Abstract
Background:
Nowadays, definitive diagnosis of numerous diseases is based on the genetic and molecular findings. Therefore, preparation of fundamental materials for these evaluations is necessary. Deoxyribonucleic acid (DNA) is the first material for the molecular pathology and genetic analysis, and better results need more pure DNA. Furthermore, higher concentration of achieved DNA causes better results and higher amplifying ability for subsequent steps. We aim to evaluate five DNA extraction methods to compare DNA intimacy including purity, concentration, and amplifying ability with each other.
Materials and Methods:
The lymphoid tissue DNA was extracted from formalin-fixed, paraffin embedded (FFPE) tissue through five different methods including phenol-chloroform as the reference method, DNA isolation kit (QIAamp DNA FFPE Tissue Kit, Qiagen, Germany), proteinase K and xylol extraction and heat alkaline plus mineral oil extraction as authorship innovative method. Finally, polymerase chain reaction (PCR) and real-time PCR method were assessed to compare each following method consider to DNA purity and its concentration.
Results:
Among five different applied methods, the highest mean of DNA purity was related to heat alkaline method. Moreover, the highest mean of DNA concentration was related to heat alkaline plus mineral oil. Furthermore, the best result in quantitative PCR was in proteinase K method that had the lowest cycle threshold averages among the other extraction methods.
Conclusion:
We concluded that our innovative method for DNA extraction (heat alkaline plus mineral oil) achieved high DNA purity and concentration.
Key words: Deoxyribonucleic acid concentration, deoxyribonucleic acid extraction, deoxyribonucleic acid purity, lymphoid tissue
INTRODUCTION
On the rise of attention in the genetic basis of diseases, the amount of genomic deoxyribonucleic acid (DNA) available from biological samples might be limit the genomic analysis practicality.1 Molecular diagnostics of human diseases can be classified into three major areas: Pathogen detection, mutation detection, and detecting the alterations in human genes that modify their functions or expression levels.2
Getting the genomic DNA from fresh or frozen tissues is required for analyses.3 Pursuant to the collections of paraffin samples in pathology institutes around the world as archives of genetic material, could provide much useful genetic information.4
Investigating DNA of materials stashed as formalin-fixed, paraffin embedded tissue (FFPET) sections in pathology laboratories has been increasingly practical to meet the requirement of rapid development of molecular morphology. Therefore, the extraction method which provides an adequate quality and quantity of DNA is critical.5 In addition, it is worth noting that the integrity of DNA is affected critically by the applied fixation method.6
To mutation detection or linkage analysis, the isolation of DNA fragments is so important. Recently, polymerase chain reaction (PCR) amplification method has revolutionized the field of molecular pathology due to its ability on specific and fast disease detection.7 PCR is a simple in vitro chemical reaction that allows the synthesis of necessarily limitless quantities of a targeted nucleic acid sequence. This is performed via the action of DNA polymerase, under the right conditions.8 The most common methods, for extraction from FFPE, was reported by 60-80% success in amplification rate.9 Therefore, the success in subsequent analysis that has good purity, integrity, and concentration for molecular diagnosis is important.10
There are different methods for DNA extraction from paraffin-embedded tissues (PETs). One of the most efficient and frequent used methods to purify DNA from PETs is phenol-chloroform method. In this study, we investigated three different DNA extraction methods then compared them with two routine methods as control groups; the first was the traditional chloroform method and the second was a commercially available DNA isolation kit to identify a simple and inexpensive protocol.
MATERIALS AND METHODS
Five fresh lymphoid tissues from patients with tonsil hypertrophy in a recent tonsillectomy surgery were obtained from ear, nose and throat. Their clinical diagnosis is confirmed after preparation of microscopic slides and hematoxylin and eosin staining by two expert pathologists. 10 millimeter thick sections of each block of PETs were accumulated in a 1.5 ml microtube. The equal numbers of sections were prepared from each case into five different tubes. One milliliter preheated xylene was added to each microtube, and then these were kept at 56°C for 10 min. Then the tubes were centrifuged at 9300 g for 5 min. The supernatant was discarded, followed by new variations of preheated xylene, until the paraffin was entirely removed. The pellet was washed in series of ethanol dilution (absolute ethanol, ethanol 95% and ethanol 70%). Every alteration was preceded by homogenization and centrifugation at 9300 g for 5 min. Five different DNA extraction methods into the following manner.
Phenol-chloroform method
It was the reference method that we used for DNA extraction from fresh samples. Therefore, 500 μl of buffer lysis (25 μl proteinase K; 20 mg/dl, 1 μl ethylenediaminetetraacetic acid (EDTA); 0.5M, 5 μl Tris-hydrochloride; pH 8, 50 μl sodium dodecyl sulfate [SDS] 10%, 420 μl distilled water, 150 μl sodium hydroxide [NaOH]; 0.1N) was added to the microtube, then centrifuged for short time (about 1 min), microtube was incubated 24 h at 56°C. After that, 440 μl of phenol was added and shake for about 5 min, centrifuged at 100,000 rpm for 10 min. The supernatant was transferred by pipetting into a new microtube, 500 μl from mixture of (phenol-chloroform-isoamyl alcohol) was added and the same was done as phenol step. Then 440 μl from mixture of phenol-chloroform with 24/1 ratio was added to the new microtube again. The same was done as phenol step; sodium acetate (3M) for about 1/10 of the entire content of microtube was added. Isopropanol for equal volume of entire microtube content and 1 μl glycogen (10 mg/ml) were added and then vertex. The sample was incubated in −20°C freezer for one night then it was put out and centrifuged as the same before, the supernatant was removed out, 1 ml ethanol 70% was appended to the precipitant, centrifuged at 14,000 rpm for 5 min, this step was re-did two to three times. The microtube was put on dry block at 55°C to vapor alcohol and 50 μl distilled water or Tris-EDTA buffer was added.
QIAamp deoxyribonucleic acid formalin-fixed, paraffin embedded tissue kit
Direct DNA extraction with QIAamp DNA FFPET kit was done. The extraction and purification procedure was performed conforming to the manufacturer's instructions (QIAamp DNA FFPE Tissue Kit, Qiagen, Germany).
Proteinase K and xylol extraction method
In this method, 1 ml of phosphate-buffered saline was appended to micro-tube, centrifuged at 14,000 rpm for 15 min, the supernatant removed; this step was repeated one more time. Other steps were similar to phenol-chloroform method.
Heat alkaline extraction method
In this method, 500 μl of NaOH (0.1M), 50 μl of SDS 10% were added to spacemen, respectively. Microtube was put on the dry block at 100°C for 20 min then in room temperature (RT), for 15 min. Then 500 μl of phenol was added and centrifuged at 12,000 rpm for 10 min. Other steps were similar to phenol-chloroform method.
Heat alkaline plus mineral oil extraction method
In this method, 300 μl of mineral oil and ethanol 100% were added to the microtube then incubated for 30 min at RT, then followed by centrifugation at 14,000 rpm for 10 min. The supernatant was removed; the previous stage exactly and separately was done for ethanol 90% and 70% either. After removing the supernatant, 500 μl NaOH (0.1M) and 50 μl SDS 10% were added into microtube on dry block at 100°C for 20 min and 15 min at RT. Other steps were similar to phenol-chloroform method.
Evaluation of extracted deoxyribonucleic acid
After extraction with these five different methods, we assessed:
Assessment of deoxyribonucleic acid content and deoxyribonucleic acid purity
For assessment of DNA content (DNA yield), bioanalyzer (NanoDrop 2000 Spectrophotometer, Thermo, Wilmington, USA) that worked based on spectrophotometry was used.
Assessment of deoxyribonucleic acid amplification ability
For assessment of DNA amplification capability, PCR on extracted DNAs with utilization of a housekeeping gene (β-globin gene) was carried out. For this purpose, three sizes of β-globin, primer gene with 110 bp, 268 bp, and 530 bp length which produced in Metabion Company (Germany) were used.
PCR was applied by the thermocycler (Veriti-Applied Biosystems instrument, USA) and PCR method previously described [Figure 1].6
Figure 1.
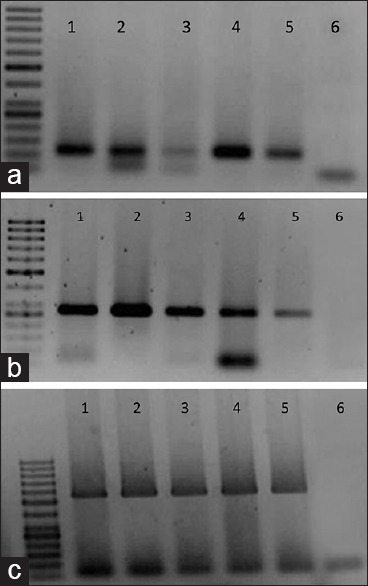
5 different extraction methods for three sizes, (a) Figure: 110 bp size, (b) 256 and (c) 512bp of amplified β-globin gene primers. 1. Phenol-chloroform method, 2. QIAamp DNA FFPE Tissue Kit method, 3. Proteinase K and xylol method, 4. Heat alkaline method, 5. Heat alkaline plus mineral oil method and 6 is Negative control
Real-time polymerase chain reaction method
Amplifying ability of extracted DNA assessed by real-time PCR that previously described.4,11 Real-time PCR for B-actin gene was performed in the StepOne ABI detection system (USA).
Statistical analysis
Data analysis was carried out by SPSS statistical software version 11.5 (SPSS Inc., Chicago, IL, USA). Numerical data are expressed as mean ± standard deviation or as proportions of the sample size. We used Chi-square test to compare means for comparison of qualitative variables such as PCR results in different fixatives. All data were checked for normality by Kolmogorov-Smirnov test. For quantitative variables such as DNA purity, ANOVA test, or Kruskal-Wallis test due to parametric or nonparametric distribution of our data was performed. P < 0.05 was considered significant.
RESULTS
We had two categories, fresh and formalin-fixed samples. The total amount and purity of DNA from each method and category are shown in Table 1.
Table 1.
Statistical analysis between different DNA extraction methods for fresh and fixed tissues.
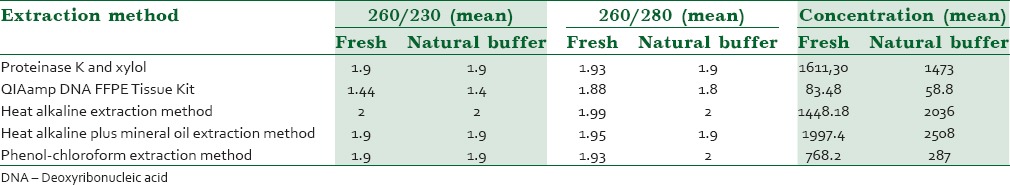
All five extraction methods produced good yields of DNA (above nearly 90 mg). The highest mean purity (260/230: 2) was related to heat alkaline protocol in both categories. Furthermore, significant difference in DNA concentration between five DNA extraction methods (P < 0.05). Moreover, the highest mean concentration was related to heat alkaline with mineral oil method (1997 ± 161 ng/μl and 2508 ± 161 ng/μl).
Statistical analysis on comparing DNA intimacies (concentration, purity, amplifying ability) in formalin-fixed tissues with fresh tissues as a gold standard issue showed that there was not any significant difference in DNA purity between formalin-fixed and fresh tissues. However, there was significant difference in DNA concentration between formalin-fixed and fresh tissues (P = 0.01).
Three figures show the electrophoretic pattern of DNA recovered by the five extraction methods. The electrophoresis pattern was identical for all methods used.1
In amplifying the 256 bp and 512 bp fragment of the β-globin gene, proteinase K transcends on other extraction methods, qua in all five different fixatives the highest successful amplification with 256 and 512 bp fragment of the β-globin gene was in samples extracted via proteinase K method, as it was mentioned in Table 2.
Table 2.
Statistical analysis for PCR amplification between different Extraction methods
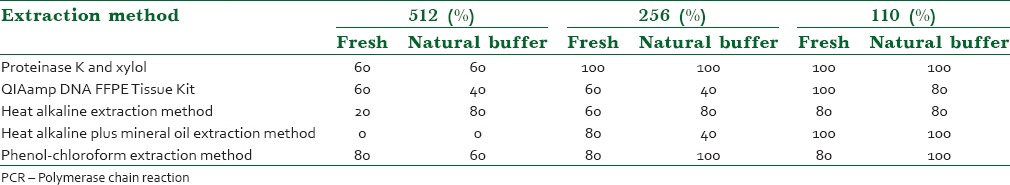
After statistical analysis, there was no significant difference between five different extraction methods for three sizes (110, 256, 512 bp) of amplified β-globin gene primers (P = 0.37).
There was a Significant difference in cycle threshold (Ct) of real-time PCR (P = 0.001). The lowest Ct was in proteinase K method.
DISCUSSION
FFPET represent the largest original of archived biological material for genomic and molecular pathology studies and the only DNA resource available in several cases.2,12 On the other hand, we know that definitive diagnosis of numerous diseases is based on their genetic and molecular findings. Now PCR-based techniques coupled with new developments in the extraction of DNA from FFPEs enable pathologists to use such archival material for a variety of purposes.1,13 Therefore, preparation of basic materials for these evaluations is necessary. According to the previous studies, the highest concentration of achieved DNA yielded the better results and the highest amplifying ability for subsequent steps. Although PETs are regarded commonly as presenting damage incurred during the fixation and embedding processes. It has been demonstrated that obtained DNA from PETs are suitable for use in PCR.1
The presented protocol in this study satisfies these features of quality and quantity. We demonstrated that the obtained DNA was efficiently used in PCR and real-time reactions. In this study, we demonstrated heat alkaline plus mineral oil extraction method had the highest quality and quantity DNA among the other applied different DNA extraction methods. As the highest mean of DNA purity was in heat alkaline method. Highest mean of DNA concentration was related to heat alkaline plus mineral oil (our authorship method). Furthermore, the best results in quantitative PCR were in proteinase K method that had the lowest Ct averages between other extraction methods.
The first report about the use of mineral oil as a deparaffinizing reagent was published in December 2009 by Lin et al.6 Both mineral oil and paraffin have been used in PCR for many years, and it was already known that neither affects the efficiency of PCR; moreover, paraffin can be dissolved in mineral oil entirely. Due to these reasons, we presumed that mineral oil could be applied to eliminate wax effectively from FFPE samples without affecting DNA quality for downstream PCR and genotyping tests.
Lin et al. extracted DNA from 140 long-term archived FFPE samples applying a simple although efficacious deparaffinization method, eliminating the wax with mineral oil, and a commercially existent DNA extraction kit. DNA quality was pursuant experimented in a genotyping test with 14 microsatellite markers. High-quality DNA was obtained with a mean PCR success rate of 97% (range: 88-100%) across markers. Their results suggested that DNA extracted using this novel method is likely to be suitable for genetic studies involving DNA fragments <200 bp.6,11
We also used mineral oil in one of our extraction methods and to the best of our knowledge; it is the first report of this extraction method that is synchronous usage of mineral participant with heat alkaline protocol.
Cao et al. presented a comparison of methods for DNA extraction from PETs and buccal cells. They concluded that among three methods modified phenol-chloroform protocol, simple boiling method and DNA Extraction Mini Kit, both the simple boiling method and the phenol-chloroform method are better methods for DNA isolation from FFPET, besides the phenol-chloroform method is the best method for DNA extraction from buccal cells.14,15
Cler et al. study for comparison of five extracting DNA methods from paucicellular clinical samples found that the performance of the three commercial kits was superior to either phenol-chloroform extraction or single step proteinase K digestion. QIAamp and Puregene DNA extraction methods are well-suited for the procurement of paucicellular clinical samples for PCR-based tests.16
Farrugia et al. compared the efficiency of three DNA extraction and purification protocols from two various free flow electrophoresis tissue substrates, heart, and liver, by quantitative PCR and multiplex amplification. They showed that the method, using phenol-chloroform and the QIAamp DNA Mini Kit (Qiagen, Germany), was the most efficient DNA extraction and purification method and that the DNA quantity extracted from liver is statistically more important than that extracted from heart.17,18
One of the most important reasons that we selected the commercial QIAamp kit for our study was many articles, referred previously that realized that this kit is one of the most authentic kits between hundreds of commercial kits.
Shi et al. designed several studies that presented variously DNA extraction methods. Their results showed that boiling tissue sections in 0.1M NaOH, KOH or its complex retrieval solutions was made higher efficiencies and better quality of DNA compared to lysis buffer or chemical solutions alone.19,20
Achievement of successful PCR was a little different in three primer sizes. Amplifying the 110 bp fragment of the β-globin gene was successful in most of samples (all fixatives with all extraction method) qua more than 90% were amplified. Hence, in subsequent studies if our target be a little size amplicon there is not any difference to use.
Therefore, that we compare our fixed tissues with fresh tissue as a gold standard, so there was no significant difference between fresh and all fixed tissues in DNA purity (P ≥ 0.05).
CONCLUSION
It must be noted that our innovative method for DNA extraction (heat alkaline plus mineral oil) as the first innovative extraction, have excellent results especially in the achievement of high DNA concentration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Santos S, Sá D, Bastos E, Guedes-Pinto H, Gut I, Gärtner F, et al. An efficient protocol for genomic DNA extraction from formalin-fixed paraffin-embedded tissues. Res Vet Sci. 2009;86:421–6. doi: 10.1016/j.rvsc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Sun W. Molecular Diagnostics: Techniques and Applications for the Clinical Laboratory. 1st ed. London: Academic; 2010. Nucleic extraction and amplification. [Google Scholar]
- 3.Rivero ER, Neves AC, Silva-Valenzuela MG, Sousa SO, Nunes FD. Simple salting-out method for DNA extraction from formalin-fixed, paraffin-embedded tissues. Pathol Res Pract. 2006;202:523–9. doi: 10.1016/j.prp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Votavova H, Forsterova K, Stritesky J, Velenska Z, Trneny M. Optimized protocol for gene expression analysis in formalin-fixed, paraffin-embedded tissue using real-time quantitative polymerase chain reaction. Diagn Mol Pathol. 2009;18:176–82. doi: 10.1097/PDM.0b013e31818d1091. [DOI] [PubMed] [Google Scholar]
- 5.Shibutani M, Uneyama C, Miyazaki K, Toyoda K, Hirose M. Methacarn fixation: A novel tool for analysis of gene expressions in paraffin-embedded tissue specimens. Lab Invest. 2000;80:199–208. doi: 10.1038/labinvest.3780023. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Kennedy SH, Svarovsky T, Rogers J, Kemnitz JW, Xu A, et al. High-quality genomic DNA extraction from formalin-fixed and paraffin-embedded samples deparaffinized using mineral oil. Anal Biochem. 2009;395:265–7. doi: 10.1016/j.ab.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonds LA, Barnes P, Foucar K, Sever CE. Acetic acid-zinc-formalin: A safe alternative to B-5 fixative. Am J Clin Pathol. 2005;124:205–11. doi: 10.1309/29DA-CY9K-BHNW-4BG6. [DOI] [PubMed] [Google Scholar]
- 8.McPherson RA, Pincus MR. Henry's Clinical Diagnosis and Management by Laboratory Methods. New York: Sunder; 2012. [Google Scholar]
- 9.Coura R, Prolla JC, Meurer L, Ashton-Prolla P. An alternative protocol for DNA extraction from formalin fixed and paraffin wax embedded tissue. J Clin Pathol. 2005;58:894–5. doi: 10.1136/jcp.2004.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasiri H, Forouzandeh M, Rasaee MJ, Rahbarizadeh F. Modified salting-out method: High-yield, high-quality genomic DNA extraction from whole blood using laundry detergent. J Clin Lab Anal. 2005;19:229–32. doi: 10.1002/jcla.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra KT, Gulati U, Balzer B, Wu HY. Comparison of DNA extraction methods from formalin-fixed, paraffin embedded tissue and their impact on real-time PCR-based mutation assays. J Med Diagn Methods. 2012;1:1–6. [Google Scholar]
- 12.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–53. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 13.Feeney-Burns L. The early years of research. Prog Clin Biol Res. 1985;190:3–23. [PubMed] [Google Scholar]
- 14.Cao W, Hashibe M, Rao JY, Morgenstern H, Zhang ZF. Comparison of methods for DNA extraction from paraffin-embedded tissues and buccal cells. Cancer Detect Prev. 2003;27:397–404. doi: 10.1016/s0361-090x(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 15.Edwin Shiaw CS, Shiran MS, Cheah YK, Tan GC, Sabariah AR. Evaluation of DNA and RNA extraction methods. Med J Malaysia. 2010;65:133–7. [PubMed] [Google Scholar]
- 16.Cler L, Bu D, Lewis C, Euhus D. A comparison of five methods for extracting DNA from paucicellular clinical samples. Mol Cell Probes. 2006;20:191–6. doi: 10.1016/j.mcp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Farrugia A, Keyser C, Ludes B. Efficiency evaluation of a DNA extraction and purification protocol on archival formalin-fixed and paraffin-embedded tissue. Forensic Sci Int. 2010;194:e25–8. doi: 10.1016/j.forsciint.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Niland EE, McGuire A, Cox MH, Sandusky GE. High quality DNA obtained with an automated DNA extraction method with 70+year old formalin-fixed celloidin-embedded (FFCE) blocks from the indiana medical history museum. Am J Transl Res. 2012;4:198–205. [PMC free article] [PubMed] [Google Scholar]
- 19.Shi SR, Datar R, Liu C, Wu L, Zhang Z, Cote RJ, et al. DNA extraction from archival formalin-fixed, paraffin-embedded tissues: Heat-induced retrieval in alkaline solution. Histochem Cell Biol. 2004;122:211–8. doi: 10.1007/s00418-004-0693-x. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, et al. Tissue handling and specimen preparation in surgical pathology: Issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929–35. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]


