Abstract
Background
Minimizing time to Human Immunodeficiency Virus (HIV) viral suppression is critical in pregnancy. Integrase strand transfer inhibitors (INSTIs), like raltegravir, are known to rapidly suppress plasma HIV ribonucleic acid (RNA) in nonpregnant adults. There is limited data in pregnant women.
Objective
We describe time to clinically relevant reduction in HIV RNA in pregnant women using INSTI-containing and non-INSTI-containing ART options.
Study Design
We conducted a retrospective cohort study of pregnant HIV-infected women in the U.S. from 2009 to 2015. We included women who initiated antiretroviral therapy (ART), intensified their regimen or switched to a new regimen due to detectable viremia (HIV RNA > 40c/mL) at ≥ 20 weeks gestation. Among women with a baseline HIV RNA permitting one-log reduction, we estimated time to one-log RNA reduction using the Kaplan-Meier estimator comparing women starting/adding an INSTI in their regimen versus other ART. To compare groups with similar follow-up time, we also conducted a subgroup analysis limited to women with ≤14 days between baseline and follow-up RNA data.
Results
This study describes 101 HIV-infected pregnant women from 11 U.S. clinics. Seventy-five percent (76/101) women were not taking ART at baseline; 24 were taking non-INSTI containing ART, and 1 received zidovudine monotherapy. Thirty-nine percent (39/101) of women started an INSTI-containing regimen or added an INSTI to their ART regimen. Among 90 women with a baseline HIV RNA permitting one-log reduction, the median time to one-log RNA reduction was 8 days [Interquartile Range (IQR): 7, 14] in the INSTI group versus 35 days [IQR: 20, 53] in the non-INSTI ART group (p<0.01). In a subgroup of 39 women with first and last RNA measurements ≤14 days apart, median time to one-log reduction was 7 days [IQR: 6, 10] in the INSTI group versus 11 days [IQR: 10, 14] in the non-INSTI group (p<0.01).
Conclusion
ART that includes INSTIs appears to induce more rapid viral suppression than other ART regimens in pregnancy. Inclusion of an INSTI may play a role in optimal reduction of HIV-RNA for HIV-infected pregnant women presenting late to care or failing initial therapy. Larger studies are urgently needed to assess the safety and effectiveness of this approach.
Keywords: HIV, pregnancy, integrase inhibitors
Introduction
In the past twenty years, tremendous progress has been made in the United States in the field of HIV and pregnancy. Current U.S. Perinatal HIV Guidelines, the American College of Obstetricians and Gynecologists, and the World Health Organization (WHO) now recommend initiation of antiretroviral therapy (ART) as early as possible during pregnancy due to prolific amounts of data demonstrating lower risk of mother-to-child HIV transmission (MTCT) with earlier viral suppression.1 In women with suppressed HIV viral loads, the risk of mother-to-child HIV transmission (MTCT) is less than 2%, however, the higher a woman’s viral load at the time of delivery, the more likely her chance of transmitting to her infant.2,3 Unsuppressed HIV viral load at the time of delivery remains one of the most important risk factors for perinatal HIV transmission.4–6 Despite massive public health efforts in the US, perinatal HIV transmission still occurs, often among women who present late in pregnancy with a high viral load due to antiretroviral drug resistance issues, non-adherence to prescribed ART or late entry into HIV care.6 Challenges also remain in settings where provider adherence to HIV perinatal guidelines may be suboptimal.5
Options for women who present with high viral loads close to the time of delivery are limited to planning a cesarean delivery and potentially switching the women to an antiretroviral that may rapidly decrease her viral load. Raltegravir, an integrase strand transfer inhibitor (INSTI) antiretroviral agent, has been shown to rapidly decrease time to virologic suppression and, in combination with other antiretroviral drugs, is recommended as a first-line option for nonpregnant patient populations.7 In August 2015, raltegavir was upgraded to a preferred agent for use of INSTI-based combination ART in pregnancy.1 Prior to that, it was considered an alternative option in pregnant women, although clinically it has been used in women who required rapid virologic suppression late in pregnancy. Data from published case series demonstrate rapid reductions in viral load during pregnancy, although no guidelines currently recommend it as a first line therapy to rapidly suppress HIV viral loads among women close to the time of delivery.8–12 In this study we describe time to clinically relevant reduction in HIV RNA in pregnant women using INSTI-containing and non-INSTI-containing ART options.
Materials and Methods
We conducted a retrospective cohort study of pregnant HIV-infected women receiving prenatal care at 11 tertiary care centers in the United States between July 1, 2009 and June 30, 2015. The cohort included HIV-infected pregnant women with HIV RNA levels ≥ 48 copies/mL at ≥ 20 0/7 weeks gestation and their HIV-exposed neonates. Women were included if they had an intervention to change their treatment during this time period. Women were described as ART initiators if they were not currently taking ART. This group included women who were either ART naïve or had stopped taking previously prescribed medications. Women who were currently taking ART were described as either changing (stopping current ART and starting a new regimen) or intensifying their regimen (adding new medications to existing ART regimen). Women were excluded if they did not have at least 2 HIV RNA measurements - one corresponding to the time prior to the intervention and at least one after the intervention.
A request for participation was enlisted on the University of California San Francisco (UCSF)-Infectious Disease Society for Obstetrics and Gynecology (IDSOG) Reproductive Infectious Disease listserv. Researchers from 11 academic medical centers agreed to collaborate. Institutional Review Board approval or exemption was completed at each of the 11 sites. Demographic data, medical history, laboratory testing of mother and infant pairs were collected via chart review and recorded on a standardized form at each site. An AIDS diagnosis was defined as history of CD4 count of less than 200 cells/μl. Each site reviewed its own records for inclusion and exclusion criteria. De-identified data were sent to the University of North Carolina.
Baseline characteristics of women with an INSTI-containing intervention versus a non-INSTI-containing intervention were compared using standard statistical tests, including chi-square or Fisher’s exact tests for categorical factors and Wilcoxon rank sum tests for continuous measures. Our primary outcome of interest was time (in days) from ART intervention to a one-log decrease in viral load. Time from ART intervention until a one-log decrease in viral load as well as cumulative incidence curves, were estimated using the Kaplan-Meyer estimator. Women who did not achieve a one-log decrease in viral load were censored. Women who had a baseline viral load greater than 398 copies/mL (2.6 log10 copies/mL) were eligible for the analysis for a reduction in one log10, since the lower limit of assay detection was 40 (1.6 log10 copies/mL). The log-rank test was used to test for differences in time to each outcome comparing those initiating/adding an INSTI and those initiating/adding a non-INSTI regimen.
Results
Baseline characteristics
One hundred and one pregnant women met eligibility criteria from 11 sites. Characteristics of all pregnant women at baseline (time of ART intervention at ≥ 20 weeks gestation) are reported in Table 1. The median gestational age at time of ART intervention was 29.0 weeks (interquartile range [IQR]: 26.0–33.6). The median viral load at time of ART intervention was 16,030 copies/mL (IQR: 3,370–46,271). Sixty-three (62%) of the pregnant women were diagnosed with HIV prior to the index pregnancy and the remaining 38 (38%) were diagnosed during the index pregnancy.
TABLE 1.
Characteristics of HIV-infected pregnant women at late pregnancy antiretroviral therapy intervention, 2009–2015.
| Overall (N=101) |
INSTI (N=39) |
Other (N=62) |
p-value | |
|---|---|---|---|---|
| Race & Ethnicity | ||||
| White, non-Hispanic | 16 (16) | 5 (13) | 11 (18) | 0.47 |
| White, Hispanic | 10 (10) | 4 (11) | 6 (10) | |
| Black | 73 (72) | 28 (72) | 45 (73) | |
| Other | 2 (2) | 2 (5) | 0 (0) | |
| Parity* | ||||
| 0 | 38 (38) | 17 (44) | 21 (34) | 0.63 |
| ≥1 | 62 (61) | 22 (56) | 40 (65) | |
| Maternal Age (years)* | 27 (23, 32) | 29 (23, 34) | 26 (23, 31) | 0.33 |
| Gestational Age (weeks) | 29.0 (26.0–33.6) | 33.6 (29.9–36.1) | 27.5 (25.4–30.7) | <0.01 |
| Body Mass Index (kg/m2) | 29.6 (26.3–35.2) | 27.7 (24.4–35.0) | 30.5 (26.6–35.3) | 0.26 |
| No ART at baseline† | 76 (75) | 20 (51) | 56 (90) | <0.01 |
| Initial HIV RNA | ||||
| (log10) | 4.2 (3.5–4.7) | 4.3 (3.5–4.9) | 4.1 (3.4–4.6) | 0.21 |
| (copies/mL) | 16,030 (3,370–46,271) | 21,278 (3,370–71,660) | 14,033 (2,500–35,570) | |
| AIDS Diagnosis* | 43 (43) | 23 (60) | 20 (32) | <0.01 |
| Maternal Comorbidity | ||||
| Hepatitis B | 5 (5) | 2 (5) | 3 (5) | 0.95 |
| Hepatitis C | 10 (10) | 2 (5) | 8 (13) | 0.31 |
| Substance Use | 19 (19) | 7 (18) | 12 (19) | 0.86 |
| Depression/mental illness | 28 (28) | 14 (36) | 14 (23) | 0.15 |
Categorical variables are expressed as N (%) and continuous variables are expressed as median (IQR).
One missing observation.
Women on ART were only included if they were not suppressed after 20 weeks gestation and thus additional ART was added to their regimen or their regimen was switched.
An INSTI was initiated/added in 39/101 (39%) women (Table 1). There were no significant differences in age, race/ethnicity, parity, initial HIV RNA, or maternal comorbidities between the INSTI and non-INSTI groups (Table 1). The median gestational age at inclusion in the study was 33 weeks in the INSTI-group compared to 27 weeks in the non-INSTI group (p<0.01). Twenty-three (60%) women who initiated/added an INSTI-based regimen had an AIDS diagnosis compared to twenty (32%) women who initiated/added other ART (p<0.01). The majority of women had genotype testing performed either prior to pregnancy (28%) or during pregnancy (70%).
ART regimens
ART initiation
Of the 101 pregnancies, 76 (76%) women were not taking any ART regimen prior to intervention. All ART regimens that were initiated during the study included two nucleoside reverse transcriptase inhibitors (NRTIs) such as tenofovir/emtricitabine or zidovudine/lamivudine. Of these 76 women, 6 women initiated on 2NRTI+INSTI-based regimens (5 raltegravir, 1 elvitegravir/cobistat). Fourteen women initiated 2NRTIs + INSTI + PI (6 lopinavir/raltegravir, 3 atazanavir/raltegravir, 1 atazanavir/dolutegravir, 3 darunavir/raltegravir, 1 darunavir/raltegravir). Forty-nine women inititied 2NRTI+PI-based regimens (37 lopinivir, 6 atazanavir, 6 darunavir). When used, all protease inhibitors (PIs) were boosted with ritonavir. One woman initiated a 2NRTI+ non-nucleoside reverse transriptase inhibitor (NNRTI)-based regimen (efavirenz) and 5 women initiated a single tablet regimen of tenofovir/emtricitabine combined with the NNRTI rilpivirine. One initiated a 3-NRTI based regimen (abacavir/lamivudine/zidovudine).
Regimen change or intensification
Of the 25 remaining women who were taking any antiretroviral medications at the time of inclusion in our study, 23 had been prescribed a ritonavir-boosted PI-based regimen (9 on atazanavir, 6 on darunavir, 8 on lopinavir) in addition to dual NRTIs. One woman had been prescribed abacavir/lamivudine/zidovudine and one was presented taking zidivudine only. At the time of inclusion of the study, 18 women changed regimens completely and 7 had drugs added to intensify their regimen. Of the 18 who changed regimens, 12 changed to INSTI-based regimens and 6 changed to other regimens. Of the 7 who had drugs added to their regimen, all had an INSTI (6 raltegravir, 1 dolutegravir) added.
One log10 reduction in HIV RNA or viral load
Ninety-two (91%) of 101 women were eligible for inclusion in the analysis for a one-log reduction in viral load. Eighty-eight percent (81/92) of the women eligible for this analysis had at least a one log10 reduction by their last viral load measure before delivery.
The median time to a visit where viral load was reduced by one log10 varied based on whether or not women initiated a INSTI-based regimen or non-INSTI regimen (8 days [IQR: 7, 14] versus 35 days [IQR: 20, 53] respectively, p<0.01, Figure 1a). Of note, women on INSTI-based regimens had an average of one viral load measurement every 13 days, while the average rate of assessment among women on non-INSTI-based regimens was one viral load measurement every 23 days [rate ratio [95% CI]=1.8 (1.4, 2.2)]. Therefore, in order to compare groups with similar levels of follow-up and viral load assessment, we conducted a subgroup analysis among women with a follow-up visit after an initial baseline assessment of less than 14 days (n=39). Among this subgroup, the median time to a visit where there was a one log10 reduction in viral load varied based on whether they initiated an INSTI-based regimen or non-INSTI regimen (7 days [IQR: 6, 10] and 11 [IQR: 10, 14] respectively, Figure 2; log-rank p <0.01).
FIGURE 1.
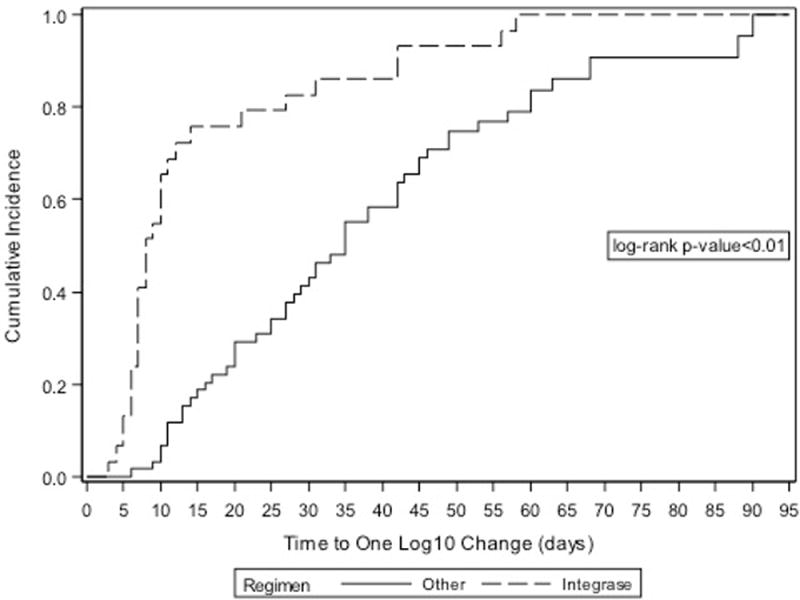
Cumulative incidence of one log10 viral load reduction among HIV-infected pregnant women initiating antiretroviral therapy in late pregnancy.
FIGURE 2.
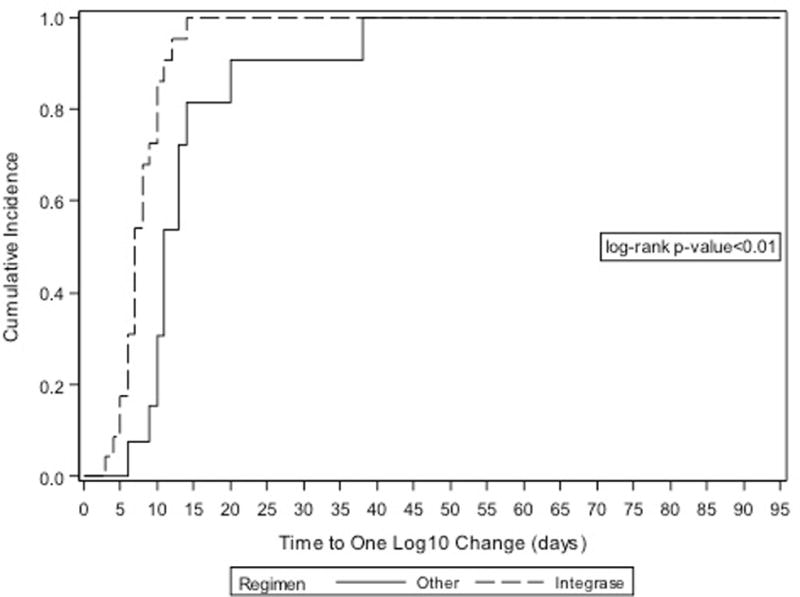
Cumulative incidence of one log10 viral load reduction among HIV-infected pregnant women initiating antiretroviral therapy in late pregnancy among subgroup with comparable follow up.
Delivery
The median time from ART intervention to delivery was 57 days (IQR: 34–84) and the median reduction in viral load from ART intervention to last viral load measurement was 14,779 copies/mL (IQR: 3,290–42,786) (Table 2). Thirty-five (35%) women’s viral loads were suppressed to <40 copies/mL based on their last viral load measurement reported before delivery. Although there was not a statistically significant difference in the amount of viral load reduction in the two groups, women in the INSTI group had a shorter ART duration prior to delivery (p<0.01) and lower CD4 nadir during pregnancy (p=0.02,Table 2).
TABLE 2.
Characteristics of HIV-infected pregnant women at delivery, 2009–2014.
| Overall (N=101) |
INSTI (N=39) |
Other (N=62) |
p-value | |
|---|---|---|---|---|
| Total | ||||
| Mode of delivery | ||||
| Vaginal | 49 (49) | 23 (59) | 26 (42) | 0.05 |
| Elective C-section | 36 (36) | 14 (36) | 22 (35) | |
| Labored C-section | 16 (16) | 2 (5) | 14 (23) | |
| Gestational Age (weeks) | ||||
| <37 | 21 (21) | 7 (18) | 14 (23) | 0.58 |
| 37–41 | 80 (79) | 32 (82) | 48 (77) | |
| Pregnancy CD4 nadir (cells/μl) | 275 (164–458) | 195 (82–397) | 297 (196–463) | 0.02 |
| Duration of ART (days)* | 57 (34–84) | 35 (8–59) | 71 (47–86) | <0.01 |
| Median VL reduction | 14,779 | 20,650 | 12,926 | 0.21 |
| IQR | (3,290–42,786) | (3,350–59,610) | (2,480–35,442) | |
| range | (25–633,885) | (25–633,885) | (71–143,023) |
Categorical variables are expressed as N (%) and continuous variables are expressed as median (IQR).
Time from ART intervention to delivery; does not include ART use prior to late pregnancy intervention.
There were no reports of laboratory abnormalities or complications attributed to ART use in the cohort. Nine of 101 women were diagnosed with preeclampsia/gestational hypertension and six of 101 presented with premature rupture of membranes (PPROM or PROM) or preterm labor. A total of 21/101 (21%) women delivered prematurely (< 37 weeks gestation).
Follow up data were available on 86/101 (85%) of the neonates from birth to at least 4 months of age. Seventy of the neonates had sufficient results to be diagnosed as HIV-uninfected. Fifteen neonates had only one negative HIV RNA result. One neonate was diagnosed as HIV-infected. The mother was diagnosed with HIV during the index pregnancy which was complicated by depression. She was initiated on zidovudine/lamivudine/boosted lopinavir at 31 weeks with a viral load of 21,685 copies/mL. A second viral load of 2,171 copies/mL was documented 42 days later at 37 weeks gestation, when she was delivered by elective cesarean section due to preeclampsia. Of note, she had an active genital HSV infection at 34 weeks. The neonate’s birth HIV DNA PCR testing was positive, and this was presumed to be an intrauterine infection. The following complications were observed in other neonates (count reported in parentheses): anemia (2), hyperbilirubinemia (1), club foot (1), group B streptococcal septicemia (1), respiratory syncytial virus (1), pseudohypoaldosteronism (1), upper respiratory infection (1), small for gestational age (2), hyperbilirubinemia (1), neonatal opiate withdrawal (1), and congenital heart defect (1).
Case reports of dolutegravir
Four of the 39 INSTI-based regimens included dolutegravir. Two patients initiated a dolutegravir-containing regimen (dolutegravir/darunavir/ritonavir/truvada and truvada/atazanavir/ritonavir/dolutegravir), one patient switched from abacavir/atazanavir/ritonavir to dolutegravir/tenofovir/emtricitabine, and the last had dolutegravir added to her abacavir/atazanavir/ritonavir regimen. The baseline viral load for these patients was 34,000, 2,000, 14,554 and 46,271 copies/mL, respectively. All of their interventions occurred late in the third trimester (range 33–36 weeks), and all had only one follow-up viral load (range 6–8 days). All four women experienced at least a one-log reduction in viral load to 1,080, 42, 20, 3,485 copies/mL, respectively. No maternal side effects were noted; one neonate was small for gestational age and was diagnosed with hyperbilirubinemia. All neonates exposed to dolutegravir were HIV-uninfected.
Comment
Principle findings
Our retrospective study demonstrates that a wide variety of ART regimens are prescribed across the U.S. for management of women whose HIV-1 viral load is not suppressed late pregnancy. This study supports previously published case reports of rapid decline in HIV-1 viral load after treatment with INSTI-containing regimens.8,9,11,12 All of the observed ART regimens were effective in decreasing HIV viral load in a relatively short time period, but the addition of an integrase inhibitor suppressed viral load more quickly. This is consistent with prior literature describing the effect of INSTI-based ART in pregnancy, however, a strength of our study is that we compared INSTI- and non-INSTI-containing ART directly.8,9,11–14 Additionally, we describe the clinical practice of using regimens that did not fall under 1st line recommended maternal treatment at the time of the study.1 According to these data, pregnant HIV-infected women are prescribed newer potent ART options or regimens that are convenient once-a-day options, likely in order to promote adherence. Our study also includes one of the few clinical descriptions of dolutegravir use in pregnancy.
Meaning of observations as it relates to other studies and research implications
This study contributes to the growing literature on raltegravir in pregnancy. Studies of pregnant HIV-infected women have described high rates of viral suppression, good tolerance to the medication, and ready cross-over into the placenta despite median raltegravir area under the curve concentrations that were 29–50% lower than non-pregnant women.15–17 The previous collection of case reports has also shown rapid decline in plasma viral loads similar to our study.15,16,18–20,21,22,23 A single case report described maternal hepatic toxicity, which diminished after discontinuation of raltegravir. Monitoring of transaminases should occur with INSTI use during pregnancy.24 Our study included 39 women exposed to an INSTI without apparent ART related side effects; however due to the retrospective nature of this study, there may be missing or unavailable data. Our sample size was smaller than the large studies needed to detect rare events. The national Antiretroviral Pregnancy Registry has not reported congenital anomalies or adverse neonatal outcomes associated with INSTI use in more than 350 pregnant women thus far.18 Dolutegravir and Elvitegravir are considered U.S Food and Drug Administration (FDA) pregnancy category B and Raltegravir is pregnancy category C.
Use of raltegravir was associated with a rapid decrease in viral load in our study. However, raltegravir is a twice-daily medication, which may be associated with noncompliance. Raltegravir was studied as a once daily medication (800 mg daily) compared to twice daily (400 mg bid) in a double-blinded randomized controlled noninferiority study – both in combination with tenofovir and emtricitabine in non-pregnant adults.25 This study demonstrated longer time to viral suppression with once-daily dosing, particularly in individuals with HIV RNA >100,000 copies/ml or CD4 counts <200 cells/mm3 prior to initiating therapy. Among other participants, the response rate for once-daily raltegravir was similar to other recommended ART regimens. The authors were not able to comment on which regimen would be superior if there were drug adherence challenges.26 Notably, 80% of the participants were male, and pregnant women were excluded. Further study of once-daily dosed INSTI’s in pregnant women should be pursued.
There are insufficient data to recommend use of other INSTI’s such as dolutegravir or elvitegravir in pregnancy.18 One of the major benefits of dolutegravir and elvitegravir is their once-daily dosing schedule. A single case report describes pharmacokinetics of dolutegravir in the newborn after maternal exposure.27 Our study includes 4 women who took dolutegravir and 1 woman who took elvitegravir. All of these women demonstrated rapid viral load reduction and no adverse maternal or neonatal outcomes that could linked to medication exposure. Further studies on the safety and efficacy of these regimens in pregnancy are needed as the once a day dosing of these medications is appealing.
Clinical Implications
While HIV testing and treatment campaigns have dramatically reduced vertical infections in resource-rich settings, they still occur.5 Limited access to prenatal care, inadequate HIV testing in pregnancy, and psychosocial stressors and mental health issues affecting adherence continue to cause delayed diagnoses and/or late engagement in HIV care. A case series of perinatal HIV transmissions between 2005 to 2012 reported that 24 of 27 transmission were related to failure in healthcare delivery and uptake.5 Vigilant screening for HIV in pregnancy and resources to support pregnant HIV-infected mothers during pregnancy must be in place to eliminate MTCT.
Providers must also be aware of resources and updates to current guidelines1 Raltegravir is now a preferred agent if an INSTI is used in pregnancy.1 Though dosed twice daily, raltegravir is well tolerated and has a high barrier to developing drug resistance. Intravenous administration of intrapartum zidovudine continues to be recommended in women whose viral loads have not been consistently suppressed during pregnancy and elective cesarean delivery should be offered to women whose viral load is ≥ 1,000 copies/mL.1 However, late term or intrapartum interventions will not prevent antepartum transmission as in the case of the one known HIV-infected neonate in the cohort. Only early, aggressive control of viremia will prevent intrauterine infections. The Perinatal HIV/AIDS hotline (888-448-8765) is available to care providers 24 hours a day for consultation regarding HIV-infected pregnant mothers and neonates in the U.S.
Limitations
Our retrospective study is limited in that we could not control for timing of viral load measurements. Our results are potentially biased by differential outcome assessment. As indicated by our subgroup analysis of women who had two viral load measures less than 14 days apart, we may have seen a more rapid drop in the non-INSTI group in the larger analysis if viral load measurements had occurred on a similar timeline as the INSTI group. Ideally, if there had been more time prior to the completion of the pregnancies, we could have made a more direct comparison of time to viral suppression between the two groups rather than being limited to a one-log decrease in viremia. However, a reduction in viral load is a significant factor in decreasing risk of MTCT.28 The risk of perinatal transmission decreased as viral load decreased in United Kingdom/Ireland population-based surveillance. MTCT rates were 0.09%, 1.0%, and 2.6% for women with viral loads of <50, 50–399, and 400–999 copies/mL, respectively (p<0.001).28 There was a difference in timing of regimens (33.6 weeks for INSTI; 27.5 weeks for other regimens) in our study; this may imply that there was a bias in choosing INSTI’s in women who were later in pregnancy or had higher viral loads. Though all centers had access to INSTI’s, we also do not have data on the rationale for choice of regimen. The small sample size of this study also limited the ability to control for other potential confounders and explore issues such the preterm labor rate of 21%. Despite these limitations, this study also contributes important data on a difficult to study population.
Conclusions
These study findings should direct future research efforts. The INSTI class of medication could play a significant role not only in resource-rich settings, but also in low-resource settings where women may only access prenatal care in the 3rd trimester or not start ART until close to delivery. A well-tolerated, rapid-acting ART option with a high threshold for development of resistance mutations could be ideal in these settings to reduce risk of MTCT. Decreasing viremia prior to delivery may modify the risk not only of infant HIV infection, but also of adverse maternal outcomes by decreasing need for elective cesarean delivery. Additionally, the potential role of INSTI-containing regimens to rapidly reduce viral load in settings where maternal complications, preterm labor or premature rupture of membranes may shorten pregnancy unexpectedly is appealing. It is critical that providers caring for HIV-infected pregnant women are aware of INSTI use as a potential strategy for rapid virologic suppression in pregnancy which is ideal at any gestational age to prevent both antepartum and intrapartum infection. Further data is needed regarding the efficacy, safety and tolerability of INSTIs during pregnancy.
Footnotes
Conflict of Interest Statement: The authors report no relevant financial conflicts of interest.
This abstract has been accepted for oral presentation at the Society of Maternal Fetal Medicine Conference in Feb 2016, Atlanta, GA.
Contributor Information
Lisa RAHANGDALE, Department of Obstetrics & Gynecology, University of North Carolina School of Medicine, Chapel Hill, NC.
Ms. Jordan CATES, Department of Epidemiology, University of North Carolina Gillings School of Public Health, Chapel Hill, NC.
JoNell POTTER, Department of Obstetrics and Gynecology; University of Miami Miller School of Medicine, Miami, FL.
Martina L. BADELL, Emory University Department of Gynecology and Obstetrics, Division of Maternal-fetal Medicine.
Dominika SEIDMAN, Department of Obstetrics, Gynecology and Reproductive Sciences; University of California San Francisco.
Emilly S. MILLER, Department of Obstetrics & Gynecology, Division of Maternal Fetal Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Jenell S. COLEMAN, Department of Gynecology and Obstetrics, Johns Hopkins University, Baltimore, MD.
Gweneth B. LAZENBY, Department of Obstetrics and Gynecology, Medical University of South Carolina, Charleston, SC.
Judy LEVISON, Department of Obstetrics & Gynecology, Baylor College of Medicine, Houston, TX.
William R. SHORT, Department of Medicine, University of Pennsylvania Perelmen School of Medicine, Philadelphia, PA (Formerly at Sidney Kimmel Medical College at Thomas Jefferson University).
Sigal YAWETZ, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Andrea CIARANELLO, Medical Practice Evaluation Center, Division of Infectious Diseases, Massachusetts General Hospital.
Elizabeth LIVINGSTON, Department of Obstetrics & Gynecology; Duke University, Durham, NC.
Lunthita DUTHELY, Department of Obstetrics and Gynecology; University of Miami, Miami, FL.
Bassam H. RIMAWI, Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, GA.
Jean R ANDERSON, Department of Gynecology and Obstetrics, Johns Hopkins University, Baltimore, MD.
Elizabeth M STRINGER, Department of Obstetrics & Gynecology, University of North Carolina School of Medicine, Chapel Hill, NC.
References
- 1.Panel of Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. 2015 http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed Aug 8, 2015.
- 2.Townsend CL, Cortina-Borja M, Peckem CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy intervention in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 3.Neisham S, Taylor A, Lampe MA, et al. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012;130:738–744. doi: 10.1542/peds.2012-0194. [DOI] [PubMed] [Google Scholar]
- 4.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infact transmision of human immunodeficiency virus type 1 with zidovudine treatment. Pediatri AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 5.Camacho-Gomez AF, Kingbo M-H, Boylan A, Eckard AR, Chahroudi A, Chakraborty R. Missed opportunities for prevention of mother-to-child transmission in the United States: a review of cases from the state of Georgia, 2005–2012. AIDS. 2015;29 doi: 10.1097/QAD.0000000000000710. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz IT, Leister E, Kacanek DK, et al. Factors associated with lack of viral suppression at delivery among highly active antiretroviral therapy-naive women with HIV: A cohort study. Ann Intern Med. 2015;162:90–99. doi: 10.7326/M13-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. 2015 https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed Aug 30, 2015.
- 8.Westling K, Pettersson K, Kaldma A, Naver L. Rapid decline in HIV viral load when introducting raltegravir-containing antiretroviral treatment late in pregnancy. AIDS Patient Care STDs. 2012;26:714–717. doi: 10.1089/apc.2012.0283. [DOI] [PubMed] [Google Scholar]
- 9.Nobrega I, Travassos AG, Haguihara T, Amorim F, Brites C. Short Communication: Use of raltegravir in late-presenting HIV-infected pregnant women. AIDS Res Hum Retroviruses. 2013;29:1451–1454. doi: 10.1089/aid.2013.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shust GF, Jao J, Rodriguez-Caprio G, et al. Salvage regimens containing darunavir, etravirine, raltegravir, or enfurvitide in highly treatment-experience perinatally infected pregnant women. J Pediatric Infect Dis Soc. 2014;3:246–250. doi: 10.1093/jpids/pit019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Halsema C, Whitfield T, Lin N, Ashton K, Torkington A, Ustianowski A. Five years’ real-life experience with raltegravir in a large HIV centre. Int J STD AIDS. 2015 doi: 10.1177/0956462415584485. [DOI] [PubMed] [Google Scholar]
- 12.Boucoiran I, Tulloch K, Pick N, et al. A case series of third-trimester raltegravir initiation: Impact on maternal HIV-1 viral load and obstetrical outcomes. Can J Infect Dis Med Microbiol. 2015;26:145–150. doi: 10.1155/2015/731043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read PJ, Mandalia S, Khan P, et al. When should HAART be initiated in pregnancy to achieve an undetectable HIV viral load by delivery? AIDS. 2012;26:1095–1103. doi: 10.1097/QAD.0b013e3283536a6c. [DOI] [PubMed] [Google Scholar]
- 14.Aziz N, Sokoloff A, Kornak J, et al. Time to viral load suppression in antiretroviral-naive and -experience HIV-infection pregnant women on highly active antiretroviral therapy: implications for pregnant women presenting late in gestation. BJOG. 2013;120:1534–1547. doi: 10.1111/1471-0528.12226. [DOI] [PubMed] [Google Scholar]
- 15.Watts DH, Stek A, Best BM, et al. Raltegravir pharmacokinetics during pregnancy. J Acquir Immune Defic Syndr. 2014;67:375–381. doi: 10.1097/QAI.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blonk M, Colbers A, Hidalgo-Tenorio C, et al. Raltegravir in HIV-1 infected pregnant women: Pharmacokinetics, safety and efficacy. Clin Infect Dis. 2015 doi: 10.1093/cid/civ366. [DOI] [PubMed] [Google Scholar]
- 17.McKeown DA, Rosenvinge M, Donaghy S, et al. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS. 2010;24:2416–2418. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 18.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Reprot for 1 January 1989 through 31 January 2015. 2015 http://www.APRegistry.com. Accessed July 13, 2015.
- 19.Taylor N, Touzeau V, Geit M, et al. Raltegravir in pregnancy: a case series presentation. Int J STD AIDS. 2011;22:358–360. doi: 10.1258/ijsa.2011.010469. [DOI] [PubMed] [Google Scholar]
- 20.Pinnetti C, Baroncelli S, Villani P, et al. Rapid HIV-RNA decline following addition of raltegravir and tenofovir to ongoing highly active antiretroviral therapy in a women presenting with high-level HIV viraemia at weet 38 of pregnancy. J Antimicrob Chemother. 2010;65:2050–2052. doi: 10.1093/jac/dkq264. [DOI] [PubMed] [Google Scholar]
- 21.Jaworsky D, Thompson C, Yudin MH, et al. Use of newer antiretroviral agents, darunavir and etravirine, with or without raltegravir, in pregnancy: a report of two cases. Antivir Ther. 2010;15:677–680. doi: 10.3851/IMP1558. [DOI] [PubMed] [Google Scholar]
- 22.Cha A, Shaikh R, Williams S, Berkowitz LL. Rapid reduction in HIV viral load in late pregnancy with raltegravir: a case report. J Int Assoc Provid AIDS Care. 2013;12:312–314. doi: 10.1177/2325957413488176. [DOI] [PubMed] [Google Scholar]
- 23.DeHoffer L, Di Biagio A, Bruzzone B, et al. Use of raltegravir in a late presenter HIV-1 woman in advanced gestational age: a case report and literature review. J Chemother. 2013;25:181–183. doi: 10.1179/1973947812Y.0000000066. [DOI] [PubMed] [Google Scholar]
- 24.Renet S, Closon A, Brochet MS, Bussieres JF, Boucher M. Increase in transaminase levels following the use of raltegravir in a woman with a high HIV viral load at 35 weeks of pregnancy. J Obstet Gynaecol Can. 2013;35:68–72. doi: 10.1016/s1701-2163(15)31051-3. [DOI] [PubMed] [Google Scholar]
- 25.Eron JJ, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled phase 3 non-inferiority trial. Lancet Infect Dis. 2011;11:907–915. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]
- 26.Adams JL, Greener BN, Kashuba ADM. Pharmacology of HIV integrase inhibitors. Curr Opin HIV AIDS. 2012;7:390–400. doi: 10.1097/COH.0b013e328356e91c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pain JB, Le MP, Caseris M, et al. Pharmacokinetics of dolutegravir in a premature neonate after HIV treatment intensification during pregnancy. Antimicrob Agents Chemother. 2015;59:3660–3662. doi: 10.1128/AAC.00173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend CL, Byrne L, Corina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS. 2014;28:1049–1057. doi: 10.1097/QAD.0000000000000212. [DOI] [PubMed] [Google Scholar]


