5′-to-3′ communication. Likewise, the communication induced by these interactions influences a variety of processes linked to the use and fate of the RNA that contains them. Recent studies are revealing how dynamic these interactions are, possibly changing in response to cellular conditions or to link various phases of the mRNA’s life, from translation to decay. Thus, 5′-to-3′ communication is about more than just making a closed circle; the RNA elements and associated proteins are key players in controlling gene expression at the post-transcriptional level.
Keywords: messenger RNA, polyadenylate tail, 7-methylguanosine, mRNA circularization
Introduction
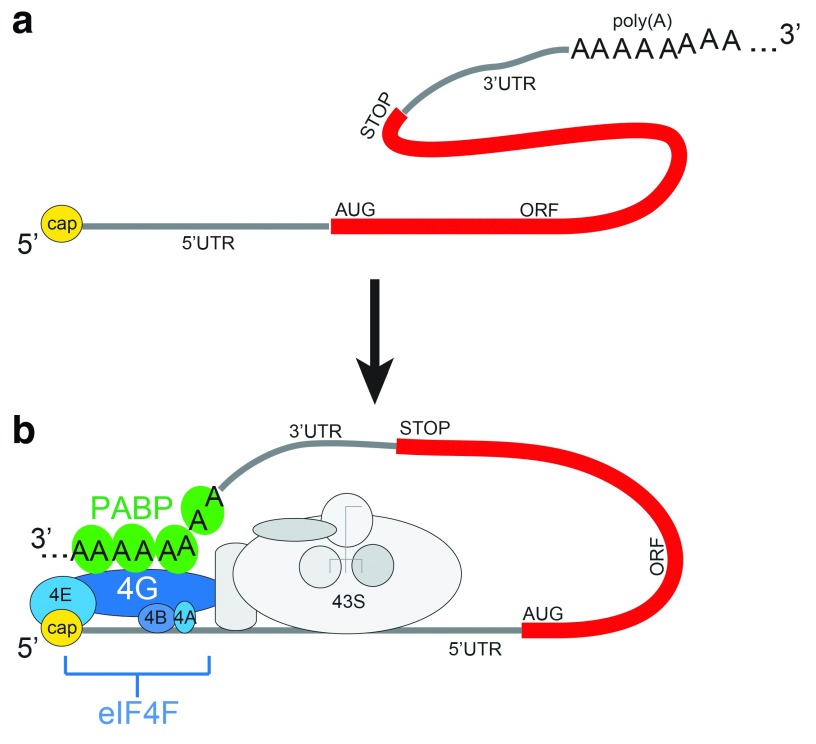
Messenger RNAs (mRNAs) provide the template for synthesis of proteins; by definition, they contain an open reading frame (ORF) that encodes an amino acid sequence. However, encoding a protein is not enough: the use and fate of specific eukaryotic mRNAs must be controlled within the overall strategy used by cells to regulate gene expression. Much of the regulatory power is conferred by essential cis-acting sequences and structures in the mRNA’s untranslated regions (UTRs), which reside both 5′ (upstream) and 3′ (downstream) of the ORF. Also important for regulation, the vast majority of mature eukaryotic mRNAs have a modified nucleotide (generally a 7-methylguanosine, or m7G) on their 5′ terminus and a polyadenylate (poly[A]) tail on their 3′ end ( Figure 1a). These features control the fate of the mRNA, in part through long-range communication between the 5′ and 3′ ends. Often described as “mRNA circularization”, this phenomenon is central in controlling a number of post-transcriptional events. In addition to cellular mRNAs, many viral genomic RNAs use communication between their 5′ and 3′ ends, illustrating how useful, important, and ubiquitous this strategy is.
Figure 1. Canonical 5′-to-3′ communication in eukaryotic messenger RNAs (mRNAs).
( a) Diagram of a mature eukaryotic mRNA. The 5′ modified nucleotide cap is shown in yellow, the open reading frame (ORF) in red, and the start codon AUG, translation termination codon (STOP), poly(A) tail, and untranslated regions (UTRs) are labeled. ( b) Diagram of 5′-to-3′ communication in the context of translation initiation. Poly(A)-binding protein (PABP) is shown in green, the eukaryotic initiation factor (eIF) 4F complex is in shades of blue, and its components are labeled. The 43S complex is depicted in shades of gray. poly(A), polyadenylate.
In this review, we discuss various strategies used by mRNAs and viral RNAs to enable communication between their 5′ and 3′ ends. We present these interactions in the context of the ways that they direct RNA function, including driving efficient translation, regulating mRNA stability and turnover, and promoting viral replication. We do not attempt a comprehensive assessment of all related literature, but rather present some illustrative examples to show how evolution has crafted a variety of ways to confer 5′-to-3′ communication, how useful this strategy is, and the dynamic nature of these interactions. We also highlight a few emerging ideas and areas that remain mysterious, encouraging further investigation.
5′-to-3′ communication used in translational control
Overview of eukaryotic translation initiation: canonical and non-canonical
Probably the most well-known use of 5′-to-3′ communication is to promote or regulate translation of eukaryotic mRNAs. Although some have urged caution in broadly accepting that the 3′ end of an mRNA affects initiation from the 5′ end 1, continued experimentation supports the existence of this communication. To explain how 5′-to-3′ communication can occur, we first outline the canonical initiation mechanism. The eukaryotic initiation factor (eIF) 4F complex, which contains the cap-binding protein eIF4E and scaffold protein eIF4G, binds at the 5′ end 2– 4. Bound eIF4F recruits the 43S complex, which contains the 40S ribosomal subunit 3. This complex then scans the 5′ UTR to find the appropriate start codon 4, where GTP hydrolysis, release of eIFs, and 60S subunit joining create a translationally competent 80S ribosome 5– 7 (reviewed in 8– 10). As an alternative to this canonical cap- and scanning-dependent pathway, some mRNAs and viral RNAs contain an internal ribosome entry site (IRES) that directs translation initiation using a 5′ end-independent mechanism. IRES RNAs often use a subset of the canonical eIFs and RNA structures to drive translation initiation (reviewed in 11, 12).
5′-to-3′ communication in translation using canonical translation factors
A ubiquitous and important form of mRNA 5′-to-3′ communication depends on the 5′ cap and poly(A) tail ( Figure 1). The presence of these two signals on opposite ends of an mRNA synergistically enhances the rate of translation of that mRNA 13, 14. The model is that eIF4F binds to the cap at the 5′ end while multiple poly(A)-binding proteins (PABPs) bind the poly(A) tail and eIF4G, physically linking the 5′ and 3′ ends ( Figure 1b) 15, 16. Functionally, the PABP-eIF4G interaction is associated with favorable subunit binding at the 5′ end 17, 18, and it increases the affinity of cap-binding protein eIF4E for the cap 19. The PABP-eIF4G interaction can be regulated, supporting the importance of the 5′-to-3′ communication. For example, PABP-interacting proteins 1 and 2 (Paip1 and 2) either up- or down-regulate translation of an mRNA message by modulating the 5′-to-3′ communication 20– 22.
The PABP-eIF4G interaction within a single mRNA is the “closed loop model” 23, and it is proposed that the physical proximity between the 5′ and 3′ ends favors the transfer of terminating ribosomes from the 3′ end of the ORF to reinitiate at the 5′ end of the mRNA 14, 15, 24. One piece of evidence in support of this model comes from the observation that ribosomes within mature polysomes have a rate of exchange with surrounding free ribosomes that is slow enough to suggest reinitiation on an mRNA 25. In addition, the 5′-to-3′ interaction may serve as a quality-control mechanism to favor translation of full-length, mature mRNAs 26.
The canonical PABP-eIF4G circularization strategy appears to be a generally important mechanism, but care must be taken not to oversimplify its details or nuances. For example, the synergistic increase in translation efficiency conferred by the cap and poly(A) tail is not strictly contingent upon circularization. Specifically, addition of a poly(A) RNA to translationally competent extract in trans stimulates translation of a capped and non-polyadenylated mRNA to a similar degree as when the poly(A) tail is present in cis; this may be due to increased affinity of eIF4E for the cap 27. Also, in a series of recent publications, it has been observed that mRNAs go through changes in their global architectures over time and as the number of loaded ribosomes increases—first forming circles, then “double-row” structures, then complex “helical” structures where the ends may no longer interact—and there is evidence of similarly dynamic mRNA “closed-loops” in living cells 25, 28– 30. Even more interesting, when an mRNA is altered to prevent the PABP-eIF4G interaction, only initial rounds of translation initiation were slowed; the formation of higher-order polysome structures eventually occurred 25, 31. This suggests that the PABP-eIF4G interaction might be important for “kick-starting” the initial rounds of translation but is less important during steady-state translation on large polysomes, where other forces dictate higher-order architecture. This in turn suggests a dynamic model for 5′-to-3′ communication linked to the maturation of polysomes and perhaps other yet-to-be-determined signals. Clearly, there is much more to be learned even in regard to the canonical eIF4G-PABP interaction, especially surrounding events in live cells and how this relates to other events in the mRNA life, such as decay.
Non-canonical 5′-to-3′ communication strategies using proteins
Exploration of 5′-to-3′ communication starts with the canonical PABP-eIF4G interaction ( Figure 2a) but does not end there (pun intended). Other strategies include those used by IRES RNA-containing transcripts of viral origin that operate without a cap. For example, the viral RNA of poliovirus (and related viruses) is not capped, but translation of the viral genome is still enhanced by a 3′ poly(A) tail 32. The poliovirus IRES RNA directly binds eIF4G, which could interact with PABP bound to the poly(A) tail ( Figure 2b) 33– 36. However, during infection, a viral protease cleaves eIF4G and while a fragment of the factor binds the IRES, it cannot bind PABP. Thus, an alternative mechanism includes 5′-to-3′ communication between poly(rC)-binding protein (PCBP) bound to the 5′ cloverleaf structure, upstream of the IRES, and PABP bound to the 3′ poly(A) tail ( Figure 2b) 37– 39. In addition, it has been shown that sequences upstream of the poly(A) tail, within the 3′ UTR, can enhance translation in the absence of the poly(A) tail, perhaps by using other proteins 40. Thus, even within this one IRES-containing virus, there may be multiple ways that 5′-to-3′ end communication can be achieved. In addition, viral RNAs with a 5′ viral protein of the genome (VPg) in place of a 5′ cap structure, such as members of the Calciviridae family, might use VPg recruitment of the eIF4F complex (in particular, eIF4G, along with 4A and PABP) to mediate end-to-end communication 41. These different strategies may be used during different stages of viral infection, suggesting temporally dynamic interactions that respond to changing cellular conditions and help coordinate different viral processes, such as replication (discussed in more depth later in this review).
Figure 2. Diverse 5′-to-3′ communication strategies.
( a) The canonical eIF4G-PABP interaction, shown as in Figure 1b. ( b) Two potential strategies used by poliovirus. ( c) Strategy used by the serine hydroxymethyltransferase 1 IRES-containing mRNA. ( d) Strategy used by rotavirus. CL, cloverleaf; CUGBP1, CUG-binding protein 1; hnRNP H2, heterogeneous nuclear ribonucleoprotein H2; IRES, internal ribosome entry site; NS3, non-structural protein 3; ORF, open reading frame; PABP, polyadenylate-binding protein; PCBP, poly(rC)-binding protein.
Viral IRESs are not the only places where alternative methods of 5′-to-3′ communication are found, as cellular mRNAs with IRESs also exhibit different strategies. An interesting example is found in the cellular c-myc mRNA, which contains an IRES that binds initiation factors, including eIF4G, and translation is enhanced by the poly(A) tail but without needing PABP 42. In this case (and many others), the precise mechanism of 5′-to-3′ communication is mysterious. Another intriguing example is the cellular mRNA for serine hydroxymethyltransferase 1, which does not require eIF4G or PABP. Rather, proposed structures in the 5′ and 3′ UTRs bind to heterogeneous nuclear ribonucleoprotein H2 (hnRNP H2) and CUG-binding protein 1 (CUGBP1), respectively, which mediate IRES-driven translation initiation via direct protein-protein contact during states of decreased cap-driven translation initiation ( Figure 2c) 43.
Although IRESs are logical places to use alternative modes of 5′-to-3′ communication, non-canonical mechanisms are also found in viral and cellular transcripts that do not contain IRESs. Rotaviruses are capped but do not have a poly(A) tail; rather, viral non-structural protein 3 (NSP3) binds near the 3′ end and interacts with eIF4G ( Figure 2d) 43– 46. Whereas earlier studies concluded that NSP3 was not involved in translation based on a partial knockdown 47, another interpretation of these data is that NSP3 is a potent translation enhancer, such that a 10-fold decrease in the amount of NSP3 levels still supports viral protein synthesis 48. Likewise, the cellular mRNAs that encode for histones have a highly conserved stem-loop structure at their 3′ ends rather than a poly(A) tail. This structure binds stem-loop-binding protein (also called hairpin-binding protein), which facilities interactions between initiation factors at the 5′ end 49. Clearly, the advantageous effect of 5′-to-3′ communication can be achieved with a variety of combinations of mRNA intrinsic elements (5′ cap, poly(A), and RNA structure), used with canonical factors (eIF4G and PABP), or several “non-canonical” proteins, or both. These mRNAs and viral RNAs illustrate how, despite the existence of the general eIF4G-PABP strategies, there are idiosyncratic methods of achieving 5′-to-3′ communication that add layers of regulatory complexity, which may be linked to cell type, cell conditions, and so on. No doubt there are many more strategies to be discovered and understood.
Binding initiation factors at one end for use at the other: RNA-RNA communication and other strategies
The above examples illustrate how 5′ and 3′ ends communicate through various combinations of RNA-encoded signals and proteins. Interesting variations on this theme are RNA signals near the 3′ end of an RNA that bind translation-essential proteins to be used at the 5′ end ( Figure 3). Examples of this phenomena are found in plant-infecting viruses, whose genomic RNAs are often not capped or poly(A)-tailed. Rather than evolving an IRES, these viruses have structured RNA elements in their 3′ UTRs called cap-independent translation enhancers (CITEs) (reviewed in 50, 51). CITEs are distinct from IRESs in that CITEs themselves do not bind ribosomes in the proper location or context for translation initiation. The structures of CITEs and the mechanisms by which they operate are diverse and fall into several classes. This has been well reviewed recently 51; therefore, we present just a few examples.
Figure 3. A strategy used by some CITE RNAs.
(Top) The CITE element (green) is located in the 3′ untranslated region where it can bind a eukaryotic initiation factor (eIF) (in this case, eIF4G associated with eIF4E, both in blue). (Bottom) Base-pairing between a hairpin (HP) in the CITE and a HP near the 5′ end (orange) results in delivery of the factor to the 5′ end, where it can serve in translation initiation along with the 43S complex (shown in dashed gray). Other CITEs use diverse strategies. CITE, cap-independent translation enhancer.
CITEs generally eschew the use of proteins to achieve long-range communication, relying instead on direct RNA-RNA base-pairing between distal sequences. This streamlined approach often consists of a hairpin structure in the 3′ UTR that is complementary to four to eight bases in another short hairpin structure in the 5′ UTR (reviewed in 52). One of the best-characterized examples is from the non-capped and non-polyadenylated Barley yellow dwarf virus (BYDV), whose 3′ UTR contains a proposed cruciform-like secondary structure referred to as a BYDV-like element (BTE). One stem-loop of the BTE can base-pair to five nucleotides in a short 5′ UTR hairpin, creating a “kissing” interaction 53. Because the BTE binds to eIF4G directly, this provides a way to deliver eIF4G to the 5′ UTR 54, 55 and also possibly binds directly to the 18S rRNA through base-pairing to recruit the ribosome to the 5′ end ( Figure 3) 56. In fact, direct RNA-ribosome interaction is an emerging mechanism in viral and cellular mRNA translation 57. Another class of CITEs are Panicum mosaic virus-like translational enhancers (PTEs), which functionally replace a cap structure by directly binding eIF4E 58– 60. Although the high-resolution structure of a PTE-eIF4E complex has not been solved, it has been proposed that these RNAs form a compact fold in which a conserved guanine base is extruded from the structure to be recognized by the initiation factor 61. Consistent with the trend of many 3′ CITEs, there are sequences in the PTE that are complementary to sequences in the 5′ end, and these are likely part of the mechanism for bringing eIF4E to the 5′ end to be used in translation initiation.
The CITEs mentioned above have a fairly well-established strategy to link their 5′ and 3′ ends, but the mechanism for other CITEs is less straightforward. For example, the T-shaped structure (TSS) CITEs found in the genome of several viruses, including Turnip crinkle virus, are proposed to fold into a three-dimensional structure that resembles a tRNA 62, 63 which binds to 80S ribosomes and 60S subunits 64. Unlike the case in many other CITEs, there is no clear sequence complementarity between the TSS and sequence in the 5′ end; the current model is that 5′-to-3′ communication occurs through an unusual ribosome subunit-subunit interaction of the 3′ bound 60S subunit with a 40S subunit bound to the 5′ end 65. Clearly, the details of these interactions deserve continued exploration.
End-to-end communication and messenger RNA turnover
Thus far, we have focused on the use of 5′-to-3′ communication in translation, but in fact the m7G cap structure and poly(A) tail serve more than one purpose: recruitment of translation machinery for protein synthesis and also protection against degradation of the RNA by exonucleases (reviewed in 66, 67). Consistent with this, communication between cap-bound and poly(A)-bound proteins can regulate RNA turnover. In the dominant pathway for mRNA decay, an mRNA that is destined for decay first has its poly(A) tail progressively shortened by deadenylating enzymes (deadenylation-dependent decay) 68. This shortening is accompanied by the loss of bound PABP, disrupting the 5′-to-3′ communication, likely loss of eIF4E affinity for the cap, enzymatic decapping, and degradation by the 5′-to-3′ exonuclease Xrn1 69, 70. In a less used deadenylation-independent decay pathway, a stem-loop structure in the 3′ UTR of the mRNA binds a factor that enhances decapping (Edc3) at the 5′ end (and subsequent degradation) 68. The fact that translation and mRNA decay are both influenced by 5′-to-3′ communication connects and coordinates these processes within an overall regulatory strategy.
End-to-end communication promotes viral replication
Unlike mRNAs, the genomic RNAs of positive-sense single-stranded viruses must be replicated to make a negative-sense RNA intermediate that serves as a template for many copies of the positive-sense RNA. Communication between the 5′ and 3′ ends is essential for this, and several strategies exist to achieve it. Again with poliovirus as an example, the RNA-dependent RNA polymerase (RNAP) must initiate minus-strand synthesis from the poly(A) tail. To do this, the cloverleaf RNA structure within the 5′ end of the viral RNA binds to PCBP and also to RNAP; both interact with poly(A)-binding protein 1, which binds the poly(A) tail 71, 72. These interactions support a model in which the protein-protein bridge helps specifically deliver RNAP recruited via unique structures in the 5′ end, to the 3′ end. This helps to distinguish poliovirus RNA from other poly(A) RNAs and ensures that only full-length poliovirus RNAs are replicated 72. A different mechanism with a similar outcome is found in Dengue virus (a flavivirus). In Dengue, direct base-pairing that forms between sequences in the 5′ and 3′ UTRs of the viral RNA do not appear to have a role in directing translation but are important for viral replication 73, 74. Specifically, a 5′ stem-loop functions as a promotor to bind the RNAP and the base-pairing then is thought to deliver the RNAP to the 3′ UTR to commence minus-strand synthesis 75, 76.
A general theme in many viral RNAs is that 5′-to-3′ communication plays a role in both translation and replication. Dynamic changes of the interactions between the 5′ and 3′ ends (either different RNA-RNA interactions or differentially bound bridging protein interactions) have been proposed as mechanisms to organize these processes 77, 78. This is important because the translation and replication machinery would clash should they both simultaneously use the same copy of viral RNA. This again underscores a common theme: that 5′ and 3′ communication strategies are dynamic and diverse players in regulating the use and fate of the associated RNA.
Summary and final thoughts
In the preceding text, we have illustrated the diverse interaction strategies in which eukaryotic cellular mRNAs and some viral RNAs can promote communication between the 5′ and 3′ ends and how this relates to a number of different processes. Although there are common mechanisms by which communication is achieved, there are no “hard and fast” rules; examples of RNA-RNA, RNA-protein, and protein-protein interactions abound, and these examples use canonical factors as well as more specialized proteins. In some cases, the strategy appears to be idiosyncratic to a particular mRNA or virus, consistent with hundreds of millions of years of evolutionary tinkering and fine-tuning. Although many strategies have been described, it is clear that there are many others remaining to be discovered. Very recently, Weingarten-Gabbey et al. used a high-throughput approach to identify thousands of non-canoncial translation initiation signals in both viral and human RNAs located throughout the RNA; although interactions between these and the 5′ end have not been shown, these discoveries suggest that many more examples of long-range communication are still to be discovered 57. Also, there is much to be learned about how these interactions may be affected by changing cellular conditions, concentration of factors or RNA, cell type, time of viral infection, and so on. Even in the case of the canonical PABP-eIF4G interaction, quantitative assessment of the affinities, conformational changes and binding dynamics, and how these relate to the maturation of polysomes and transitions between different phases of the mRNA’s life cycle remain areas ripe for more exploration. Overall, there is much to learn about roles of higher-order architecture and the dynamics of these architectures. Happily, this suggests that many more exciting discoveries are on the horizon.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Katherine Fitzgerald, Program in Innate Immunity, Division of Infectious Diseases and Immunology, Department of Medicine, University of Massachusetts Medical School, Worcester, MA, USA
W. Allen Miller, Plant Pathology & Microbiology Department, Iowa State University, Ames, IA, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Kozak M: How strong is the case for regulation of the initiation step of translation by elements at the 3' end of eukaryotic mRNAs? Gene. 2004;343(1):41–54. 10.1016/j.gene.2004.08.011 [DOI] [PubMed] [Google Scholar]
- 2. Grifo JA, Tahara SM, Morgan MA, et al. : New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983;258(9):5804–10. [PubMed] [Google Scholar]
- 3. Lamphear BJ, Kirchweger R, Skern T, et al. : Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases. Implications for cap-dependent and cap-independent translational initiation. J Biol Chem. 1995;270(37):21975–83. 10.1074/jbc.270.37.21975 [DOI] [PubMed] [Google Scholar]
- 4. Pestova TV, Borukhov SI, Hellen CU: Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature. 1998;394(6696):854–9. 10.1038/29703 [DOI] [PubMed] [Google Scholar]
- 5. Kapp LD, Lorsch JR: GTP-dependent recognition of the methionine moiety on initiator tRNA by translation factor eIF2. J Mol Biol. 2004;335(4):923–36. 10.1016/j.jmb.2003.11.025 [DOI] [PubMed] [Google Scholar]
- 6. Unbehaun A, Borukhov SI, Hellen CU, et al. : Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon-anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev. 2004;18(24):3078–93. 10.1101/gad.1255704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maag D, Fekete CA, Gryczynski Z, et al. : A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol Cell. 2005;17(2):265–75. 10.1016/j.molcel.2004.11.051 [DOI] [PubMed] [Google Scholar]
- 8. Pestova TV, Kolupaeva VG, Lomakin IB, et al. : Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci U S A. 2001;98(13):7029–36. 10.1073/pnas.111145798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapp LD, Lorsch JR: The molecular mechanics of eukaryotic translation. Annu Rev Biochem. 2004;73:657–704. 10.1146/annurev.biochem.73.030403.080419 [DOI] [PubMed] [Google Scholar]
- 10. Jackson RJ, Hellen CU, Pestova TV: The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11(2):113–27. 10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filbin ME, Kieft JS: Toward a structural understanding of IRES RNA function. Curr Opin Struct Biol. 2009;19(3):267–76. 10.1016/j.sbi.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Plank TM, Kieft JS: The structures of nonprotein-coding RNAs that drive internal ribosome entry site function. Wiley Interdiscip Rev RNA. 2012;3(2):195–212. 10.1002/wrna.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michel YM, Poncet D, Piron M, et al. : Cap-Poly(A) synergy in mammalian cell-free extracts. Investigation of the requirements for poly(A)-mediated stimulation of translation initiation. J Biol Chem. 2000;275(41):32268–76. 10.1074/jbc.M004304200 [DOI] [PubMed] [Google Scholar]
- 14. Gallie DR: The cap and poly(A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991;5(11):2108–16. 10.1101/gad.5.11.2108 [DOI] [PubMed] [Google Scholar]
- 15. Tarun SZ, Jr, Sachs AB: Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996;15(24):7168–77. [PMC free article] [PubMed] [Google Scholar]
- 16. Imataka H, Gradi A, Sonenberg N: A newly identified N-terminal amino acid sequence of human eIF4G binds poly(A)-binding protein and functions in poly(A)-dependent translation. EMBO J. 1998;17(24):7480–9. 10.1093/emboj/17.24.7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarun SZ, Jr, Sachs AB: A common function for mRNA 5' and 3' ends in translation initiation in yeast. Genes Dev. 1995;9(23):2997–3007. 10.1101/gad.9.23.2997 [DOI] [PubMed] [Google Scholar]
- 18. Munroe D, Jacobson A: mRNA poly(A) tail, a 3′ enhancer of translational initiation. Mol Cell Biol. 1990;10(7):3441–55. 10.1128/MCB.10.7.3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borman AM, Michel YM, Kean KM: Biochemical characterisation of cap-poly(A) synergy in rabbit reticulocyte lysates: the eIF4G-PABP interaction increases the functional affinity of eIF4E for the capped mRNA 5′-end. Nucleic Acids Res. 2000;28(21):4068–75. 10.1093/nar/28.21.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Craig AW, Haghighat A, Yu AT, et al. : Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998;392(6675):520–3. 10.1038/33198 [DOI] [PubMed] [Google Scholar]
- 21. Martineau Y, Derry MC, Wang X, et al. : Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008;28(21):6658–67. 10.1128/MCB.00738-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derry MC, Yanagiya A, Martineau Y, et al. : Regulation of poly(A)-binding protein through PABP-interacting proteins. Cold Spring Harb Symp Quant Biol. 2006;71:537–43. 10.1101/sqb.2006.71.061 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson A, Peltz SW: Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. 10.1146/annurev.bi.65.070196.003401 [DOI] [PubMed] [Google Scholar]
- 24. Kahvejian A, Svitkin YV, Sukarieh R, et al. : Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005;19(1):104–13. 10.1101/gad.1262905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kopeina GS, Afonina ZA, Gromova KV, et al. : Step-wise formation of eukaryotic double-row polyribosomes and circular translation of polysomal mRNA. Nucleic Acids Res. 2008;36(8):2476–88. 10.1093/nar/gkm1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fatscher T, Boehm V, Weiche B, et al. : The interaction of cytoplasmic poly(A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay. RNA. 2014;20(10):1579–92. 10.1261/rna.044933.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borman AM, Michel YM, Malnou CE, et al. : Free poly(A) stimulates capped mRNA translation in vitro through the eIF4G-poly(A)-binding protein interaction. J Biol Chem. 2002;277(39):36818–24. 10.1074/jbc.M205065200 [DOI] [PubMed] [Google Scholar]
- 28. Afonina ZA, Myasnikov AG, Shirokov VA, et al. : Conformation transitions of eukaryotic polyribosomes during multi-round translation. Nucleic Acids Res. 2015;43(1):618–28. 10.1093/nar/gku1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Archer SK, Shirokikh NE, Hallwirth CV, et al. : Probing the closed-loop model of mRNA translation in living cells. RNA Biol. 2015;12(3):248–54. 10.1080/15476286.2015.1017242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Afonina ZA, Myasnikov AG, Khabibullina NF, et al. : Topology of mRNA chain in isolated eukaryotic double-row polyribosomes. Biochemistry (Mosc). 2013;78(5):445–54. 10.1134/S0006297913050027 [DOI] [PubMed] [Google Scholar]
- 31. Afonina ZA, Myasnikov AG, Shirokov VA, et al. : Formation of circular polyribosomes on eukaryotic mRNA without cap-structure and poly(A)-tail: a cryo electron tomography study. Nucleic Acids Res. 2014;42(14):9461–9. 10.1093/nar/gku599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergamini G, Preiss T, Hentze MW: Picornavirus IRESes and the poly(A) tail jointly promote cap-independent translation in a mammalian cell-free system. RNA. 2000;6(12):1781–90. 10.1017/S1355838200001679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michel YM, Borman AM, Paulous S, et al. : Eukaryotic initiation factor 4G-poly(A) binding protein interaction is required for poly(A) tail-mediated stimulation of picornavirus internal ribosome entry segment-driven translation but not for X-mediated stimulation of hepatitis C virus translation. Mol Cell Biol. 2001;21(13):4097–109. 10.1128/MCB.21.13.4097-4109.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulous S, Malnou CE, Michel YM, et al. : Comparison of the capacity of different viral internal ribosome entry segments to direct translation initiation in poly(A)-dependent reticulocyte lysates. Nucleic Acids Res. 2003;31(2):722–33. 10.1093/nar/gkf695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheung P, Lim T, Yuan J, et al. : Specific interaction of HeLa cell proteins with coxsackievirus B3 3'UTR: La autoantigen binds the 3' and 5'UTR independently of the poly(A) tail. Cell Microbiol. 2007;9(7):1705–15. 10.1111/j.1462-5822.2007.00904.x [DOI] [PubMed] [Google Scholar]
- 36. Souii A, M'hadheb-Gharbi MB, Gharbi J: Cellular Proteins Act as Bridge Between 5′ and 3′ Ends of the Coxsackievirus B3 Mediating Genome Circularization During RNA Translation. Curr Microbiol. 2015;71(3):387–95. 10.1007/s00284-015-0866-y [DOI] [PubMed] [Google Scholar]
- 37. Sweeney TR, Abaeva IS, Pestova TV, et al. : The mechanism of translation initiation on Type 1 picornavirus IRESs. EMBO J. 2014;33(1):76–92. 10.1002/embj.201386124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogram SA, Spear A, Sharma N, et al. : The 5'CL-PCBP RNP complex, 3' poly(A) tail and 2A pro are required for optimal translation of poliovirus RNA. Virology. 2010;397(1):14–22. 10.1016/j.virol.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kempf BJ, Barton DJ: Poliovirus 2A Pro increases viral mRNA and polysome stability coordinately in time with cleavage of eIF4G. J Virol. 2008;82(12):5847–59. 10.1128/JVI.01514-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dobrikova E, Florez P, Bradrick S, et al. : Activity of a type 1 picornavirus internal ribosomal entry site is determined by sequences within the 3' nontranslated region. Proc Natl Acad Sci U S A. 2003;100(25):15125–30. 10.1073/pnas.2436464100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung L, Bailey D, Leen EN, et al. : Norovirus translation requires an interaction between the C Terminus of the genome-linked viral protein VPg and eukaryotic translation initiation factor 4G. J Biol Chem. 2014;289(31):21738–50. 10.1074/jbc.M114.550657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thoma C, Fraterman S, Gentzel M, et al. : Translation initiation by the c-myc mRNA internal ribosome entry sequence and the poly(A) tail. RNA. 2008;14(8):1579–89. 10.1261/rna.1043908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox JT, Stover PJ: Mechanism of the internal ribosome entry site-mediated translation of serine hydroxymethyltransferase 1. J Biol Chem. 2009;284(45):31085–96. 10.1074/jbc.M109.035576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piron M, Vende P, Cohen J, et al. : Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998;17(19):5811–21. 10.1093/emboj/17.19.5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vende P, Piron M, Castagne N, et al. : Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3' end. J Virol. 2000;74(15):7064–71. 10.1128/JVI.74.15.7064-7071.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Groft CM, Burley SK: Recognition of eIF4G by rotavirus NSP3 reveals a basis for mRNA circularization. Mol Cell. 2002;9(6):1273–83. 10.1016/S1097-2765(02)00555-5 [DOI] [PubMed] [Google Scholar]
- 47. Montero H, Arias CF, Lopez S: Rotavirus Nonstructural Protein NSP3 is not required for viral protein synthesis. J Virol. 2006;80(18):9031–8. 10.1128/JVI.00437-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gratia M, Sarot E, Vende P, et al. : Rotavirus NSP3 Is a Translational Surrogate of the Poly(A) Binding Protein-Poly(A) Complex. J Virol. 2015;89(17):8773–82. 10.1128/JVI.01402-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rattray AM, Muller B: The control of histone gene expression. Biochem Soc Trans. 2012;40(4):880–5. 10.1042/BST20120065 [DOI] [PubMed] [Google Scholar]
- 50. Nicholson BL, White KA: 3' Cap-independent translation enhancers of positive-strand RNA plant viruses. Curr Opin Virol. 2011;1(5):373–80. 10.1016/j.coviro.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 51. Simon AE, Miller WA: 3′ cap-independent translation enhancers of plant viruses. Annu Rev Microbiol. 2013;67:21–42. 10.1146/annurev-micro-092412-155609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller WA, White KA: Long-distance RNA-RNA interactions in plant virus gene expression and replication. Annu Rev Phytopathol. 2006;44:447–67. 10.1146/annurev.phyto.44.070505.143353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guo L, Allen EM, Miller WA: Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol Cell. 2001;7(5):1103–9. 10.1016/S1097-2765(01)00252-0 [DOI] [PubMed] [Google Scholar]
- 54. Wang S, Browning KS, Miller WA: A viral sequence in the 3′-untranslated region mimics a 5′ cap in facilitating translation of uncapped mRNA. EMBO J. 1997;16(13):4107–16. 10.1093/emboj/16.13.4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Treder K, Kneller EL, Allen EM, et al. : The 3′ cap-independent translation element of Barley yellow dwarf virus binds eIF4F via the eIF4G subunit to initiate translation. RNA. 2008;14(1):134–47. 10.1261/rna.777308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma SD, Kraft JJ, Miller WA, et al. : Recruitment of the 40S ribosome subunit to the 3′-untranslated region (UTR) of a viral mRNA, via the eIF4 complex, facilitates cap-independent translation. J Biol Chem. 2015;290(18):11268–81. 10.1074/jbc.M115.645002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weingarten-Gabbay S, Elias-Kirma S, Nir R, et al. : Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science. 2016;351(6270): pii: aad4939. 10.1126/science.aad4939 [DOI] [PubMed] [Google Scholar]
- 58. van Lipzig R, Gultyaev AP, Pleij CW, et al. : The 5′ and 3′ extremities of the satellite tobacco necrosis virus translational enhancer domain contribute differentially to stimulation of translation. RNA. 2002;8(2):229–36. 10.1017/S1355838202018071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Z, Treder K, Miller WA: Structure of a viral cap-independent translation element that functions via high affinity binding to the eIF4E subunit of eIF4F. J Biol Chem. 2009;284(21):14189–202. 10.1074/jbc.M808841200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Meulewaeter F, Danthinne X, van Montagu M, et al. : 5′- and 3′-sequences of satellite tobacco necrosis virus RNA promoting translation in tobacco. Plant J. 1998;14(2):169–76. 10.1046/j.1365-313X.1998.00104.x [DOI] [PubMed] [Google Scholar]
- 61. Wang Z, Parisien M, Scheets K, et al. : The cap-binding translation initiation factor, eIF4E, binds a pseudoknot in a viral cap-independent translation element. Structure. 2011;19(6):868–80. 10.1016/j.str.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCormack JC, Yuan X, Yingling YG, et al. : Structural domains within the 3′ untranslated region of Turnip crinkle virus. J Virol. 2008;82(17):8706–20. 10.1128/JVI.00416-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zuo X, Wang J, Yu P, et al. : Solution structure of the cap-independent translational enhancer and ribosome-binding element in the 3′ UTR of turnip crinkle virus. Proc Natl Acad Sci U S A. 2010;107(4):1385–90. 10.1073/pnas.0908140107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stupina VA, Meskauskas A, McCormack JC, et al. : The 3′ proximal translational enhancer of Turnip crinkle virus binds to 60S ribosomal subunits. RNA. 2008;14(11):2379–93. 10.1261/rna.1227808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stupina VA, Yuan X, Meskauskas A, et al. : Ribosome binding to a 5′ translational enhancer is altered in the presence of the 3′ untranslated region in cap-independent translation of turnip crinkle virus. J Virol. 2011;85(10):4638–53. 10.1128/JVI.00005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilusz CJ, Wormington M, Peltz SW: The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol. 2001;2(4):237–46. 10.1038/35067025 [DOI] [PubMed] [Google Scholar]
- 67. Meyer S, Temme C, Wahle E: Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit Rev Biochem Mol Biol. 2004;39(4):197–216. 10.1080/10409230490513991 [DOI] [PubMed] [Google Scholar]
- 68. Garneau NL, Wilusz J, Wilusz CJ: The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8(2):113–26. 10.1038/nrm2104 [DOI] [PubMed] [Google Scholar]
- 69. Muhlrad D, Decker CJ, Parker R: Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′-->3′ digestion of the transcript. Genes Dev. 1994;8(7):855–66. 10.1101/gad.8.7.855 [DOI] [PubMed] [Google Scholar]
- 70. Braun JE, Truffault V, Boland A, et al. : A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat Struct Mol Biol. 2012;19(12):1324–31. 10.1038/nsmb.2413 [DOI] [PubMed] [Google Scholar]
- 71. Gamarnik AV, Andino R: Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J Virol. 2000;74(5):2219–26. 10.1128/JVI.74.5.2219-2226.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Herold J, Andino R: Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol Cell. 2001;7(3):581–91. 10.1016/S1097-2765(01)00205-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alvarez DE, Lodeiro MF, Filomatori CV, et al. : Structural and functional analysis of dengue virus RNA. Novartis Found Symp. 2006;277:120–32; discussion 132–5, 251–3. 10.1002/0470058005.ch9 [DOI] [PubMed] [Google Scholar]
- 74. Friebe P, Peña J, Pohl MO, et al. : Composition of the sequence downstream of the dengue virus 5′ cyclization sequence (dCS) affects viral RNA replication. Virology. 2012;422(2):346–56. 10.1016/j.virol.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alvarez DE, Filomatori CV, Gamarnik AV: Functional analysis of dengue virus cyclization sequences located at the 5′ and 3′UTRs. Virology. 2008;375(1):223–35. 10.1016/j.virol.2008.01.014 [DOI] [PubMed] [Google Scholar]
- 76. Friebe P, Harris E: Interplay of RNA elements in the dengue virus 5′ and 3′ ends required for viral RNA replication. J Virol. 2010;84(12):6103–18. 10.1128/JVI.02042-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Edgil D, Harris E: End-to-end communication in the modulation of translation by mammalian RNA viruses. Virus Res. 2006;119(1):43–51. 10.1016/j.virusres.2005.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Romero-López C, Berzal-Herranz A: A long-range RNA-RNA interaction between the 5′ and 3′ ends of the HCV genome. RNA. 2009;15(9):1740–52. 10.1261/rna.1680809 [DOI] [PMC free article] [PubMed] [Google Scholar]