Abstract
Background:
Preeclampsia (PE) is the second most common cause of maternal death after obstetric hemorrhage in Africa, a resource-limited region. This study was designed to examine the potential usefulness of a single screening plasma plasminogen activator inhibitor-1 (PAI-1) and fibronectin (FN) level for the prediction of PE in pregnant women.
Materials and Methods:
In a cohort of 180 pregnant women who were normotensive at baseline, venous blood samples were obtained before 20 weeks of gestation for the assay of plasma levels of PAI-1 and FN levels measured by enzyme-linked immunoassay technique. Twenty nonpregnant normotensive women were also evaluated as a control group. Outcomes of gestation were evaluated and correlated with the plasma levels of PAI and FN measured at mid-trimester. Mean plasma values of PAI-1 and FN were also compared between the different outcome groups.
Results:
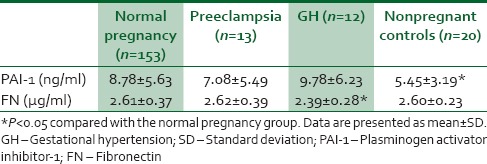
Plasma PAI-1 level was significantly higher in the pregnant women (8.68 ± 0.56 ng/ml) than in nonpregnant controls (5.55 ± 0.32 ng/ml) (P = 0.01). However, plasma FN did not show any significant difference in pregnant women (2.60 ± 0.37 μg/ml) and nonpregnant controls (2.60 ± 0.23 μg/ml) (P = 0.9). Mid-trimester mean plasma PAI-1 level measured in women who developed PE (7.08 ± 5.49 ng/ml, n = 12) and gestational hypertension (GH) (9.78 ± 6.2 ng/ml, n = 13) was not significantly different in comparison to normotensive pregnant women (8.78 ± 5.63 ng/ml, n = 153) (P = 0.75). Likewise, the mean FN level in women who developed PE was also not significantly different from nonpreeclamptics; however, the FN level in the pregnant women who developed GH was significantly different from women who remained normotensive throughout pregnancy (P = 0.02).
Conclusion:
Single mid-trimester assessment of PAI-1 and FN levels in maternal plasma was not found to be useful in predicting PE as an outcome of pregnancy in the study population.
Key words: Fibronectin, plasminogen activator inhibitor-1, preeclampsia
INTRODUCTION
Preeclampsia (PE) is the second most common cause of maternal death in Africa after obstetric hemorrhage.1 In Nigeria, the prevalence of PE has been reported to range from 5.6% to 7.6%.2,3,4 One of the challenges of modern obstetric care is to find a way to identify those women who are at increased risk of developing PE, permitting early treatment and possible prevention of life-threatening clinical disease.5
A connection between plasminogen activator inhibitor-1 (PAI-1) and PE has been established where significantly increased levels of the protein were found to be associated with thrombotic alterations of the placenta in cases of PE.6
Tjoa et al. and7 Gredmark et al.8 reported that longitudinal assessment of the change in total fibronectin (FN) levels predicted PE. However, in a resource poor country like Nigeria with high maternal mortality, it would be worthwhile to assess the predictive value of a single measurement of the markers of endothelial dysfunction in predicting PE.
The present study determines the levels of FN and PAI-1 at mid-trimester and analyzes the potential of these markers of endothelial dysfunction in predicting PE.
MATERIALS AND METHODS
This prospective study of 180 singleton pregnancies was carried out at a University Teaching Hospital in Nigeria. The research was approved by the Health Research Ethics Committee of the hospital with reference number, Ref. No. ADM/DCST/221. Only participants who gave written informed consent and satisfied the study inclusion criteria were consecutively enrolled into the study.
Inclusion criteria included normotensive gestation <20 weeks and exclusion criteria were chronic hypertension, multiple pregnancy, underlying disease history, drug consumption, familial history of hypertension, heart disease, and chronic renal disease.
All the women were enrolled between 9th and 20th week of gestation to exclude already established preeclamptics. Weight, height, and urinalysis were determined at enrollment (baseline). In addition, 4.5 ml (9 volumes) of venous blood was collected by venepuncture into vacuum tubes containing 0.5 ml (1 volume) of 0.129 M trisodium citrate and the plasma was separated after 2500 g centrifugation for 10 min and stored at − 40°C till analyzed for maternal plasma FN9 and PAI-110 levels by enzyme-linked immunosorbent assay (ELISA) (AssayMax PAI-1 and FN ELISA kit manufactured by Assaypro laboratory company, St. Charles, USA). They were then followed up till delivery when data on the outcome of delivery were collected for analysis. The three main outcome groups of pregnant women were normal, pregnancy induced hypertension (PIH) with gestational hypertension (GH).
Normal pregnancy was defined as a singleton pregnancy that occurred in a normotensive woman without proteinuria, who remained normotensive and delivered after 36 weeks of pregnancy, whereas gestational hypertension (GH) was defined as having a blood pressure higher than 140/90 measured on two separate occasions after 20 weeks of gestation, more than 6 h apart, without the presence of protein in the urine. PE was diagnosed with the presence of hypertension (two separate readings taken at least 6 h apart of 140/90 or more) and proteinuria (≥300 mg in a 24 h urine collection, or over 30 mg/dl [2+]) by urinary dipstick according to (American college of Obstetricians and Gynecologists) ACOG, both occurring after 20 weeks gestation.11
Statistical analysis
Data were analyzed using SPSS version 17.0 (Statistical Package for Social Sciences Inc., Chicago, Il, USA). The data were tested for normal distribution and Student's t-test or Chi-square test was used for comparison of two groups where appropriate. A P > 0.05 was considered statistically significant.
RESULTS
Out of the 180 participants, 178 (98.9%) were followed to delivery, whereas 2 (1.1%) were lost to follow-up. Out of the 178 pregnant women, 153 (85.9%) had a normal delivery. Thirteen (7.3%) developed PE and 12 (6.7%) developed GH. An overall incidence of PE of 7.3% was thus reported in this study.
The mean ages of the 178 pregnant women who had an eventful delivery and 20 age-matched controls were 30.6 ± 4.5 years and 30.2 ± 5.5 years, respectively (P = 0.78). The overall mean gestation age at enrollment was 17.6 ± 4.2 weeks.
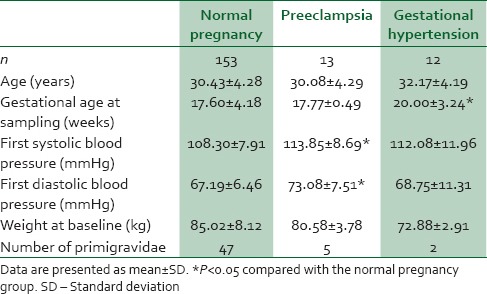
Table 1 shows the clinical characteristics of the participants. Of the 178 pregnancies, 13 developed PE and 12 developed GH. No difference was found in the average maternal age, and weight at baseline. A higher proportion of primigravidae was found in the PE group. The blood pressure was significantly higher in the PE group in comparison to the normal pregnancy group (P = 0.02).
Table 1.
Clinical characteristics of the patient population of pregnant women
Mid-trimester PAI-1 and FN levels of women who subsequently developed PE, GH, and those who remained normotensive are shown in Table 2. The mean PAI-1 concentration in the normal pregnant participants was significantly higher than the nonpregnant controls (P = 0.00) also noteworthy is the fact that the FN level in the pregnant women who developed GH was significantly different from women who remained normotensive throughout pregnancy (P = 0.02). However, there was no other significant difference in PAI-1 level between any of the groups that developed PIH and participants that remained normotensive throughout pregnancy.
Table 2.
Maternal serum plasminogen activator inhibitor-1 and fibronectin levels of women who developed preeclampsia and who remained normotensive
DISCUSSION
Placental hypoperfusion and ischemia, followed by the release of a number of vasoactive factors that alter the endothelial function are characteristic of PE and serve as the basis for examination of endothelial plasma markers such as FN and PAI-1.
In different studies, both total and cellular FN levels were found to be elevated before onset of PE.6,12,13 In the present study, no significant alteration was found in mid-trimester plasma levels of FN in women who later developed PE and those who remained normotensive throughout pregnancy. This discrepancy in observation can probably be linked to the fact that in these previous studies, longitudinal sampling was done at gestational ages >20 weeks, and in our study, the mean gestational age was 17 weeks. However, our findings were in agreement with those of Tjoa et al. and Gredmark et al. who reported that longitudinal assessment of the change in total FN levels predicted PE slightly better than cross-sectional analysis of FN at any gestational age.7,8
PAI-1 plasma levels in our study were found not to be significantly different in PE compared to levels of healthy controls. Similarly, Djurovic et al. showed no differences in plasma concentration of PAI-1 at the 18th gestational week between women who subsequently developed PE and matched healthy controls.14 In contrast, Swellan et al. and Bodova et al. found PAI-1 plasma levels to be significantly higher in PE at the 24th and 26th gestational weeks compared to levels in healthy controls.6,12
An overall incidence of PE of 7.3% was reported in this study. This agrees with an earlier study5 in the same community in which an incidence rate of 7.6% in 1803 pregnancies was reported.
Plausible reasons for failure to demonstrate the utility of PAI-1 and FN in predicting PE noted in this study may largely be due to the fact that the present study examined a convenience sample, future studies using population-based random sample, may provide stronger evidence on the associations between PAI-1, FN, and PE.
In addition, sampling was done before 20 weeks of gestation. The point in pregnancy when significant alterations in plasma levels of PAI-1 and FN occurred might have been missed. For future studies, sampling done after 20 weeks of gestation may demonstrate this supposed predictive value of PAI-1 and FN in the development of PE..
CONCLUSION
Although other studies have reported some predictive value of FN and PAI-1 levels, this study did not show that single measurement of PAI-1 and total FN in maternal plasma in early or mid-trimester is useful in predicting PE in the obstetric population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: A systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Bagga R, Aggarwal N, Chopra V, Saha SC, Prasad GR, Dhaliwal LK. Pregnancy complicated by severe chronic hypertension: A 10-year analysis from a developing country. Hypertens Pregnancy. 2007;26:139–49. doi: 10.1080/10641950701204588. [DOI] [PubMed] [Google Scholar]
- 3.Alphonsus NO, Okolo AA. Perinatal outcome in patients with pre-eclampsia in Benin City, Nigeria. Trop J Obstet Gynaecol. 2004;21:148–52. [Google Scholar]
- 4.Anorlu RI, Iwuala NC, Odum CU. Risk factors for pre-eclampsia in Lagos, Nigeria. Aust N Z J Obstet Gynaecol. 2005;45:278–82. doi: 10.1111/j.1479-828X.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 5.Belo L, Santos-Silva A, Rumley A, Lowe G, Pereira-Leite L, Quintanilha A, et al. Elevated tissue plasminogen activator as a potential marker of endothelial dysfunction in pre-eclampsia: Correlation with proteinuria. BJOG. 2002;109:1250–5. doi: 10.1046/j.1471-0528.2002.01257.x. [DOI] [PubMed] [Google Scholar]
- 6.Bodova KB, Biringer K, Dokus K, Ivankova J, Stasko J, Danko J. Fibronectin, plasminogen activator inhibitor type 1 (PAI-1) and uterine artery Doppler velocimetry as markers of preeclampsia. Dis Markers. 2011;30:191–6. doi: 10.3233/DMA-2011-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjoa ML, Oudejans CB, van Vugt JM, Blankenstein MA, van Wijk IJ. Markers for presymptomatic prediction of preeclampsia and intrauterine growth restriction. Hypertens Pregnancy. 2004;23:171–89. doi: 10.1081/PRG-120028292. [DOI] [PubMed] [Google Scholar]
- 8.Gredmark T, Bergman B, Hellström L. Total fibronectin in maternal plasma as a predictor for preeclampsia. Gynecol Obstet Invest. 1999;47:89–94. doi: 10.1159/000010069. [DOI] [PubMed] [Google Scholar]
- 9.Hamed S, Ullmann Y, Egozi D, Daod E, Hellou E, Ashkar M, et al. Fibronectin potentiates topical erythropoietin-induced wound repair in diabetic mice. J Invest Dermatol. 2011;131:1365–74. doi: 10.1038/jid.2011.15. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-García B, Madrigal-Matute J, Moreno JA, Martin-Ventura JL, López-Franco O, Sastre C, et al. TWEAK-Fn14 interaction enhances plasminogen activator inhibitor 1 and tissue factor expression in atherosclerotic plaques and in cultured vascular smooth muscle cells. Cardiovasc Res. 2011;89:225–33. doi: 10.1093/cvr/cvq278. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of obstetricians and gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 12.Swellam M, Samy N, Wahab SA, Ibrahim MS. Emerging role of endothelial and inflammatory markers in preeclampsia. Dis Markers. 2009;26:127–33. doi: 10.3233/DMA-2009-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dane C, Buyukasik H, Dane B, Yayla M. Maternal plasma fibronectin and advanced oxidative protein products for the prediction of preeclampsia in high risk pregnancies: A prospective cohort study. Fetal Diagn Ther. 2009;26:189–94. doi: 10.1159/000259317. [DOI] [PubMed] [Google Scholar]
- 14.Djurovic S, Clausen T, Wergeland R, Brosstad F, Berg K, Henriksen T. Absence of enhanced systemic inflammatory response at 18 weeks of gestation in women with subsequent pre-eclampsia. BJOG. 2002;109:759–64. doi: 10.1111/j.1471-0528.2002.01330.x. [DOI] [PubMed] [Google Scholar]


