Abstract
Background:
Infectious complications in tissue expander (TE) breast reconstruction can be devastating and costly. Therefore, to optimize care, we examined patient’s demographics, microbiology of TE infections, and the efficacy of empiric antimicrobial regimens and thereafter generated an algorithm for the treatment of these complex infections.
Methods:
We retrospectively reviewed all patients who underwent TE breast reconstruction between 2003 and 2012 and analyzed those patients who developed a “definite” device-related infection leading to TE explantation and had a positive intraoperative culture.
Results:
A total of 3,082 patients underwent immediate breast reconstruction with TE. Of these, 378 patients (12.3%) developed an infection, 189 (6.1%) eventually proceed with explantation, and 118 (3.8%) had a positive intraoperative culture. Gram-positive organisms caused 73% of infections, and Gram-negative organisms caused 27% of infections. Narrow-spectrum empiric antimicrobials with predominantly Gram-positive coverage were deemed appropriate in only 62% of cases, and those with Gram-negative coverage were appropriate in 46%. Broad-spectrum antimicrobials were used in 47% of cases, mainly recommended by infectious disease specialists, and were considered appropriate in >90% of the occasions.
Conclusions:
Current empiric antibiotic regimens do not cover the vast spectrum of organisms causing TE infections. To increase the salvage rate of an infected TE, at the first sign of infection, in addition to benefiting with an infectious diseases consultation, empiric coverage with broad-spectrum antibiotics active against biofilm-embedded organisms should be administered.
Postoperative infection in tissue expander (TE)-based breast reconstruction represents a devastating complication to the patient and a substantial financial strain on the health care system. When a periprosthetic TE infection is diagnosed, empiric antimicrobial therapy is initiated because specific culture data are not available. Insightful clinical judgment is required to balance the appropriateness of empiric antibiotic coverage against commonly encountered pathogens while limiting the potential of increased resistance and side effects from the excessive administration of broad-spectrum antimicrobial drugs.
The Surgical Care Improvement Project, initiated in 2005, established guidelines for preoperative and postoperative antibiotic prophylaxis, recommending administration of systemic antibiotics before making the incision in clean breast and axillary surgeries and discontinuation of the antibiotics within 24 hours after the procedure is complete.1 However, these guidelines do not address antibiotic usage in breast reconstruction with TEs, permanent implants, and drainage catheters, all of which increase the risk of infection because of the presence of foreign bodies and a thin soft-tissue envelope. Survey data from the American Society of Breast Surgeons indicated that 58% of physicians prescribed prolonged postoperative antibiotics in patients undergoing breast reconstruction immediately after mastectomy. In addition, recent survey data from the American Society of Plastic Surgeons showed that 72% of physicians continued antibiotic prophylaxis in patients who had undergone breast reconstruction until the drainage catheters were removed.2,3 This practice is in part supported by studies demonstrating that microorganisms causing surgical site infections (SSIs) are most commonly those colonizing the drain tubes and that increased duration of drain tube implantation is associated with an increase rate of SSIs.3,4
Despite the widespread practice of maintaining postoperative antibiotic prophylaxis in patients who have undergone breast reconstruction, the reported incidence of TE infection ranges from 2.5% to 24%.5–10 Risk factors associated with TE infections include tobacco use, increased body mass index, history of radiation therapy, axillary lymph node dissection, breast reconstruction immediately after mastectomy, use of surgical drains, use of acellular dermal matrices, and adjuvant chemotherapy.5,7,9–14 Despite these known risk factors for infection, many have begun questioning the extended use of perioperative antibiotics owing to the lack of evidence-based guidelines for their use and the risk of increasing the number of resistant organisms. Recently, McCullough et al15 identified increased rates of microorganism resistance to first-generation cephalosporins in TE SSIs in 20.5% of preoperative and 54.5% of postoperative cases. This increase in resistance to commonly used prophylactic antibiotics underscores the importance of optimizing antibiotic choice when evaluating trends of pathogens resulting in implant-based infections.
Also, many of the empiric antimicrobial regimens currently used by surgeons were selected several decades ago. As a result of recent changes in microflora antibiotic susceptibility patterns and extensive use of perioperative antimicrobial regimens, the empiric antimicrobials that are commonly used today may be outdated. Furthermore, biofilm-embedded organisms attached to the TE, living in the extracellular matrix, are much more resistant to the inhibitory and bactericidal effects of several antibiotics than are free-floating planktonic organisms. These biofilm-embedded organisms necessitate the use of antimicrobial regimens that are not only active against the microorganisms but also able to penetrate the biofilm matrix. Once established, biofilm-related infections are often impossible to eradicate without removing the associated foreign medical device.16 Therefore, to evaluate the efficacy of the empiric antimicrobial regimens currently used for the treatment of TE-related infections, we examined the current microbiology of TE infections resulting in removal of the TE and the efficacy of the empiric antimicrobial treatment regimens currently used at our institution once an infection is suspected. We then used these data to generate an algorithm for adequately managing infections and avoiding eventual explantation of the breast TE.
METHODS
Patient Population
We retrospectively reviewed the medical records of all patients who underwent breast reconstruction with TE immediately after mastectomy between January 2003 and December 2012 at The University of Texas MD Anderson Cancer Center, Houston, Tex. We identified all patients in this group who developed an SSI and subsequent TE-related infection. All infections fulfilled the strict definition of a deep SSI involving an implant, as determined by the Centers for Disease Control and Prevention. According to this definition, patients met at least 1 of the following criteria: purulent drainage, spontaneous dehiscence or surgically opened incision with positive cultures, evidence of abscess, or diagnosis by the surgeon of a deep infection occurring up to 1 year postoperatively.17 Because several patients may develop signs and symptoms that may mimic a local infection such as radiation-induced dermatitis, increased erythema and pain secondarily to rapid increase in pressure of the infused TE, superficial skin flap necrosis, or even acellular dermal matrix (ADM)-related hyperemia, to include only patients who had developed a “definite” TE infection and avoid confounding our analysis, unless a patient had their implant removed because of infection and had a positive intraoperative culture, these patients were not included in our study.
Data Collection
We reviewed data collected in a prospectively maintained database, including patient demographic information, baseline comorbidities, breast cancer characteristics, cancer treatments, timing of TE placement, operative approach, and postoperative complications. We also documented the use of perioperative and empirical antimicrobials, which were all confirmed via electronic chart review and inpatient and outpatient pharmacy records. The empiric antibiotics used were considered appropriate if the organism causing the TE infection was sensitive to the empiric antibiotic used. A broad-spectrum antibiotic was defined as one that is active against both Gram-positive and Gram-negative bacteria, as opposed to a narrow-spectrum antibiotic that is effective against specific families of bacteria. We also gathered information on intraoperative culture results, as well as the time from TE placement to infection, explanation, and subsequent reconstruction. Moreover, to determine trends in the evolution of microorganisms over time, we evaluated the incidence and distribution of the most common causative organisms by year. Our study was approved by the MD Anderson Institutional Review Board.
Statistical Analysis
We used descriptive statistics to summarize the data, including median with range for continuous variables lacking normal distribution and percentages for categorical variables. For 3-group comparisons, we used the Kruskal-Wallis test to compare nonparametric continuous variables and the chi-square test or Fisher exact test to compare categorical variables. When a significant result (P < 0.05) was detected, pairwise comparisons were performed, in which Wilcoxon rank-sum tests were used for continuous variables and the chi-square or Fisher exact test was used for categorical variables. The α levels of the post hoc pairwise comparisons were adjusted using a sequential Bonferroni method to control for type I error. All tests except those in the pairwise comparisons were 2-sided tests with a significance level of 0.05. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, N.C.).
RESULTS
Clinical Characteristics
A total of 3,082 patients underwent immediate breast reconstruction with TE between 2003 and 2012. Of these, 378 (12.3%) developed an SSI. TE explantation was required in 189 patients (6.1%) owing to unresponsiveness to antimicrobial treatment or advanced stage of infection. Of the 189 patients who underwent explantation, 118 (62%) had positive intraoperative cultures (Tables 1 and 2). The mean age at the time of TE infection was 52 years (range, 28–76 years). Approximately 60% of the patients were white, 31% were Hispanic, and 9% were black or Asian. A total of 36 patients (31%) had developed an infection within the first 30 days postoperatively, 47 patients (40%) within 31 to 90 days, and 35 patients (30%) developed an infection after 90 days postoperatively (P = 032). Although >70% of the patients had no metastatic lymph node disease, the involvement of lymphoid tissues or use of preoperative chemotherapy did not affect the timing of TE infections (P = 0.51 for lymph node involvement and P = 0.6 for chemotherapy). However, infections often occurred within the early postoperative period (<30 days) in patients with the largest chest circumferences and those with increased body mass index, although these associations were not statistically significant (P = 0.09 for chest circumference and P = 0.06 for body mass index). Patients with relatively large tumors and those who received postoperative radiotherapy were significantly more likely to develop an infection during the late postoperative period (>90 days) than the early postoperative period (P < 0.02).
Table 1.
Demographic and Clinical Characteristics of Patients with Tissue Expander Infections Occurring in Different Postoperative Periods
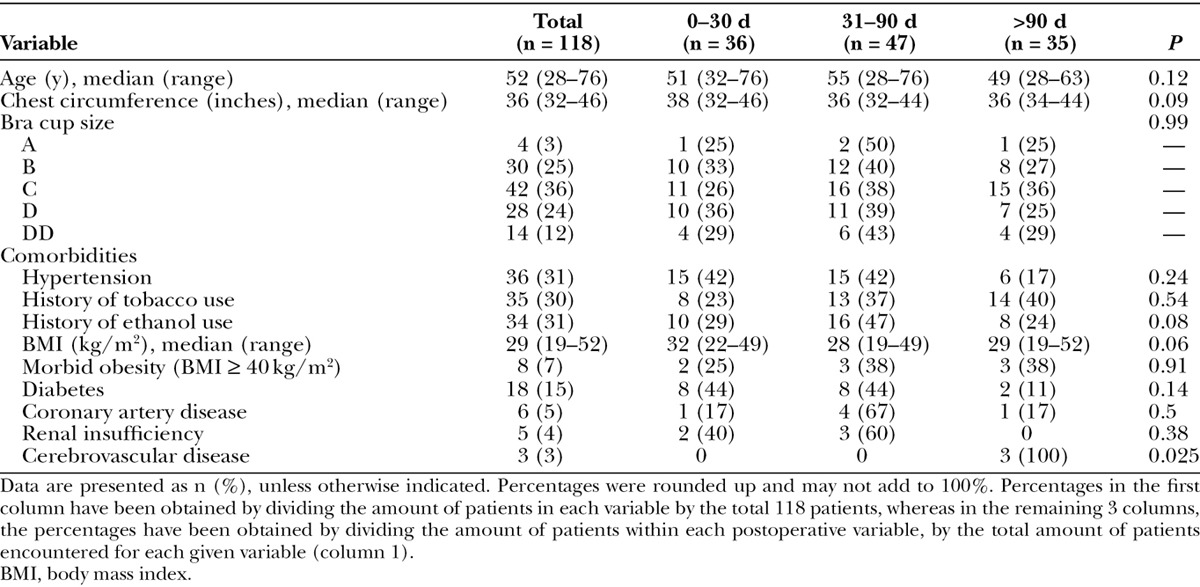
Table 2.
Cancer, Treatment, and Surgical Characteristics of Patients with Infections Occurring in Different Postoperative Periods
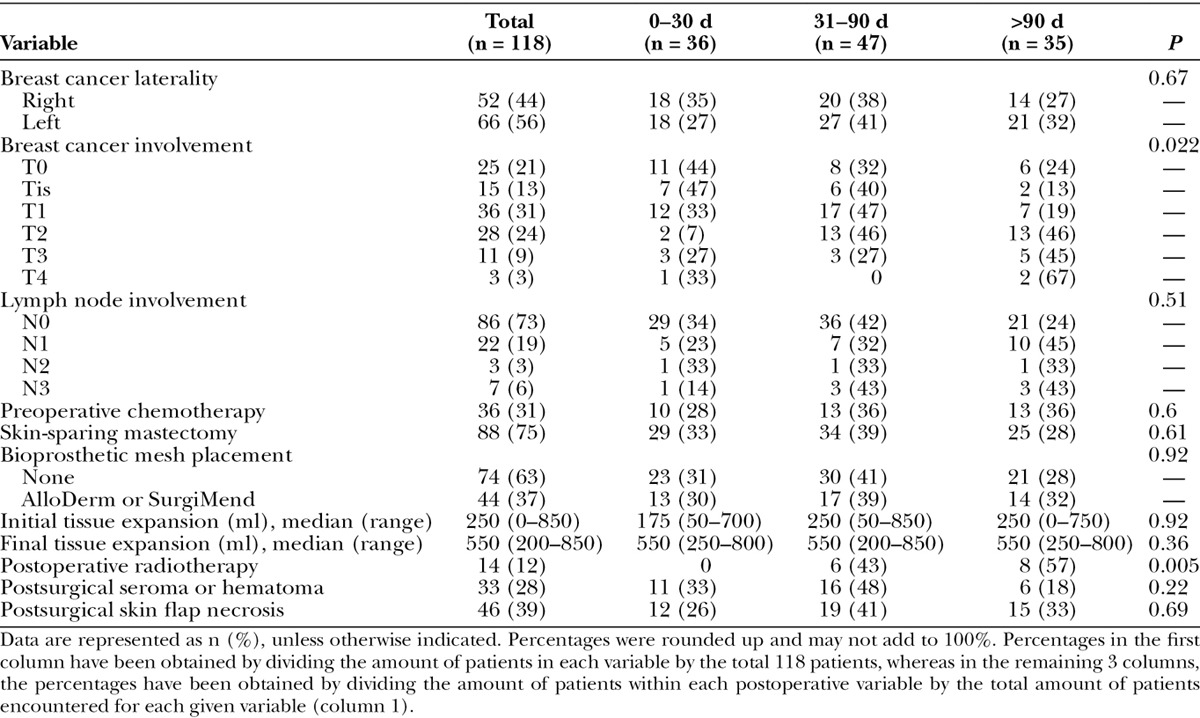
In addition, more than 70% of the patients had a skin-sparing mastectomy. An ADM was used in 44 cases (37%), and despite being a risk factor for seromas and persistence of the use of a drainage catheter, the use of this bioprosthetic mesh did not appear to be a risk factor for early infections (P = 0.92). Similarly, initial and final expansion volumes did not predict risk of early or late TE infection (P > 0.36). Furthermore, almost 40% of the patients who developed a TE infection had developed some degree of skin flap necrosis, and 28% developed a postsurgical seroma or hematoma, requiring drainage catheter placement for an extended period.
Prophylactic Antimicrobial Regimens
All patients received a standardized perioperative systemic antimicrobial therapy; the most common drugs used were cefazolin (81%), clindamycin (6%), and ampicillin-sulbactam (4%). Thereafter at the discretion of the surgeon, more than 85% of the patients underwent subpectoral TE pocket irrigation with a broad-spectrum antimicrobial solution. The most commonly used regimen was bacitracin plus polymyxin B (66%), followed by bacitracin plus cefazolin and gentamicin (9%) and vancomycin plus ciprofloxacin (4%). After surgery, oral prophylactic antibiotics were used in 78% of cases, either for a week or until the drainage catheters were removed. The most commonly prescribed postsurgical antimicrobial drugs were cephalexin (26%), cefadroxil (22%), amoxicillin/clavulanate (8%), trimethoprim/sulfamethoxazole (6%), and clindamycin (4%).
Time to Infection and Implant Removal
Once an infection occurred, the median time from initial reconstructive surgery to infection was 47 days (range, 13–309 days, with a 25th and 75th percentile of 26 and 107 days, respectively). Overall, the median time from infection to implant removal was 4 days (range, 0–136 days, with a 25th and 75th percentile of 2 and 8 days, respectively).
Microorganisms
Gram-positive organism infections were identified in 73% of patients, and Gram-negative infections were identified in 27% (Table 3). A polymicrobial infection was identified in 20 patients (17%). Methicillin-resistant Staphylococcus epidermidis (24%) was the most frequently encountered organism, followed by methicillin-sensitive Staphylococcus aureus (15%), Pseudomonas spp. (14%), and methicillin-resistant S. aureus (12%). The frequency of each causative microorganism remained relatively stable throughout the period studied; methicillin-resistant S. epidermidis was the most common microorganism throughout. These also remained stable when stratified by different postoperative time periods (Fig. 1). Of interest, Pseudomonas and other facultative Gram-negative rods were predominant at 30 days after surgery (accounting for 31% of TE infections), as well as at 90 days (27% of infections) and 180 days (25% of infections; Fig. 1). Furthermore, during the past 8 years of our study, the incidence of Gram-negative TE infections ranged from 24% to 44% of all TE infections (Fig. 2). Of note, similar to the proportion of patients without ADMs, of the 37 patients with an ADM, 62% had a Gram-positive infection and 35% had a Gram-negative infection with 6 (16%) had a polymicrobial infection.
Table 3.
Microorganisms Causative for Breast Tissue Expander-Related Infections
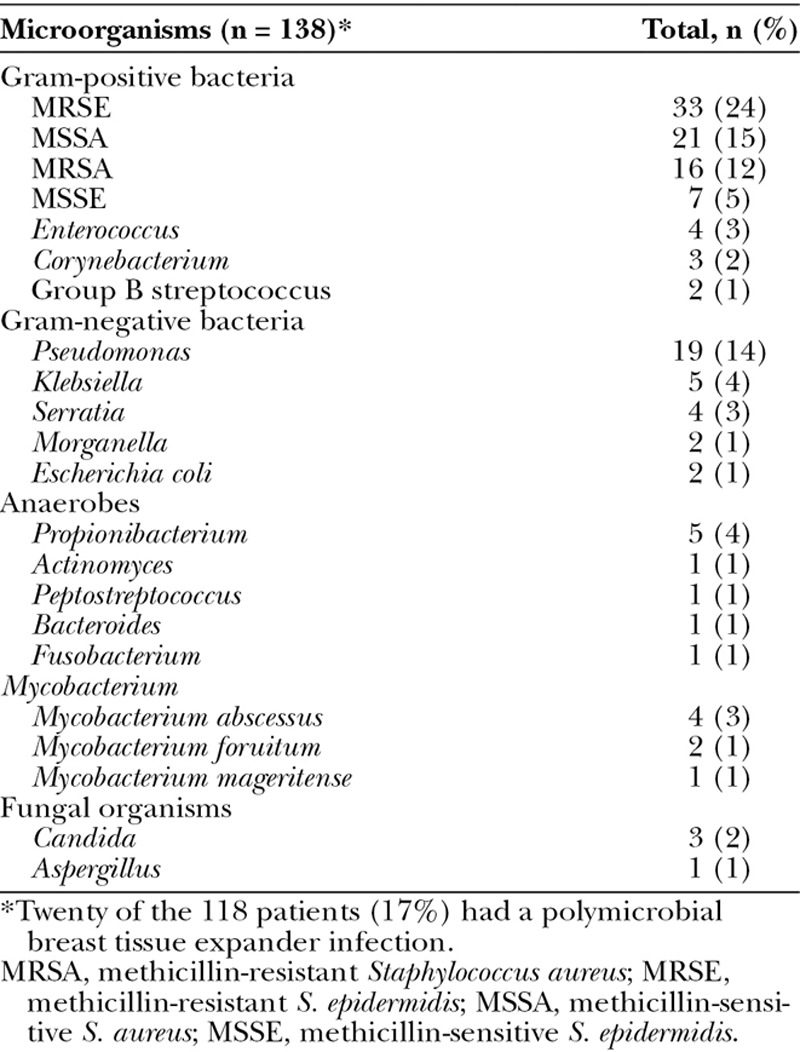
Fig. 1.
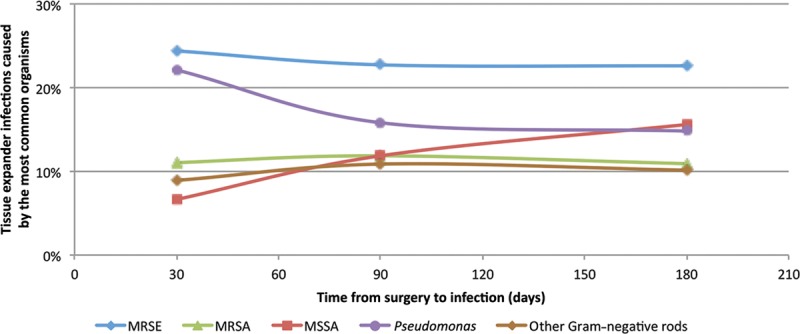
Time from surgery to infection for the most common organisms causing tissue expander infections in our study population. The numerator and denominator for these percentages represent the total number of patients included for that specific time period. Other Gram-negative rods include Klebsiella, Serratia, Morganella, Proteus, and Escherichia coli. MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant S. epidermidis; MSSA, methicillin-sensitive S. aureus.
Fig. 2.
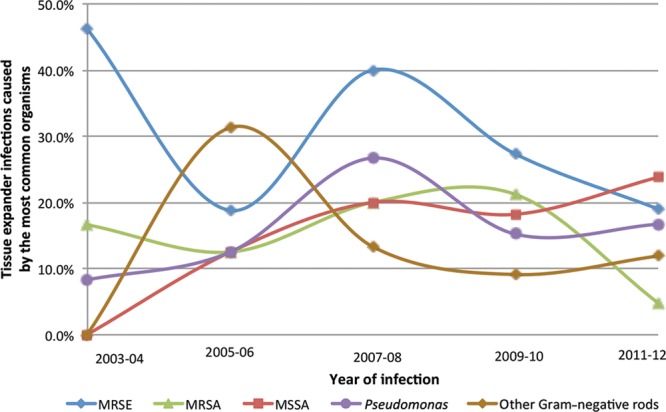
Proportion of the most common organisms causing tissue expander infections in different years of our study. The numerator and denominator for these percentages represent the total number of patients included for that specific time period. Other Gram-negative rods include Klebsiella, Serratia, Morganella, Proteus, and Escherichia coli. MRSA, methicillin-resistant Staphylococcus aureus; MRSE, methicillin-resistant S. epidermidis; MSSA, methicillin-sensitive S. aureus.
Empiric Antimicrobial Regimens
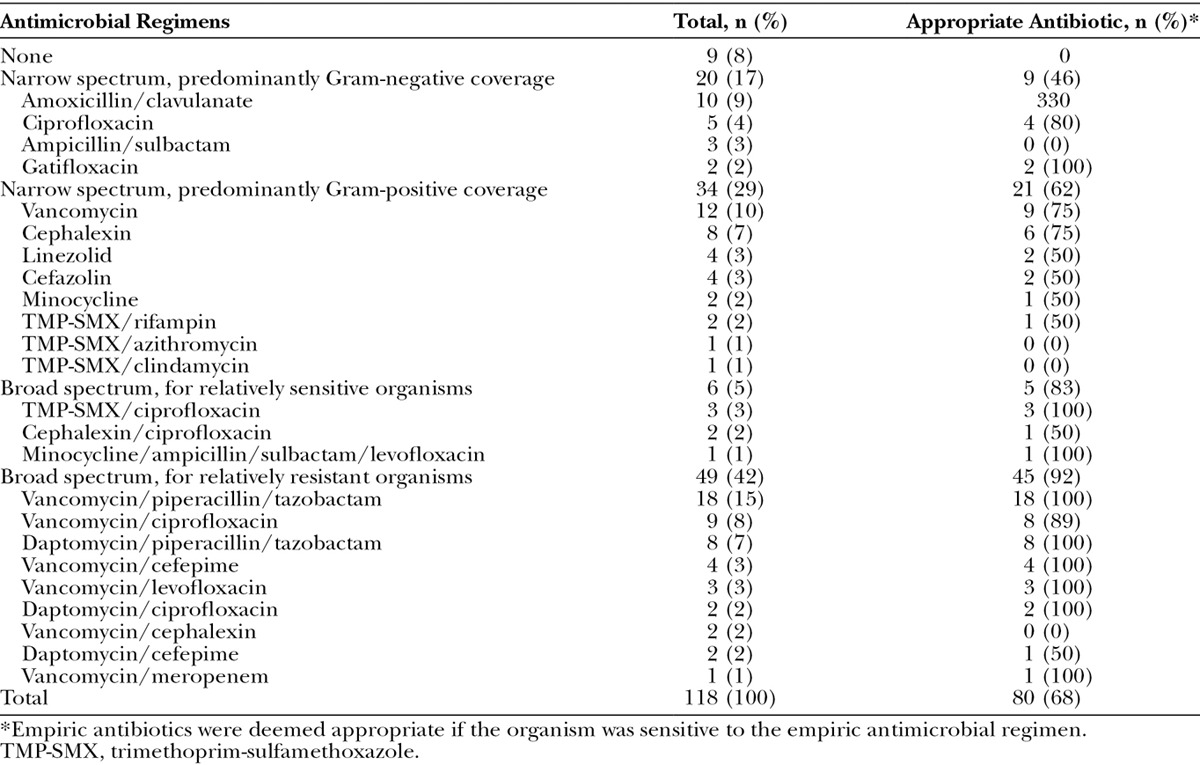
Multiple empiric antimicrobial regimens were used to treat TE infections (Table 4). The most ineffective empiric regimens were categorized as narrow spectrum, such as the isolated use of vancomycin (12 cases, 10%), amoxicillin/clavulanate (10 cases, 8%), cephalexin (8 cases, 7%), or ciprofloxacin (5 cases, 4%). As a group, narrow-spectrum antimicrobial regimens with predominantly Gram-negative coverage (20 cases, 17%) were deemed appropriate in only 45% of cases, and narrow-spectrum antimicrobial regimens with predominantly Gram-positive coverage (34 cases, 29%) were appropriate in only 62% of cases. In contrast, as a group, the use of broad-spectrum antimicrobial regimens (55 cases, 47%) was considered appropriate in more than 90% of the cases in which they were used. The most commonly used regimens were the combination of vancomycin plus piperacillin and tazobactam (18 cases, 15%), vancomycin plus ciprofloxacin (9 cases, 8%), and daptomycin plus piperacillin and tazobactam (8 cases, 7%). Interestingly, we discovered that the majority of plastic surgeons were providing a narrow-spectrum empiric antibiotic regimen, compared with the infectious disease specialists whom were consulted on all of the patients in whom broad-spectrum antimicrobials were recommended.
Table 4.
Empiric Antimicrobials Used to Treat Breast Tissue Expander-Related Infections in Our Study Population
Subsequent Breast Reconstruction
Once the infection was adequately treated, 60 patients (51%) decided to proceed with a subsequent reconstructive procedure. The median time from implant removal to subsequent reconstruction was 180 days (range, 30–1,232 days). Twenty-eight patients (47%) proceeded with an implant-based reconstruction, 22 patients (37%) underwent a flap reconstruction, and 10 patients (17%) underwent a combined implant-flap reconstructive procedure. Patients who underwent a flap reconstruction were almost equally split in terms of the type of flap; approximately one-third had an extended latissimus dorsi flap, one-third had a deep inferior epigastric perforator flap, and one-third had a muscle-sparing transverse rectus abdominis myocutaneous flap.
DISCUSSION
The rate of postoperative TE-related infections continues to remain unacceptably high despite the use of several prophylactic antimicrobial regimens. Once an infection occurs, from the very onset, it is paramount to optimize the empiric antibiotic regimen to successfully salvage the implant, decrease overall morbidity, and avoid delays in adjuvant therapy. Our results demonstrate that empiric antimicrobial regimens currently used for the treatment of TE infections are diverse and do not generally cover the spectrum of organisms that cause TE infections, including methicillin-resistant staphylococci and Gram-negative rods.
Similar to previous studies, our data show that the organisms causing TE infection and implant loss are usually resistant to commonly used postoperative prophylactic regimens that target predominantly Gram-positive bacteria, including first-generation cephalosporins.1,15,18,19 In addition, approximately two-thirds of these infections occur more than 30 days after surgery, which is often beyond when most drains have been removed and antibiotic regimens discontinued. It has been speculated that prophylactic antibiotics prevent early infections caused by nonresistant bacteria while selecting for resistant organisms that can lead to late infection.15
Consensus has not been reached on either the use of postoperative prophylactic antibiotics or the appropriate overall duration needed to prevent infections.3,18 Several studies have revealed that following Surgical Care Improvement Project guidelines in prosthetic breast reconstruction and withholding postoperative prophylactic antibiotics can increase a patient’s SSI reoperation risk 4.7 times. In addition, stopping antibiotic prophylaxis after 24 hours could raise the rate of infection requiring the removal of a TE from 7.9% to 31%.20,21 In these studies, the bacteria isolated in the groups not treated with an extended course of postoperative prophylactic antibiotics were more diverse than those seen in other studies in which patients used long-term postoperative antibiotics.15,20–23
Targeting postoperative prophylaxis to patients at high risk for infection has been suggested as an alternative to universal postoperative prophylaxis in prosthetic breast reconstruction to prevent adverse outcomes from extended use, such as increasing resistance, allergic reactions, and other infections associated with antibiotic use.15 A heightened awareness of those patients who are at an increased risk for infections including those who are obese, smokers, those with mastectomy skin necrosis, or a need for radiation therapy should be considered for prolonged use of prophylactic postoperative antibiotics.5,9,23–26
Once an SSI or TE infection is diagnosed, it is paramount to treat the infection with an empiric broad-spectrum antimicrobial regimen that will not only cover the most common causative organisms but also remain active among the organisms embedded in an early biofilm infection. Therefore, after reviewing the perioperative and postoperative antimicrobials used in our patient population, as well as the organisms responsible for TE infections, we have developed a comprehensive treatment algorithm (Fig. 3) to provide a more consistent approach to a patient presenting with a breast TE infection.27
Fig. 3.
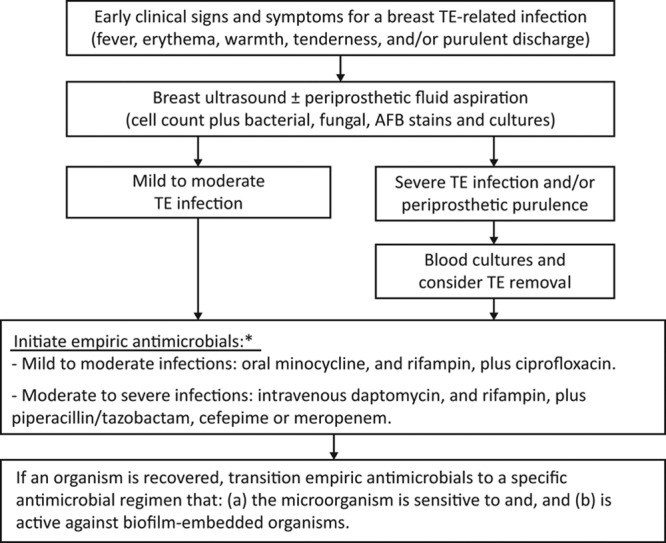
Algorithm for the treatment of TE-related infections. *Before the administration of the empiric antimicrobials, the physician should review (a) the patient’s prophylactic antimicrobials, (b) antibiotic allergies, and (c) potential for significant drug-drug interactions.
In our algorithm, we encourage all patients to proceed with a breast ultrasound. If any periprosthetic fluid is observed, ideally the fluid should be aspirated and submitted for microbiological analysis to determine whether bacteria, fungi, or acid-fast bacilli is present.28,29 In the meantime, while awaiting culture results, and after reviewing the patient’s medication and allergy profile, including any antimicrobial drugs used in the patient’s perioperative and postoperative prophylactic regimen, depending on the severity of the infection, the physician should prescribe either an oral or intravenous empiric antimicrobial regimen that targets methicillin-resistant staphylococci and Gram-negative rods including Pseudomonas. Furthermore, the empiric antimicrobial regimen should favor antibiotics that are active against the bacteria embedded in the biofilm. For example, for Gram-positive coverage, oral minocycline plus rifampin or intravenous daptomycin plus rifampin should be favored over vancomycin or linezolid.30–34 For Gram-negative coverage, quinolone, such as ciprofloxacin, should be prioritized over a β-lactam antimicrobial regimen.35–39 If the culprit organism has been eventually recovered, usually within the first 48 hours, the empiric regimen should be transitioned to a specific biofilm-active antibiotic treatment regimen dictated by the microorganism’s antimicrobial susceptibility panel, and hence, the probability for antimicrobial resistance should be extremely low. Throughout this time period, to optimize patient’s care and increase the salvage rate of TE infections, based on the experience of other complex device-related infections40,41 and knowledge of broad-spectrum antimicrobial coverage and activity of antibiotics within the biofilm matrix, the assistance of an infectious diseases consultant is highly beneficial.
One limitation of our study is the retrospective nature of the data collection. Also, because the majority of our patients had an immediate reconstructive procedure, our algorithm may not apply to patients who have had a delayed reconstructive procedure. Furthermore, to avoid including patients with a noninfectious inflammatory syndrome, and confounding our results, we did not include patients whose SSIs were adequately treated with empiric antibiotics in the absence of any culture data but only included patients who had developed a “definite” TE infection that led to removal of the TE with positive intraoperative cultures. Also, we cannot comment on the potential that withholding prophylaxis would have caused a different number of infections and TE losses. Evidence from a randomized controlled trial would be required to challenge the well-established practice patterns, which are currently based on anecdotal experience rather than evidence-based data.20 In addition, because many of our patients with cancer were not health care naive and may be colonized with hospital-acquired resistant organisms, the antimicrobial trends seen in our patient population might not accurately reflect national trends in TE infections. Therefore, to validate our recommendations, we are currently planning to proceed with a prospective clinical study.
CONCLUSIONS
To salvage an infected breast TE, based on individual hospital epidemiological data, the most common organisms causative for TE infection should be empirically covered. At our institution, methicillin-resistant staphylococci and Gram-negative rods, including Pseudomonas, were responsible for more than 60% of all TE infections across different postoperative time periods and years of our study. Therefore, these organisms should be empirically covered by broad-spectrum antibiotics that can also act on biofilm-embedded organisms, rather than by traditional narrow-spectrum regimens such as first-generation cephalosporins alone. Once an organism has been identified, the empiric antimicrobial regimen should be transitioned to a specific biofilm-active antimicrobial regimen dictated by the microorganism’s antimicrobial susceptibility panel. Finally, to optimize the care of these patients, it is of benefit to have an infectious diseases consultant assist in the care of these complex medical device-related infections.
Footnotes
Presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 8, 2014, Wash., DC.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the University of Texas MD Anderson Cancer Center.
REFERENCES
- 1.Fry DE. Surgical site infections and the surgical care improvement project (SCIP): evolution of national quality measures. Surg Infect (Larchmt) 2008;9:579–584. doi: 10.1089/sur.2008.9951. [DOI] [PubMed] [Google Scholar]
- 2.Brahmbhatt RD, Huebner M, Scow JS, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012;19:3205–3211. doi: 10.1245/s10434-012-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66:460–465. doi: 10.1097/SAP.0b013e31820c0593. [DOI] [PubMed] [Google Scholar]
- 4.Crosby MA, Dong W, Feng L, et al. Effect of intraoperative saline fill volume on perioperative outcomes in tissue expander breast reconstruction. Plast Reconstr Surg. 2011;127:1065–1072. doi: 10.1097/PRS.0b013e31820436fa. [DOI] [PubMed] [Google Scholar]
- 5.Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. doi: 10.1097/01.PRS.0000070727.02992.54. [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825–831. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RW, Berkowitz RL, Bolding F. Infection following breast reconstruction. Ann Plast Surg. 1989;23:284–288. doi: 10.1097/00000637-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Spear SL, Majidian A. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants: a retrospective review of 171 consecutive breast reconstructions from 1989 to 1996. Plast Reconstr Surg. 1998;101:53–63. doi: 10.1097/00006534-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790–1796. doi: 10.1097/PRS.0b013e3181bf80aa. [DOI] [PubMed] [Google Scholar]
- 10.Handel N, Jensen JA, Black Q, et al. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg. 1995;96:1521–1533. doi: 10.1097/00006534-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 12.Araco A, Gravante G, Araco F, et al. Infections of breast implants in aesthetic breast augmentations: a single-center review of 3,002 patients. Aesthetic Plast Surg. 2007;31:325–329. doi: 10.1007/s00266-006-0156-y. [DOI] [PubMed] [Google Scholar]
- 13.Degnim AC, Scow JS, Hoskin TL, et al. Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations. Ann Surg. 2013;258:240–247. doi: 10.1097/SLA.0b013e31828c0b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandeweyer E, Deraemaecker R, Nogaret JM, et al. Immediate breast reconstruction with implants and adjuvant chemotherapy: a good option? Acta Chir Belg. 2003;103:98–101. doi: 10.1080/00015458.2003.11679374. [DOI] [PubMed] [Google Scholar]
- 15.McCullough MC, Chu CK, Duggal CS, et al. Antibiotic prophylaxis and resistance in surgical site infection after immediate tissue expander reconstruction of the breast. Ann Plast Surg. 2014;73(2):1–5. doi: 10.1097/SAP.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 16.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28:1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 17.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1–13. doi: 10.1097/PRS.0b013e3182729c39. [DOI] [PubMed] [Google Scholar]
- 19.Weichman KE, Levine SM, Wilson SC, et al. Antibiotic selection for the treatment of infectious complications of implant-based breast reconstruction. Ann Plast Surg. 2013;71:140–143. doi: 10.1097/SAP.0b013e3182590924. [DOI] [PubMed] [Google Scholar]
- 20.Clayton JL, Bazakas A, Lee CN, et al. Once is not enough: withholding postoperative prophylactic antibiotics in prosthetic breast reconstruction is associated with an increased risk of infection. Plast Reconstr Surg. 2012;130:495–502. doi: 10.1097/PRS.0b013e31825dbefe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avashia YJ, Mohan R, Berhane C, et al. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;131:453–461. doi: 10.1097/PRS.0b013e31827c6d90. [DOI] [PubMed] [Google Scholar]
- 22.Ahn CY, Ko CY, Wagar EA, et al. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98:1225–1229. doi: 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 24.Leyngold MM, Stutman RL, Khiabani KT, et al. Contributing variables to post mastectomy tissue expander infection. Breast J. 2012;18:351–356. doi: 10.1111/j.1524-4741.2012.01253.x. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121:1886–1892. doi: 10.1097/PRS.0b013e31817151c4. [DOI] [PubMed] [Google Scholar]
- 26.Peled AW, Stover AC, Foster RD, et al. Long-term reconstructive outcomes after expander-implant breast reconstruction with serious infectious or wound-healing complications. Ann Plast Surg. 2012;68:369–373. doi: 10.1097/SAP.0b013e31823aee67. [DOI] [PubMed] [Google Scholar]
- 27.Viola GM, Raad II, Rolston KV. Breast tissue expander-related infections: perioperative antimicrobial regimens. Infect Control Hosp Epidemiol. 2014;35:75–81. doi: 10.1086/674390. [DOI] [PubMed] [Google Scholar]
- 28.Macadam SA, Mehling BM, Fanning A, et al. Nontuberculous mycobacterial breast implant infections. Plast Reconstr Surg. 2007;119:337–344. doi: 10.1097/01.prs.0000244924.61968.d2. [DOI] [PubMed] [Google Scholar]
- 29.Vinh DC, Rendina A, Turner R, et al. Breast implant infection with Mycobacterium fortuitum group: report of case and review. J Infect. 2006;52:e63–e67. doi: 10.1016/j.jinf.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Singh R, Ray P, Das A, et al. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65:1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Martínez JM, Ballesta S, García I, et al. [Activity and penetration of linezolid and vancomycin against Staphylococcus epidermidis biofilms]. Enferm Infecc Microbiol Clin. 2007;25:425–428. doi: 10.1157/13108705. [DOI] [PubMed] [Google Scholar]
- 32.Darouiche RO, Dhir A, Miller AJ, et al. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170:720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 33.Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2005;49:2467–2473. doi: 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raad I, Hanna H, Jiang Y, et al. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob Agents Chemother. 2007;51:1656–1660. doi: 10.1128/AAC.00350-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiecińska-Piróg J, Bogiel T, Gospodarek E. Effects of ceftazidime and ciprofloxacin on biofilm formation in Proteus mirabilis rods. J Antibiot (Tokyo) 2013;66:593–597. doi: 10.1038/ja.2013.59. [DOI] [PubMed] [Google Scholar]
- 36.Bowler LL, Zhanel GG, Ball TB, et al. Mature Pseudomonas aeruginosa biofilms prevail compared to young biofilms in the presence of ceftazidime. Antimicrob Agents Chemother. 2012;56:4976–4979. doi: 10.1128/AAC.00650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drago L, Mattina R, Legnani D, et al. Modulation of biofilm of strains isolated from patients with chronic obstructive pulmonary disease by levofloxacin, moxifloxacin, ciprofloxacin, amoxicillin/clavulanic acid and ceftriaxone. Int J Immunopathol Pharmacol. 2011;24:1027–1035. doi: 10.1177/039463201102400420. [DOI] [PubMed] [Google Scholar]
- 38.Naves P, Del Prado G, Ponte C, et al. Differences in the in vitro susceptibility of planktonic and biofilm-associated Escherichia coli strains to antimicrobial agents. J Chemother. 2010;22:312–317. doi: 10.1179/joc.2010.22.5.312. [DOI] [PubMed] [Google Scholar]
- 39.Bret L, Di Martino P. Effect of ceftazidime, amikacin and ciprofloxacin on biofilm formation by some enterobacterial clinical isolates. Chemotherapy. 2004;50:255–259. doi: 10.1159/000081947. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 41.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]


