Abstract
Background
In contrast to percutaneous atrial septal occluder device, surgical patch closure of atrial defects was known to be no infective endocarditis risk.
Case presentation
We herein report the first case of late endocarditis of surgical patch closure of atrial septal defects occurred at 47-year after surgery. On September 2014, a 56-year-old immunocompetent French Caucasian man was admitted into the Emergency Department for 3-week history of headache, acute decrease of psychomotor performance and fever at 40 °C. The diagnosis has been evoked during his admission for the management of a brain abscess and confirmed using 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT). Bacterial cultures of surgical deep samples of brain abscess were positive for Streptococcus intermedius and Aggregatibacter aphrophilus as identified by the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and confirmed with 16S rRNA gene sequencing. The patient was treated by antibiotics for 8 weeks and surgical patch closure removal.
Conclusions
In summary, late endocarditis on surgical patch and on percutaneous atrial septal occluder device of atrial septal defects is rare. Cardiac imaging by the 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT) could improve the diagnosis and care endocarditis on surgical patch closure of atrial septal defects while transthoracic and transesophageal echocardiography remained difficult to interpret.
Keywords: Infective endocarditis, Atrial septal defects, Surgical patch closure, Aggregatibacter aphrophilus, Streptococcus intermedius, PET scan, 18F-FDG-PET/CT, Brain abscess, Diagnosis, Treatment, Infection, Bacteria, Human
Background
Infectious complication of atrial septal occlude device is rare that represented about 0.1 % of cases [1]. We herein report the first case of endocarditis at 47-year after surgical closure of atrial septal defects discovered by positron emission tomography with 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT).
Case presentation
On September 2014, a 56-year-old immunocompetent French Caucasian man was admitted into the Emergency Department for 3-week history of headache, acute decrease of psychomotor performance and fever at 40 °C. In his medical past-history, he underwent a surgical patch closure of atrial septal defects for patent foramen ovale at 9 years old and dental care at 5 months before his admission. Laboratory investigations revealed an elevated leukocyte count at 12.6 × 109/L with an elevated neutrophil count at 8.4 × 109/L, a normal platelet count at 180 × 109/L, an elevated C reactive protein rate at 97 mg/L and negative blood culture. A brain magnetic resonance imaging (MRI) revealed a right occipital cerebral abscess (27 × 36 × 43 mm, hyper intense T2) (Fig. 1).
Fig. 1.
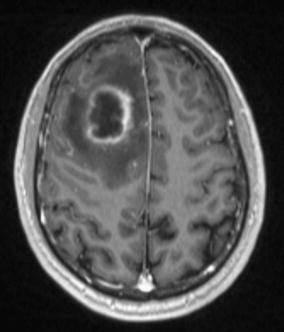
A brain magnetic resonance imaging (MRI) showing a unique brain abscess (27 × 36 × 43 mm)
He underwent surgical brain abscess drainage. Bacterial cultures of surgical deep samples of brain abscess were positive for Streptococcus intermedius and Aggregatibacter aphrophilus as identified by the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and confirmed with molecular identification (16 s gene sequencing). The patient was treated first with an empiric antibiotherapy with cefotaxim 4 g every 8 h associated with metronidazole 500 mg every 8 h and cotrimoxazole 800/160 mg every 8 h. One week later, the antibiotic treatment was modified for ceftriaxone 2 g/day associated with metronidazole 500 mg every 8 h and rifampicin 300 mg every 8 h.
A transthoracic echocardiographic examination and a body scan revealed no abnormality. A transesophageal echocardiography showed a high right left shunt suggestive of atrial septal patch dehiscence without direct sign of. An 18F-FDG-PET/CT showed an intense linear hypermetabolism opposite the interauricular septum compatible with an endocarditis on the intracardiac device (Fig. 2). Transesophageal echocardiography after 6 weeks of antibiotic treatment showed a filamentous image opposite the mitral valve and no vegetation image was found. A second 18F-FDG-PET/CT kept showing hypermetabolism on the surgical patch closure of atrial septal defects. The patient was treated by antibiotics for 8 weeks.
Fig. 2.
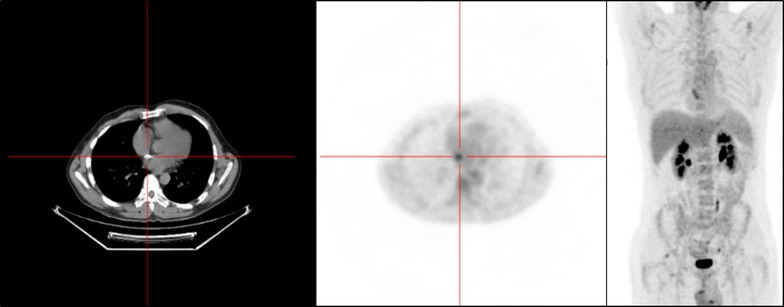
The 18F-fluorodeoxyglucose gated cardiac computed tomography (18F-FDG-PET/CT) imaging showing hypermetabolism hyperactivity around the interauricular septum
At the end of antibiotic treatment transthoracic echocardiographic examination showed an exclusive right left shunt, confirming dehiscence of the surgical patch closure of atrial septal defects. Surgical removal of surgical patch closure of atrial septal defects was necessary on April 2015 with replacement by a bovine pericardial patch. No antibiotics were given during explantation. Explanted atrial surgical patch closure of atrial septal defects looked swollen and calcified with negative bacterial cultures. The patient recovered without further complication. His hemiparesis fully disappeared; he only kept discrete amnesic and attentional failures. He presents a sequelae of attentional and memory deficits at 1 year of follow-up.
Discussion
In contrast to percutaneous atrial septal occluder device, surgical patch closure of atrial septal defects was known to be associated with no infective endocarditis risk [2]. To our best knowledge, only one case of endocarditis on surgical patch of ventricular septal defect [3] and 17 cases of bacterial endocarditis on percutaneous atrial septal occluder device [4–15] have been reported. In our case, the late onset of the infection occurred at 47-year after surgery, and it suggests that origin of endocarditis of surgical patch closure of atrial septal defects was a hematogenous spread of bacteria during the dental care at 5 months before. Diagnosis of endocarditis on atrial septal occluder device is generally done by transthoracic echocardiographic or transesophageal echocardiography. Therefore, in our case the diagnosis has been evoked during his admission for the management of a brain abscess, and established using 18F-FDG-PET/CT while transthoracic and transesophageal echocardiography remained difficult to interpret. The benefit of 18F-FDG-PET/CT has been demonstrated in the diagnosis of prosthetic valve endocarditis cardiac device-related endocarditis for detect infection by highlighting inflammatory leukocytes express a high density of glucose transporters and are highly metabolically active with the high spatial resolution of cardiac tomography and angiography, and it was recently added to the diagnosis of infective endocarditis criteria [16, 17].
Conclusion
In summary, late endocarditis on surgical patch and on percutaneous atrial septal occluder device of atrial septal defects is rare. We believe that the contribution of 18F-FDG-PET/CT could improve the diagnosis and care of endocarditis on surgical patch closure or percutaneous atrial septal occluder device of atrial septal defects. A prolonged antibiotherapy and surgical management with surgical patch removal is a major therapeutic option of endocarditis on surgical patch closure of atrial septal defects.
Authors’ contributions
EH involved in clinical data collection and the drafting of the manuscript. PS involved in the drafting of the manuscript and manuscript revision. AR provided clinical data verification and revision of the manuscript. GH provided clinical data verification and revision of the manuscript. AS provided clinical data verification, corrected the discussion section, and approved the final version to be published. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Catherine Peruffo for her assistance in acquisition of data. The authors obtained permission from Catherine Peruffo to acknowledge.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Medical imaging data will not be shared because it is not fully anonymous.
Consent to publish
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Ethics approval and consent to participate
This study was approved by the institutional research ethics board (Comite de Protection des Personnes Sud Méditerranée 1), and written informed consent was obtained from the patient to participate to this case report and any accompanying images.
Funding
The authors have no relevant affiliations or involvement with any organization or entity with a financial interest or conflict with the subject matter or materials discussed in the manuscript. No writing assistance was utilized in the production of this manuscript.
Abbreviations
- MALDI-TOF mass spectrometry
the matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- 18F-FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- MRI
magnetic resonance imaging
Footnotes
Estelle Honnorat and Piseth Seng contributed equally to this work
Contributor Information
Estelle Honnorat, Email: estelle.honnorat@mail.ap-hm.fr.
Piseth Seng, Phone: +33 (0)4 91 38 41 24, Email: sengpiseth@yahoo.fr.
Alberto Riberi, Email: alberto.riberi@mail.ap-hm.fr.
Gilbert Habib, Email: gilbert.habib@mail.ap-hm.fr.
Andreas Stein, Email: andreas.stein@mail.ap-hm.fr.
References
- 1.Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM, Kapadia SR. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc. Interv. 2012;5:777–789. doi: 10.1016/j.jcin.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins RA, Bert AA, Buchholz B, Guarino K, Meyers M. Surgical patch closure of atrial septal defects. Ann Thorac Surg. 2004;77:2144–2150. doi: 10.1016/j.athoracsur.2003.10.105. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Yamagishi M, Yaku H. Reoperation for prosthetic ventricular septal defect patch endocarditis: long-term results with an autologous atrial septal patch. Gen Thorac Cardiovasc Surg. 2011;59:753–755. doi: 10.1007/s11748-010-0768-7. [DOI] [PubMed] [Google Scholar]
- 4.Divchev D, Podewski EK, Mengel M, Meyer GP, Drexler H, Schaefer A. Inflammatory, abscess-forming foreign body reaction mimics a thrombus formation on an atrial septal defect closure device: a commented case report. Eur J Echocardiogr. 2007;8:298–302. doi: 10.1016/j.euje.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Walpot J, Amsel B, Rodrigus I, Pasteuning WH, Koeman J, Hokken R. Late infective endocarditis of an atrial septal occluder device presenting as a cystic mass. Echocardiography. 2011;28:E131–E133. doi: 10.1111/j.1540-8175.2011.01387.x. [DOI] [PubMed] [Google Scholar]
- 6.Aruni B, Sharifian A, Eryazici P, Herrera CJ. Late bacterial endocarditis of an Amplatzer atrial septal device. Indian Heart J. 2013;65:450–451. doi: 10.1016/j.ihj.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slesnick TC, Nugent AW, Fraser CD, Cannon BC. Images in cardiovascular medicine. Incomplete endothelialization and late development of acute bacterial endocarditis after implantation of an Amplatzer septal occluder device. Circulation. 2008;117:e326–e327. doi: 10.1161/CIRCULATIONAHA.107.754069. [DOI] [PubMed] [Google Scholar]
- 8.Zahr F, Katz WE, Toyoda Y, Anderson WD. Late bacterial endocarditis of an amplatzer atrial septal defect occluder device. Am J Cardiol. 2010;105:279–280. doi: 10.1016/j.amjcard.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Calachanis M, Carrieri L, Grimaldi R, Veglio F, Orzan F. Infective endocarditis after transcatheter closure of a patent foramen ovale. Catheter Cardiovasc Interv. 2004;63:351–354. doi: 10.1002/ccd.20185. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JA, Beardslee MA, Xuuu H, Sundt TM, Lasala JM. Infective endocarditis resulting from CardioSEAL closure of a patent foramen ovale. Catheter Cardiovasc Interv. 2002;55:217–220. doi: 10.1002/ccd.2999. [DOI] [PubMed] [Google Scholar]
- 11.Balasundaram RP, Anandaraja S, Juneja R, Choudhary SK. Infective endocarditis following implantation of amplatzer atrial septal occluder. Indian Heart J. 2005;57:167–169. [PubMed] [Google Scholar]
- 12.Mitchell ARJ, Leeson P, Timperley J, Myerson SG, Becher H, Goldman J. Atrial septal endocarditis. Eur J Echocardiogr J. 2007;8:48–49. doi: 10.1016/j.euje.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Stöllberger C, Bastovansky A, Finsterer J. Fatal septicemia in a patient with cerebral lymphoma and an Amplatzer septal occluder: a case report. J Med Case Reports. 2011;5:554. doi: 10.1186/1752-1947-5-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheuerman O, Bruckheimer E, Marcus N, Hoffer V, Garty B-Z. Endocarditis after closure of ventricular septal defect by transcatheter device. Pediatrics. 2006;117:e1256–e1258. doi: 10.1542/peds.2005-2498. [DOI] [PubMed] [Google Scholar]
- 15.Krantz SB, Lawton JS. Subacute endocarditis of an atrial septal closure device in a patient with a patent foramen ovale. Ann Thorac Surg. 2014;98:1821–1823. doi: 10.1016/j.athoracsur.2013.12.079. [DOI] [PubMed] [Google Scholar]
- 16.Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–2382. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 17.Pizzi MN, Roque A, Fernández-Hidalgo N, Cuéllar-Calabria H, Ferreira-González I, Gonzàlez-Alujas MT, et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18f-fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132:1113–1126. doi: 10.1161/CIRCULATIONAHA.115.015316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Medical imaging data will not be shared because it is not fully anonymous.


