Abstract
Background:
There is a long lasting dilemma over the ideal screening and diagnostic method in gestational diabetes mellitus (GDM). Even though universal screening is commonly practiced, selective screening based on risk factors is also practiced in some center. The aim of this study is to evaluate the most appropriate method to screen GDM in high-risk pregnant women in Sri Lanka.
Methods:
This study was a clinic-based, cross-sectional study conducted in a tertiary referral center, Sri Lanka. All women underwent 75 g oral glucose tolerance test at 24–28 weeks of gestation. Diagnosis of GDM was made according to the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) and World Health Organization (WHO) criteria.
Results:
With universal screening using IADPSG criteria, 23.2% (105/452) were found to have GDM and with risk factor-based screening 20.1% (91/452) were detected to have GDM. The prevalence of GDM dropped to 18.1% when GDM was diagnosed using the WHO criteria with universal screening approach. It was further dropped to 15.7% when the WHO criteria were used along with risk factors-based screening approach.
Conclusions:
The IADPSG criteria labeled considerably higher number of women as having GDM compared to the WHO criteria. With regards to the screening methods, the risk-based screening had a lower detection rate of GDM; however, it reduced the necessity of screening of women by around 20%.
Keywords: Gestational diabetes mellitus, International Association of the Diabetes and Pregnancy Study Groups criteria, universal and selective screening, World Health Organization criteria
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that is first detected during pregnancy.[1] Although the true prevalence of GDM is unknown, GDM is estimated to affect 3–11% of pregnant women depending on the varying characteristics of the population. In Sri Lanka, the prevalence of GDM was reported to be around 10–8.5%.[2,3,4] The prevalence of GDM is rising globally, and it is in keeping with increasing trends of maternal obesity and Type 2 diabetes mellitus (T2DM). The frequency of GDM usually reflects the frequency of Type 2 diabetes in the underlying population.[5] Even though this is a global phenomenon, people live in South Asia are more affected than others.[6] Sedentary lifestyle and changes in dietary practices have contributed to the higher prevalence of obesity, a well-known cause for insulin resistance, thus, making them susceptible to gestational diabetes.[7]
Although uncomplicated GDM with mild fasting hyperglycemia is not associated with increased perinatal mortality, it can increase the risk of fetal macrosomia[8] Other possible complications of GDM include neonatal hypoglycemia, jaundice, polycythemia, and hypocalcemia.[9] GDM is also associated with higher rates of cesarean delivery and increased the incidence of maternal hypertensive disorders.[10,11] Furthermore, women with GDM have increased the risk of developing T2DM after pregnancy, and children who are born to women with GDM also have a higher risk for developing T2DM later in their life.[11,12] Even though there is conflicting evidence as to the effect of GDM management on the fetal and maternal adverse effect, recent meta-analysis and few other studies had shown a reduction of complications such as preeclampsia, shoulder dystocia, and macrosomia with the treatment of GDM.[10,11,13,14,15]
There is a long lasting dilemma over the exact method and criteria to be used when diagnosing GDM. Variety of diagnostic criteria is used to diagnose GDM, and the World Health Organization (WHO) criteria are one of the commonly used criteria globally.[16] Professional bodies such as Diabetes in Pregnancy Study Group India and Sri Lankan College of Obstetricians and Gynaecologists recommended nonfasting glucose challenge test with cutoff values similar to the WHO criteria. However, the criteria proposed by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) based on hyperglycemia and adverse pregnancy outcomes study have been accepted by a large number of scientific bodies. In 2011, the American Diabetes Association (ADA) endorsed the IADPSG criteria.[17]
While most guidelines including ADA advocate universal testing, few guidelines including National Institute for Health and Care Excellence guideline in 2015 update still recommend selective screening based on the risk factors for GDM.[18,19] These guidelines or the recommendations are derived from data of unrepresentative populations in other countries and hence should not directly apply to our population. Therefore, the best method to screen GDM in our population should be based on local data. A previous community-based study carried out in Anuradhapura district showed an inadequacy of selective screening based on conventional risk factors.[3] However, it is not prudent to apply the finding of a community-based study to antenatal clinics settings in tertiary care hospitals as the underlying prevalence of GDM in these two settings are vastly different. Due to these reasons, this study was designed to determine if the universal screening approach is superior to the selective risk factor-based screening among high-risk pregnant women followed up at tertiary care settings in Sri Lanka.
METHODS
Study design and participants
This study was conducted as a clinic-based, cross-sectional study. The sample size was calculated based on the prevalence of GDM in a previous study (21.5%)[20] with 90% statistical power. A total of 452 pregnant women were recruited for the study by simple random sampling method (every third pregnant woman registered in the clinic). Women with preexisting diabetes were excluded from the study. The Regional Ethics Committee of the Faculty of Medicine, University of Ruhuna approved the study protocol and informed written consent was taken from all participants. Pregnant women found to have GDM were referred to the antenatal clinic for follow-up and treatment.
Procedures and variables assessment
An interviewer-administered questionnaire was used to collect relevant data including age, height, weight, family history of diabetes mellitus, history of GDM in previous pregnancies, history of previous delivery of large babies, bad obstetric history (miscarriages, stillbirths, and intrauterine death [IUD]), and socioeconomic status. The body mass index (BMI) of the subjects was calculated using the height and weight.
Standard 75-g oral glucose tolerance tests (OGTTs) were performed in all women during the period of 24–28 weeks of gestation. All OGTTs were performed after an overnight fast of at least 8 h and not exceeding 14 h, and following at least 3 days of unrestricted carbohydrates (>150 g/day) and exercise as per the WHO guidelines. OGTTs were performed according to the standard protocols using the glucose oxidase enzymatic calorimetric method.
For the risk factor-based screening, pregnant women with one or more of the following risk factors were included:
History of GDM/pregnancy-induced hypertension/hypertension
Maternal age ≥35 years
Preconception BMI ≥23 kg/m2
Bad obstetric history (miscarriages, stillbirths, and IUD)
History of delivering large babies (≥3.5 kg)
Family history of first-degree relatives with diabetes mellitus/GDM.
Diagnosis of GDM was made according to IADPSG criteria when any of the following three 75 g 2 h OGTT thresholds were met or exceeded: Fasting 92 mg/dL, 1 h 180 mg/dL, 2 h 153 mg/dL, or have above thresholds in OGTT or fasting blood sugar. With the WHO criteria, GDM is diagnosed with fasting plasma glucose ≥126 and/or 2 h blood glucose following 75 g glucose load ≥140 mg/dL.
Statistical analysis
To compare the difference between the WHO criteria and IADPSG, as well as selective versus universal methods, Chi-square test was employed. A receiver operating characteristics (ROCs) curve was used to determine the most suitable 2 h value of the OGTT to detect GDM. Analysis was two-tailed and P < 0.05 was considered statistically significant. Data analysis was done using the Statistical Package for Social Science (SPSS, IBM Corp., Armonk, NY, USA) version 18 and standard statistical procedures were applied.
RESULTS
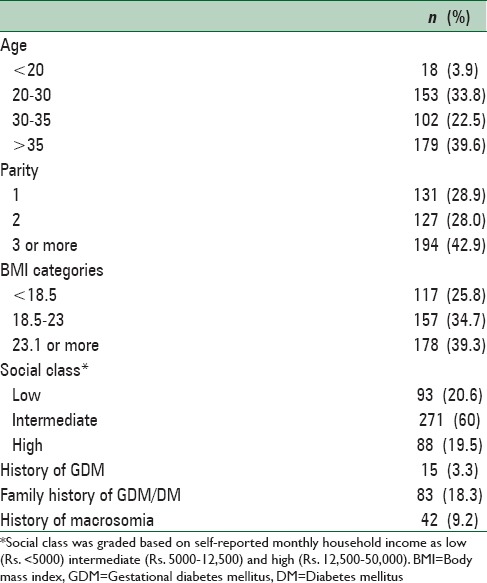
The mean age of the pregnant women was 31.3 years (standard deviation [SD] 6.3) and BMI at booking visit was 22.0 kg/m2 (SD 4.6). Primiparous women accounted for 28.9% (n = 131) of the study sample [Table 1]. For the universal screening, all 452 pregnant women were considered and for the risk factor-based screening group 359 women with at least one risk factors for GDM were selected.
Table 1.
Baseline characteristics of 452 pregnant women
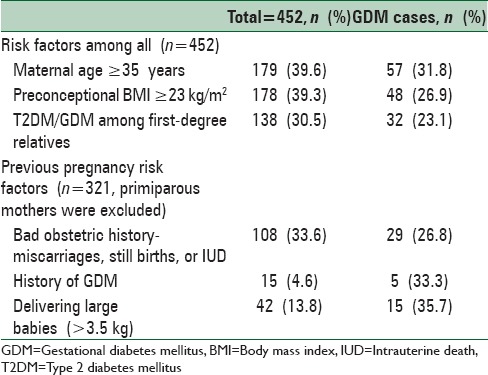
Of the risk factors of GDM assessed, 15 (4.6%) had a history of GDM, 179 (39.6%) were ≥35 years, 178 (39.3%) had BMI >23 kg/m2 at booking visit, and 138 (30.5%) had first-degree relatives with diabetes mellitus. The prevalence of the risk factors within the study population and the GDM in each risk factor groups are shown in Table 1. Out of the risk factors, women who had macrosomia in previous pregnancies had the highest GDM risk (35.7%) followed by the history of GDM (33.3%) and maternal age ≥35 years (31.8%) [Table 2].
Table 2.
Prevalence of risk factors in the study population and gestational diabetes mellitus according the risk factors
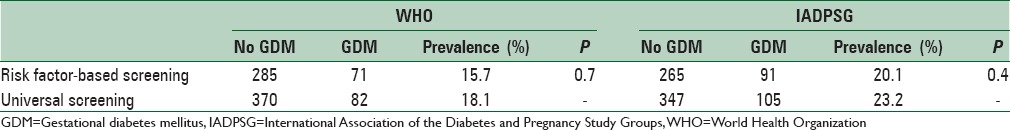
According to IADPSG criteria and with universal screening approach, 105 women were found to have GDM. Thus, the prevalence of GDM among women with universal screening was 23.2% (105/452). For the risk factor-based screening, 356 women with at least one risk factor for GDM were selected and of them, 91 had GDM. Thus, the overall prevalence of GDM with risk factor-based screening approach was 20.13% (91/452) [Table 3]. The risk factor-based screening had a sensitivity of 86% in detecting GDM cases.
Table 3.
Diagnostic performance of World Health Organization and International Association of the Diabetes and Pregnancy Study Groups criteria in the universal screening and the risk factor-based screening groups
The prevalence of GDM dropped to 18.1% when GDM was diagnosed using the WHO criteria with universal screening approach. When the WHO criteria were applied to women with risk factors only (risk factor-based screening), prevalence further dropped to 15.7%. Interestingly, FPG criterion (≥126 mg/dL) of WHO detected only six cases (1.3%) of GDM and the remaining 76 cases in the universal screening group and 65 cases in risk factor-based group were detected by 2 h value alone [Table 4].
Table 4.
Diagnostic performance of fasting plasma glucose, 1st h and 2nd h values of glucose tolerance test in detecting gestational diabetes mellitus based on the universal and the risk factor-based screening methods using World Health Organization and International Association of the Diabetes and Pregnancy Study Groups diagnostic criteria
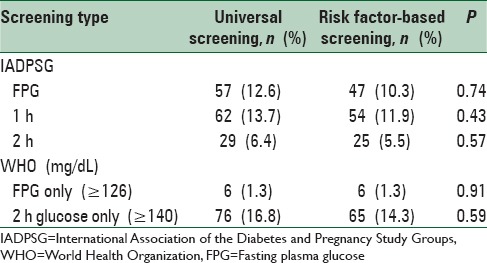
GDM prevalence based on just one abnormal fasting value was 12.6% (95% confidence interval 11.3–14.1%), detecting 54% of all women labeled as GDM by the full IADPSG criteria. With the same criteria, 62 and 29 women had GDM based on their 1 h and 2 h values, respectively. If only the fasting and 1 h value were considered, the prevalence of GDM was 22.1% (100/452), detecting 95.2% of all GDM cases by the full IADPSG criteria. Overall, just the 2 h value alone produced markedly lower prevalence of GDM (1.1%). Similar trend of FPG and 1 h value detecting the majority of GDM cases compared to 2 h value were observed in women who considered for risk factors-based analysis.
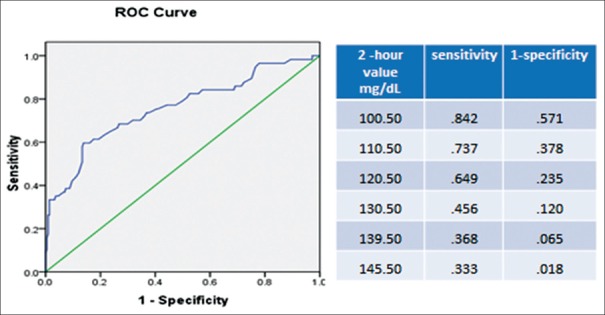
Diagnostic performance of two criteria used in this study was further assessed by the area under the ROC curve, and the ability of 2 h value of WHO criteria to predict GDM diagnosed by IADPSG criteria was 0.758 (standard error 0.039). The best cutoff point of 2 h value to predict GDM occurred at 120 mg/dL (sensitivity of 64.9%, the specificity 76.5%) [Figure 1]. The cutoff of 2 h value of 140 mg/dL used in the WHO criteria had a sensitivity of 37% and specificity of 96%, respectively [Figure 1].
Figure 1.
Receiver operating characteristics curve of 2 h value used in the World Health Organization criteria to predict gestational diabetes mellitus diagnosed by the International Association of the Diabetes and Pregnancy Study Groups criteria
DISCUSSION
There is no universally accepted “gold standard method” for the screening for GDM. There is also insufficient evidence of benefits of screening for GDM, and the insufficient evidence could partly be due to rarity of the complications and not having studies sufficiently large enough to look at these rare complications of GDM. Even though the evidence on the benefits of screening strategies for GDM remains problematic, it is a routine practice to screen pregnant women for GDM.
In this study, we used two methods of screening, namely universal and selective risk factor-based screening and two commonly used criteria IADPSG and WHO criteria. This study shows that the prevalence of GDM varied depending on the screening criteria used and screening method used. With IADPSG criteria using universal screening approach, the prevalence of GDM was 23.2%: However it dropped to 20.1% with risk factor-based screening using the same diagnostic criteria. With WHO criteria using universal approach, the prevalence of GDM was 18.1% and with risk factor-based screening it further dropped to 15.7%. Many previous studies have shown that the IADPSG criteria diagnosed considerably higher number of cases as GDM compared to other diagnostic criteria.[21,22,23] In our study, the prevalence of GDM according to the WHO criteria was 5% lower than the prevalence detected by IADPSG criteria. However, the clinical significant of higher detection rate of GDM by IADPSG is questionable.
The prevalence of GDM observed in this study is also higher than the prevalence reported in previous studies in Sri Lanka.[2,7] In 1998, GDM prevalence was reported to be 5.5% in a community-based study.[2] There could be many reasons for the dramatic increment of GDM prevalence in this study. There is accumulating evidence that GDM is a growing problem and the rise in prevalence of GDM may simply reflect the substantial increase in the community prevalence of obesity and Type 2 diabetes.[5] However, the most likely reason for the dramatic rise of the prevalence of GDM in this study is the sample of women that we studied. As the study was carried out in a tertiary care center, it is likely that women with more risk factors for GDM are concentrated in this study sample than a community-based study. Around 80% of women in this study had one or more risk factors for GDM. Thus, it is reasonable to say that the overall prevalence of GDM in a community would be considerably lower than the reported prevalence in this study. Similar to our study, previous hospital-based studies too had shown a higher prevalence of GDM.[24,25]
Our study also confirmed that universal screening is superior to selective risk factor-based screening. Numbers of previous studies too have reported similar findings. Furthermore, universal screening appears to be the most practical approach because 80% of pregnant women had at least one risk factor for GDM. Therefore, majority (80%) would anyway require GDM screening even if the selective screening is chosen. Our result also indicates that if selective screening was used, around 20% of women would not require screening and which in turn would reduce the burden of screening. If the selective risk factor-based screening is practiced in less risk population such as women attending peripheral centers or community antenatal clinics, it is likely that many women would be spared from GDM screening. Therefore, the use of selective risk factor-based screening will largely depend on the prevalence of these risk factors in the screened population.
Based on the finding of this study, we can suggest that universal screening with IADPSG criteria had a higher sensitivity of detecting GDM in tertiary care settings in Sri Lanka and is probably the most practical method for pregnant women followed up at a tertiary referral center.
CONCLUSIONS
The IADPSG criteria labeled considerably higher number of women as having GDM compared to the WHO criteria, and the universal screening had a higher detection rate of GDM than the risk-based screening. However, risk-based screening reduced the necessity of screening by around 20%. In a population like Sri Lanka, where there is a high prevalence of GDM, the number of cases missed with the risk-based screening may be significant. Therefore, universal screening may be more appropriate to use in tertiary care settings in Sri Lanka. However, if the facilities are limited, risk factor-based screening can be used in community clinics where women are less likely to have multiple risk factors for GDM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank the participants, the consultants who kindly consented to use their patients, director and other health-care professionals at Teaching Hospital Mahamodara. A special word of thank is extended to K. M. Kumuduni de Silva and K. S. M. Weerarathna for laboratory assistance, and Dr. Iresha Mampitiya Dr. S. P. Mohotti, Dr. C. M. De Silva and Dr. L. Fonseka for their assistance in conducting this study.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siribaddana SH, Deshabandu R, Rajapakse D, Silva K, Fernando DJ. The prevalence of gestational diabetes in a Sri Lankan antenatal clinic. Ceylon Med J. 1998;43:88–91. [PubMed] [Google Scholar]
- 3.Dahanayaka NJ, Agampodi SB, Ranasinghe OR, Jayaweera PM, Wickramasinghe WA, Adhikari AN, et al. Inadequacy of the risk factor based approach to detect gestational diabetes mellitus. Ceylon Med J. 2012;57:5–9. doi: 10.4038/cmj.v57i1.4193. [DOI] [PubMed] [Google Scholar]
- 4.Wijeyaratne CN, Ginige S, Arasalingam A, Egodage C, Wijewardhena K. Screening for gestational diabetes mellitus: The Sri Lankan experience. Ceylon Med J. 2006;51:53–8. doi: 10.4038/cmj.v51i2.1353. [DOI] [PubMed] [Google Scholar]
- 5.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34:173–99 vii. doi: 10.1016/j.ogc.2007.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridhar GR, Putcha V, Lakshmi G. Time trends in the prevalence of diabetes mellitus: Ten year analysis from Southern India (1994-2004) on 19,072 subjects with diabetes. J Assoc Physicians India. 2010;58:290–4. [PubMed] [Google Scholar]
- 7.Senanayake H, Seneviratne S, Ariyaratne H, Wijeratne S. Screening for gestational diabetes mellitus in Southern Asian women. J Obstet Gynaecol Res. 2006;32:286–91. doi: 10.1111/j.1447-0756.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(Suppl 1):42–6. doi: 10.4158/EP.12.S1.42. [DOI] [PubMed] [Google Scholar]
- 9.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Aktary WM, et al. Screening and diagnosing gestational diabetes mellitus. Evidence Report/Technology Assessment no. 210. AHRQ Publication no. 12(13)-E021-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2012; [Last accessed on 2015 Jan 11]. Available from: www.ncbi.nlm.nih.gov/books/NBK114844/ [Google Scholar]
- 10.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 11.HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcome (HAPO) study: Associations with neonatal anthropometrics. Diabetes. 2009;58:453–9. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maresh M. Screening for gestational diabetes mellitus. Semin Fetal Neonatal Med. 2005;10:317–23. doi: 10.1016/j.siny.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 14.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: A systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159:123–9. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 15.Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: A systematic review and meta-analysis. PLoS One. 2014;9:e92485. doi: 10.1371/journal.pone.0092485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng LC, Salmon YM. Are the WHO (1980) criteria for the 75 g oral glucose tolerance test appropriate for pregnant women? Br J Obstet Gynaecol. 1993;100:645–8. doi: 10.1111/j.1471-0528.1993.tb14231.x. [DOI] [PubMed] [Google Scholar]
- 17.Lapolla A, Dalfrà MG, Ragazzi E, De Cata AP, Fedele D. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: A retrospective study on pregnancy outcome. Diabet Med. 2011;28:1074–7. doi: 10.1111/j.1464-5491.2011.03351.x. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence (NICE) (2015) Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. Clinical guideline NG3. 2015. [Last accessed on 2015 Sep 20]. Available from www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-of-diabetes-and-its-complications-from-preconception-to-the-postnatal-period-51038446021 .
- 19.Lieberman N, Kalter-Leibovici O, Hod M. Global adaptation of IADPSG recommendations: A national approach. Int J Gynaecol Obstet. 2011;115(Suppl 1):S45–7. doi: 10.1016/S0020-7292(11)60013-1. [DOI] [PubMed] [Google Scholar]
- 20.Herath M, Weerarathna TP, Umesha D. Is nonfasting glucose challenge test sensitive enough to diagnose gestational diabetes mellitus? Int Arch Med. 2015;8:1–8. [Google Scholar]
- 21.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 22.Avalos GE, Owens LA, Dunne F. ATLANTIC DIP Collaborators. Applying current screening tools for gestational diabetes mellitus to a European population: Is it time for change? Diabetes Care. 2013;36:3040–4. doi: 10.2337/dc12-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morikawa M, Yamada T, Yamada T, Akaishi R, Nishida R, Cho K, et al. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract. 2010;90:339–42. doi: 10.1016/j.diabres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Jali MV, Desai BR, Gowda S, Kambar S, Jali SM. A hospital based study of prevalence of gestational diabetes mellitus in an urban population of India. Eur Rev Med Pharmacol Sci. 2011;15:1306–10. [PubMed] [Google Scholar]
- 25.Mohan MA, Chandrakumar A. Evaluation of prevalence and risk factors of gestational diabetes in a tertiary care hospital in Kerala. Diabetes Metab Syndr. 2016;10:68–71. doi: 10.1016/j.dsx.2015.09.002. [DOI] [PubMed] [Google Scholar]