Abstract
Introduction:
Available data indicated that diabetes mellitus (DM) increases the vulnerability of the gastric ulcers and the need of the hour is to develop effective agents to treat ulcer with diabetes for better patient compliance and cost effectiveness. The ulcer-healing properties of ethanolic extract of Caralluma attenuata (CAEt) against both chemically- and physically induced gastric ulcers in experimental rats are recently studied.
Aim:
To assess the ulcer healing potential of Ethanolic Extract of Caralluma attenuata on Experimental Diabetic Rats.
Material and Methods:
The current study aimed to evaluate ulcer healing properties of CAEt on the aspirin induced gastric ulcer in rats with streptozotocin induced DM. The hypothesis is based on the fact that DM results in compromising the mucosal defensive factors associated with delay in gastric ulcer healing, and if these changes can be corrected by using agents known for their antidiabetic and antiulcer properties. Experimental albino rats were divided into six groups. Except for Group I, other groups contained streptozotocin-induced diabetic rats. Group I (normal control) and Group II (diabetic control) were administered vehicle, Groups III and IV (diabetic experimental) were administered CAEt in dose of 100 mg/kg and 250 mg/kg, respectively, and Groups V and VI (positive controls) were respectively administered oral standard drugs omeprazole, 20 mg/kg, and tolbutamide 10 mg/kg.
Result:
The results confirmed that the CAEt significantly decreases the ulcer index (P < 0.05) in the aspirin-induced gastric ulcers and also significantly exhibit antioxidant and glucose lowering activity in the diabetic ulcer rats. The study showed that C. attenuata has the potential to be used as an antiulcer agent in experimental diabetic rats.
KEYWORDS: Antiulcer agent, Caralluma attenuate, diabetes mellitus, herbal drug
INTRODUCTION
The peptic ulcers occurring in the course of diabetic state are more severe and often associated with complications such as gastrointestinal bleeding.[1] A higher incidence of peptic ulcer disease (PUD) was reported in diabetic patients as compared to the non-diabetic patients.[2] Aggravated gastric mucosal susceptibility and delayed gastric ulcer healing to ulcerogenic drugs or stress in diabetic rats has been shown in animal studies.[3,4,5] Ulcer disease and acute gastric inflammation occur with high incidence in patients with type 2 diabetes mellitus.[6] Gastric mucosa of diabetic rats is more susceptible to ulcerogens such as non-steroidal anti-inflammatory drugs.[4,7]
Many Indian medicinal plants have been found to successfully manage diabetes. Caralluma attenuata, (Family: Asclepiadaceae) is known for its anti-hyperglycemic activity. Numerous indigenous medicinal plants have been reported to be helpful in successfully managing the diabetes. Caralluma attenuate Wight. Which belongs to the family Asclepiadaceae, is an herb growing wild in dry hill slope regions of southern India. It is known as ‘Kundaetikommu’ indigenously, and is taken raw as a cure for diabetes and the juice of the plant along with black pepper is suggested in the treatment of migraine.[8] This plant was found to be a good source of glycosides and known for its antidiabetic activity.[9] Previously, the antidiabetic potential of the ethanolic extract of C. attenuata (CAEt) in streptozotocininduced hyperglycemia and oxidative stress has been reported.[8] Recently, the ulcer healing properties of CAEt against both chemically and physically induced gastric ulcers in experimental rats were also studied.[10] The present investigation was therefore undertaken to further evaluate ulcer-healing properties of CAEt on aspirin-induced gastric ulcer in rats with streptozotocin-induced diabetes mellitus. The results of this study supported the ethnopharmacological claims of C. attenuata for its use in gastric ulcers and diabetes.
MATERIALS AND METHODS
Experimental animals
Albino rats (150–175 g) were obtained from the animal colony of National Laboratory Animal Centre, Lucknow. The animals were maintained under standard laboratory conditions and given free access to tap water ad libitum and standard feed during quarantine period. The animals were kept fasting overnight with free access to the water. All studies were performed in accordance with the guidance for care and use of laboratory animals, as laid out by the Institutional Animal Care Committee, National Botanical Research Institute, Lucknow, India (Reg. No. IAEC/NBRI/PH/6-6).
Preparation of plant extract
Fresh whole plants of C. attenuata were collected from the Southern part of India (Andhra Pradesh). The plant material was authenticated and taxonomically identified by a taxonomist in the National Botanical Research Institute, Lucknow. The ethanolic extract was prepared using the crushed and powdered shade dried plant materials with 10 ml volumes of 50% ethanol. The filtered and pooled extract was concentrated on rotavapour (Buchi, USA) and dried in a lyophilizer (Laboconco, USA) under condensed pressure.
Diabetes induction
Diabetes was induced in albino rats by injecting a freshly prepared solution of streptozotocin in 0.1 M citrate buffer (pH = 4.5). The dose of streptozotocin was calculated using 50 mg/kg of body weight and given intraperitoneally (i.p). The induction of diabetes was confirmed by measuring fasting blood glucose concentration after 72 hour of streptozotocin injection. The diabetic rats with blood glucose level above 140 mg/dl were used in the study. Overnight fasted animals were dosed as per experimental design. Aspirin at a dose of 200 mg/kg, orally was administered to all diabetic rats for initial 7 days.
Animal grouping and extract administration
All the animals were divided into six groups. Except the Group I, rest of the groups contained diabetic rats which were administrated aspirin orally at a dose 200 mg/kg for initial 7 days of the 28 day study. Group I (normal control) and Group II (diabetic control) were administered vehicle, Groups III and IV (diabetic experimental) were administered CAEt at doses of 100 mg/kg and 250 mg/kg, respectively, orally in 2% gum acacia, Groups V and VI (positive controls) were respectively administered oral standard drugs omeprazole, 20 mg/kg, and tolbutamide 10 mg/kg.
Ulcer index
Ulcer index was calculated by adding the total number and the total severity of ulcers per stomach. Ulcer severity was determined by recording the severity of each ulcer after histological confirmation as follows: 0, no ulcer; +, pin point ulcer and histological changes limited to superficial layers of mucosa and no congestion; ++, ulcer size less than 1 mm and half of the mucosal thickness showing necrotic changes; +++, ulcer size 1-2 mm with more than two-thirds of the mucosal thickness destroyed with marked necrosis and congestion, muscularis remaining unaffected; ++++, ulcer >2 mm in size or perforated with complete destruction of the mucosa with necrosis and haemorrhage, muscularis still remaining unaffected. The pooled group ulcer score was then noted.[11]
Blood glucose tolerance determination
Fasting blood samples were drawn in Eppendorf's tubes containing 50 ml of anticoagulant (10% trisodium citrate solution) on Day 1 after single administration of CAEt and on Day 28 by tail vein puncture under mild ether anaesthesia to determine the effect of CAEt administered orally, on the blood glucose level in 28 days.
Antioxidant activities
Lipid peroxidase
Malondialdehyde (expressed as n-mol per mg of proteins), a lipid peroxidase (LPO) product was assessed using 1,1,3,-tetrahydroxypropane as the standard.[12]
Superoxide dismutase activity
Methods of Kakkar et al.[13] was used to assess superoxide dismutase (SOD). The reduction of nitroblue tetrazolium to blue coloured formazan was inhibited in the presence of phenazine metasulphate. The activity was measured at 560 nm and the results have been expressed as units (U) of SOD activity/mg protein. One unit (U) of enzyme activity is defined as the enzyme concentration required for inhibiting the chromogen production by 50% in one minute under the defined assay conditions.
Catalase activity
The catalase (CAT) activity was calculated by measuring the decomposition of H2O2 at 240 nm.[14] Results are expressed as units (U) of CAT activity per mg of protein.
Statistical analysis
The results were presented as mean ± standard error of mean (SEM) and comparison between different groups was done using one-way analysis of variance (ANOVA) followed by individual comparison by least significant difference test for the determination of level of significance. A P value of <0.05 was considered as statistically significant difference between means.
RESULTS
Antiulcer and ulcer healing effects
The ulcer index in diabetic ulcer rat was found to be significantly reduced in the CAEt treated group at dose 250 mg/kg (Group IV). The ulcer index in diabetic control group II was 14.35 ± 1.86 which decreased to 7.25 ± 1.37 and 4.63 ± 0.55 (P < 0.01) for Groups III and IV administered with 100 and 250 mg/kg CAEt respectively [Figure 1]. The reduction in ulcer index of CAEt treated group (IV) at a dose of 250 mg/kg was comparable to decrease in ulcer index by omeprazole standard control group (V). The ulcer index was found to be 3.15 ± 1.00 and 14.03 ± 1.34 for omeprazole and tolbutamide standard control groups respectively.
Figure 1.
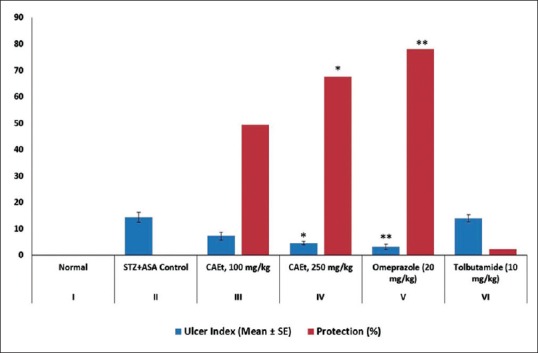
Effect of C. attenuata Extract on Aspirin-induced Gastric Ulcers in Diabetic Rats. The values represent the mean ± SEM for six rats per group. Values are expressed as mean ± SEM of six rats in each group. *P < 0.01 and **P < 0.001 compared with control group
The treatment with CAEt for 21 days exhibited visible protection of the gastric mucosa against the acid attack in diabetic rats. The CAEt at a dose of 250 mg/kg protected the gastric mucosa and cured gastric ulcerations significantly (P < 0.01).
Effect of CAEt extracts on fasting blood glucose levels
The blood glucose was estimated at Day 0 and 28 to determine the glucose tolerance effect of CAEt in experimental diabetic ulcer rats. The CAEt at dose of 100 and 250 mg/kg reduced the blood glucose concentration from 229.68 ± 3.29 mg/dL to 157.73 ± 4.41 mg/dL and 231.93 ± 4.58 mg/dL to 136.22 ± 5.30 mg/dL (P < 0.01), respectively [Figure 2]. The difference in the tolerance of glucose level of different groups was significant [P < 0.001 to 0.01, Figure 2].
Figure 2.
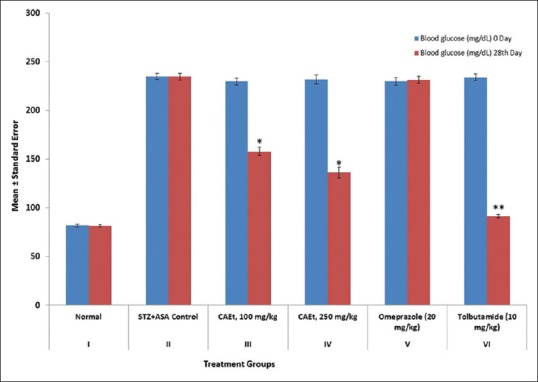
Effect of C. attenuata Extract on Blood Glucose Tolerance in Diabetic Ulcer Rats. The values represent the mean ± SEM for six rats per group. Values are expressed as mean ± SEM of six rats in each group. *P < 0.01 and **P < 0.001 compared with control group
Effect of CAEt on antioxidant enzymes
The LPO value in control group II increased to 16.75 ± 0.31 U/mg protein from 5.8 ± 0.34 U/mg protein compared to control group I [P < 0.001, Figure 3]. The SOD and CAT levels in the diabetic control group (II) decreased from 7.5 ± 0.4 to 5.1 ± 0.43 (P < 0.001) and 56.48 ± 1.44 to 28.35 ± 1.88 U/mg protein (P < 0.001), respectively when compared to Group I animals. However, the CAEt at doses of 100 and 250 mg/kg significantly increase the levels of SOD from 5.1 ± 0.43 to 8.12 ± 0.45 and to 9.27 ± 0.34 (P < 0.01 to P < 0.001) and CAT from 28.35 ± 1.88 to 57.4 ± 1.51 and to 61.52 ± 2.82 U/mg protein (P < 0.01 to P < 0.001) when compared to Group II rats. This indicates that the CAEt contributes to the antioxidant activity.
Figure 3.
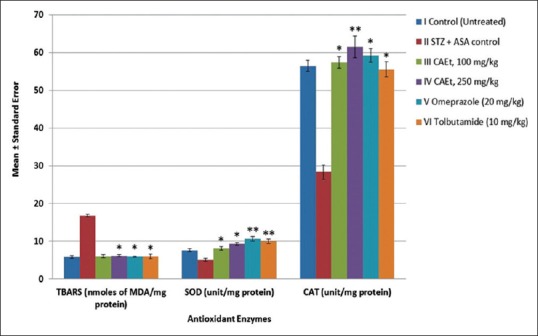
Effect of C. attenuata Extract on Antioxidant Enzymes in Aspirin-induced Gastric Ulcers in Diabetic Rats. The values represent the mean ± SEM for six rats per group. Values are expressed as mean ± SEM of six rats in each group. *P < 0.01 and **P < 0.001 compared with control group
DISCUSSION
Diabetes mellitus is a disorder characterized by increased blood glucose levels, increased production of reactive oxygen species, decreased antioxidant levels, and disturbance in lipid metabolism.[15,16] Studies show that diabetes delays ulcer healing due to the significant reduction in the gastric microcirculation around the ulcer. This possibly involves the increased release of proinflammatory cytokines such as TNF-α. In addition, the hyperglycemia and increased production of proinflammatory cytokines such as interleukin-1b and TNF-a result in sustained inflammatory reaction and thus delays healing process at the ulcer area.[3,4] The nonsteroidal anti-inflammatory drugs including aspirin have significant untoward effects such as formation of gastric mucosal bleeding erosions, ulcers, improving the ulcerogenic response to various noxious stimuli and impairment of healing of pre-existing ulcers. These actions are attributed to their topical irritating effect, reduction in the microcirculation, activation of neutrophils, enhancement in gastric motility and the reduction in mucosal generation of PGE2.[17,18,19] The CAEt may have exerted a cytoprotective effect due to its significant antioxidant property that protects the gastric mucosa against oxidative stress associated with the diabetic mellitus.[10,20] The ulcer protective effects of CAEt may also be due to its action on gastric mucosal defensive factors such as an increase in dissolved mucus and decrease in mucosal cell exfoliation[21] which can be studied further.
The CAEt exhibited a significant reduction in the ulcer index of diabetic rats. In diabetes, mucosal defensive factors play a key role in increasing tendency to gastric ulceration and this may explain the effectiveness of omeprazole in gastric ulceration in diabetic rats. The CAEt significantly restored the mucosal glycoprotein in diabetic rats. This effect was almost similar to the reference antiulcer drug omeprazole. Hence, the antiulcer activity of C. attenuata in diabetic rats has been demonstrated.
CONCLUSION
The results of the present study suggest that CAEt may prevent aspirin-induced gastric ulcer due to their antioxidant properties by increasing the SOD/CAT levels and diminishing the lipid peroxidation in the rat gastric mucosa. The results are in line with the findings of our previous work on the antidiabetic and antiulcer properties of the C. attenuata. This study shows that CAEt contains the active antiulcer and hyperglycemic moiety. These findings could lead to identification of a novel molecule from C. attenuata that would serve as a better choice of treatment of gastric ulcer in diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pietzsch M, Theuer S, Haase G, Plath F, Keyser M, Riethling AK. Results of systematic screening for serious gastrointestinal bleeding associated with NSAIDs in Rostock hospitals. Int J Clin Pharmacol Ther. 2002;40:111–5. doi: 10.5414/cpp40111. [DOI] [PubMed] [Google Scholar]
- 2.Tseng PH, Lee YC, Chiu HM, Chen CC, Liao WC, Tu CH, et al. Association of diabetes and HbA1c levels with gastrointestinal manifestations. Diabetes Care. 2012;35:1053–60. doi: 10.2337/dc11-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konturek PC, Brzozowski T, Burnat G, Szlachcic A, Koziel J, Kwiecien S, et al. Gastric ulcer healing and stress-lesion preventive properties of pioglitazone are attenuated in diabetic rats. J Physiol Pharmacol. 2010;61:429–36. [PubMed] [Google Scholar]
- 4.Harsch IA, Brzozowski T, Bazela K, Konturek SJ, Kukharsky V, Pawlik T, et al. Impaired gastric ulcer healing in diabetic rats: Role of heat shock protein, growth factors, prostaglandins and proinflammatory cytokines. Eur J Pharmacol. 2003;481:249–60. doi: 10.1016/j.ejphar.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Podvigina TT, Bagaeva TR, Morozova OIu, Filaretova LP. Gastric mucosal susceptibility for ulcerogenic effect of indometacin at different time points of streptozotocin-induced diabetes development. Ross Fiziol Zh Im I M Sechenova. 2011;97:957–67. [PubMed] [Google Scholar]
- 6.Fouad AA, Al-Sultan AI, Yacoubi MT, Gomaa W. Ameliorative effects of telmisartan in diabetic rats with indomethacin-induced gastric ulceration. Eur J Pharmacol. 2010;637:162–70. doi: 10.1016/j.ejphar.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Brzozowska I, Targosz A, Sliwowski Z, Kwiecien S, Drozdowicz D, Pajdo R, et al. Healing of chronic gastric ulcers in diabetic rats treated with native aspirin, nitric oxide (NO)-derivative of aspirin and cyclooxygenase (COX)-2 inhibitor. J Physiol Pharmacol. 2004;55:773–90. [PubMed] [Google Scholar]
- 8.Kumar P, Sharma A, Varshney P, Rao CV. Antidiabetogenic and antioxidant effect of Caralluma attenuata extract on streptozotocin induced diabetes in rats. J Pharm Res. 2013;7:257–62. [Google Scholar]
- 9.Venkatesh S, Reddy GD, Reddy BM, Ramesh M, Rao AV. Antihyperglycemic activity of Caralluma attenuata. Fitoterapia. 2003;74:274–9. doi: 10.1016/s0367-326x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 10.Garg S, Pal K, Sharma A, Garg K. Ethnopharmacological evaluation of antiulcer activity of Caralluma attenuata. Int J Pharm Life Sci. 2014;5:3585–9. [Google Scholar]
- 11.Rao CV, Sairam K, Goel RK. Experimental evaluation of Bacopa monniera on rat gastric ulceration and secretion. Indian J Physiol Pharmacol. 2000;44:435–41. [PubMed] [Google Scholar]
- 12.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 13.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 14.Aebi HE. Method of Enzymatic Analysis. Vol. 3. Germany-Deerfield, FL: VCH, Weinheim; 1983. Catalase; pp. 273–86. [Google Scholar]
- 15.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 16.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi K, Suzuki K, Yamamoto H, Araki H, Mizoguchi H, Ukawa H. Cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal anti-inflammatory drugs and gastric mucosal responses. J Physiol Pharmacol. 1998;49:501–13. [PubMed] [Google Scholar]
- 18.Wang JY, Yamasaki S, Takeuchi K, Okabe S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology. 1989;96(2 Pt 1):393–402. doi: 10.1016/0016-5085(89)91563-1. [DOI] [PubMed] [Google Scholar]
- 19.Lanza FL. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med. 1984;77:19–24. doi: 10.1016/s0002-9343(84)80014-5. [DOI] [PubMed] [Google Scholar]
- 20.Koo JR, Ni Z, Oviesi F, Vaziri ND. Antioxidant therapy potentiates antihypertensive action of insulin in diabetic rats. Clin Exp Hypertens. 2002;24:333–44. doi: 10.1081/ceh-120004795. [DOI] [PubMed] [Google Scholar]
- 21.Dorababu M, Prabha T, Priyambada S, Agrawal VK, Aryya NC, Goel RK. Effect of Bacopa monniera and Azadirachta indica on gastric ulceration and healing in experimental NIDDM rats. Indian J Exp Biol. 2004;42:389–97. [PubMed] [Google Scholar]


