Abstract
Background
Age is a known risk factor for recurrence for women with DCIS treated with breast-conserving surgery (BCS); we explored the relationship between age, other risk factors, and recurrence.
Methods
Using a prospectively maintained database of DCIS patients undergoing BCS from 1978–2010, the association of age and recurrence risk was analyzed using Kaplan–Meier estimates, multivariable analysis (MVA), and competing-risk MVA (CRMVA).
Results
2996 cases were identified. Median follow-up for those without recurrence was 75 mos; 732 had ≥10y follow-up. 363 (12%) had recurrence: 192 (53%) DCIS, 160 (44%) invasive, 11 (3%) unknown. Risk of recurrence decreased with age, even after adjustment for eight clinicopathologic variables on MVA (hazard ratios [HR] with <40y as reference: 40–49y[0.82, p=0.36], 50–59y[0.46, p=0.0005], 60–69y[0.50, p=0.003], 70–79y[0.56, p=0.02], ≥80y [0.21, p=0.0015]). This association persisted for cohorts with and without RT. Using CRMVA, the effect of age on invasive recurrence was empirically stronger than for DCIS recurrence. 10y invasive recurrence was 16% vs. 6.5% in women <40y vs. women ≥40y. Only 0.6% of the population ultimately developed distant disease; those <40y constituted 4.7% (141/2996) of the population but 21% (4/19) of those developing distant disease.
Conclusions
Risk of recurrence of DCIS decreases with age; this effect is particularly strong at the extremes of age, and is independent of other clinicopathologic factors. The oldest women are at low risk of recurrence; the youngest have a higher overall and especially invasive recurrence rate, although mortality remains low. These findings should be incorporated into risk/benefit discussions of treatment options.
Keywords: DCIS, breast-conserving surgery, age, recurrence, invasive recurrence, distant metastases
INTRODUCTION
Widespread adoption of screening mammography over the last 30 years has led to an increased detection of ductal carcinoma in situ (DCIS), such that it now accounts for over 20% of breast cancers diagnosed in the U.S.1, 2 Current treatment options for DCIS include breast-conserving surgery (BCS) alone, BCS with radiation (RT), BCS with endocrine therapy, BCS with both RT and endocrine therapy, mastectomy, and even bilateral mastectomy.3, 4 There is currently concern about overtreatment of this lesion; however, while survival is excellent with all treatments, local recurrence rates vary widely with various treatment options.5–11
Both retrospective studies12–14 and the randomized trials of RT7–10 have shown that younger age is associated with a higher risk of local recurrence for DCIS treated with BCS. A recent analysis of DCIS patients from the Surveillance, Epidemiology, and End Results (SEER) registry found an increased risk of breast cancer mortality for those <35y of age.11 However, the relationship between age and recurrence across the full spectrum of age, and the interaction between age and other clinicopathological and treatment factors has not been fully elucidated. The goal of this study was to explore the association of age with ipsilateral locoregional recurrence, both in situ and invasive, and development of distant disease.
METHODS
Following Institutional Review Board approval the outcomes of DCIS patients who underwent BCS at Memorial Sloan Kettering Cancer Center from 1978–2010 were analyzed from a prospectively maintained database. Bilateral cases of DCIS in patients with either synchronous (n=30) or metachronous (n=29) lesions were abstracted as separate cases.
Patients were grouped and analyzed according to age at the time of surgery (<40, 40–49, 50–59, 60–69, 70–79 or ≥80y). Other factors analyzed were: family history of breast cancer (defined as ≥1 first- or second-degree family member), presentation (radiological or clinical), nuclear grade (low/intermediate or high), necrosis, number of excisions required (≤2 or ≥3), margin status (positive/close (≤2mm) or negative (>2mm)), use of adjuvant RT and endocrine therapy, and treatment time period (≤1998 or ≥1999).15
The primary endpoint was time to first recurrence, categorized as DCIS or invasive recurrence. DCIS recurrence was defined as ipsilateral breast recurrence of DCIS without invasion. Invasive recurrence was defined as first recurrence as ipsilateral invasive breast cancer, ipsilateral axillary nodal recurrence without ipsilateral breast recurrence, or, in a single patient, distant recurrence in the absence of any ipsilateral recurrence or contralateral diagnosis of breast carcinoma. Time to event was defined as the interval between definitive surgery and date of first recurrence.
A secondary endpoint was development of distant disease at any time, in the absence of contralateral invasive breast cancer.
Differences in patient characteristics by age were assessed using χ2 test. Five- and 10-year Kaplan–Meier recurrence estimates by age were calculated for the entire population, as well as the cohorts with and without RT. Multivariable Cox regression analysis was used to assess the relationship between age and recurrence after adjustment for clinicopathological and treatment factors. Competing risk analysis was used to assess risk of invasive or in situ recurrence according to age univariately, and competing risk multivariable analysis was used to adjust for other factors. Women in whom the type of recurrence (invasive or DCIS) was not known were excluded from the competing risk analysis. All analyses were performed in SAS version 9.4 and R version 3.1.1.
RESULTS
2996 cases of DCIS managed with BCS were identified from 1978 to 2010. Characteristics of the entire population and the cohorts stratified by decade of age at the time of surgery are shown in Table 1. The median patient age was 57y (range 20–92). The distribution of the study population by age is shown in Supplemental Fig. 1.
TABLE 1.
Characteristics of the entire population, and by age group
| Characteristic | Total population
|
<40
|
40 – 49
|
50 – 59
|
60 – 69
|
70 – 79
|
≥80
|
p value* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=2996 | N=141 | N=704 | N=887 | N=727 | N=426 | N=111 | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| Presentation | |||||||||||||||
| Clinical | 386 | 13 | 43 | 30 | 99 | 14 | 91 | 10 | 83 | 11 | 52 | 12 | 18 | 16 | <0.0001 |
| Radiological | 2606 | 87 | 98 | 70 | 605 | 86 | 795 | 90 | 644 | 89 | 371 | 87 | 93 | 84 | |
| Unknown | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | |
|
| |||||||||||||||
| Family History | |||||||||||||||
| No | 1816 | 61 | 84 | 60 | 427 | 61 | 522 | 59 | 452 | 62 | 264 | 62 | 67 | 60 | 0.7 |
| Yes | 1163 | 39 | 54 | 38 | 266 | 38 | 358 | 40 | 262 | 36 | 154 | 36 | 42 | 38 | |
| Unknown | 44 | 1 | 3 | 2 | 11 | 2 | 7 | 1 | 13 | 2 | 8 | 2 | 2 | 2 | |
|
| |||||||||||||||
| Nuclear Grade | |||||||||||||||
| Low/intermediate | 1787 | 60 | 82 | 58 | 420 | 60 | 543 | 61 | 422 | 58 | 259 | 61 | 61 | 55 | 0.4 |
| High | 994 | 33 | 47 | 33 | 235 | 33 | 287 | 32 | 259 | 36 | 124 | 29 | 42 | 38 | |
| Unknown | 215 | 7 | 12 | 9 | 49 | 7 | 57 | 6 | 46 | 6 | 43 | 10 | 8 | 7 | |
|
| |||||||||||||||
| Necrosis | |||||||||||||||
| Absent | 1029 | 34 | 46 | 33 | 270 | 38 | 308 | 35 | 228 | 31 | 139 | 33 | 38 | 34 | 0.1 |
| Present | 1802 | 60 | 84 | 60 | 395 | 56 | 533 | 60 | 465 | 64 | 256 | 60 | 69 | 62 | |
| Unknown | 165 | 6 | 11 | 8 | 39 | 6 | 46 | 5 | 34 | 5 | 31 | 7 | 4 | 4 | |
|
| |||||||||||||||
| Number of excisions | |||||||||||||||
| ≤2 | 2775 | 93 | 130 | 92 | 634 | 90 | 820 | 92 | 676 | 93 | 406 | 95 | 109 | 98 | 0.01 |
| ≥3 | 217 | 7 | 11 | 8 | 67 | 10 | 67 | 8 | 50 | 7 | 20 | 5 | 2 | 2 | |
| Unknown | 4 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
|
| |||||||||||||||
| Margin status | |||||||||||||||
| Close/positive | 553 | 19 | 27 | 19 | 119 | 17 | 147 | 16 | 128 | 18 | 99 | 23 | 33 | 30 | 0.0003 |
| Negative | 2235 | 75 | 100 | 71 | 549 | 78 | 689 | 77 | 543 | 74 | 285 | 67 | 69 | 62 | |
| Unknown | 208 | 7 | 14 | 10 | 36 | 5 | 51 | 6 | 56 | 8 | 42 | 10 | 9 | 8 | |
|
| |||||||||||||||
| Radiation therapy | |||||||||||||||
| Yes | 1588 | 53 | 82 | 58 | 404 | 57 | 520 | 59 | 411 | 57 | 157 | 37 | 14 | 13 | <0.0001 |
| No | 1374 | 46 | 56 | 40 | 294 | 42 | 358 | 40 | 309 | 43 | 263 | 62 | 94 | 85 | |
| Unknown | 34 | 1 | 3 | 2 | 6 | 1 | 9 | 1 | 7 | 1 | 6 | 1 | 3 | 3 | |
|
| |||||||||||||||
| Endocrine therapy | |||||||||||||||
| Yes | 628 | 21 | 16 | 11 | 145 | 21 | 238 | 27 | 158 | 22 | 63 | 15 | 8 | 7 | <0.0001 |
| No | 2321 | 77 | 122 | 87 | 553 | 79 | 636 | 72 | 556 | 76 | 356 | 84 | 98 | 88 | |
| Unknown | 47 | 2 | 3 | 2 | 6 | 1 | 13 | 1 | 13 | 2 | 7 | 2 | 5 | 5 | |
|
| |||||||||||||||
| Year of surgery | |||||||||||||||
| ≤1998 | 785 | 26 | 56 | 40 | 181 | 26 | 214 | 24 | 179 | 25 | 128 | 30 | 27 | 24 | 0.0014 |
| ≥1999 | 2211 | 74 | 85 | 60 | 523 | 74 | 673 | 76 | 548 | 75 | 298 | 70 | 84 | 76 | |
χ2 test of difference between age at time surgery
The median follow-up was 75 months (range 0–30y) in patients without recurrence; 732 were followed for ≥10 years. There were 363 recurrences; 192 patients developed a DCIS ipsilateral breast recurrence. Of the 160 invasive recurrences, 147 were ipsilateral breast recurrence, 10 were concurrent breast and regional nodal recurrence, 2 had evidence of axillary recurrence only, and one had distant recurrence without evidence of locoregional recurrence or contralateral invasive disease. There were 11 cases of recurrence of unknown type. Distant disease developed in 19 patients; 16/19 occurred after an invasive locoregional recurrence.
Differences in patient, tumor and treatment characteristics by age
Women <40y constituted a smaller proportion of the population after 1999 as compared to the earlier years of the study (3.8% vs. 7.1%). Comparison across the age categories demonstrated that patients <40y more frequently presented clinically than their older counterparts, and less frequently received adjuvant endocrine therapy. Women ≥70y less frequently had ≥3 excisions, more frequently had positive or close margins, and less commonly received adjuvant RT or endocrine therapy.
Differences in recurrence rates by age
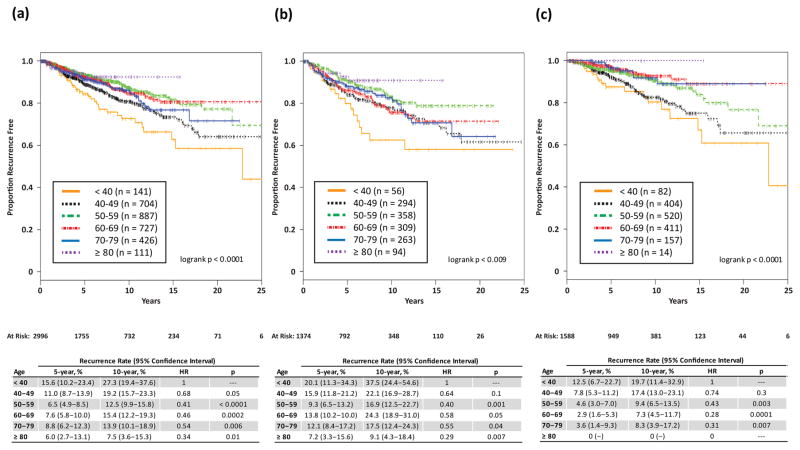
With increasing age there was a significant decrease in 10-year recurrence rates, ranging from 7.5% in women ≥80y to 27.3% in women <40y (p<0.0001) (Fig. 1a). After stratification by use of radiation, the association of decreasing recurrence rates with increasing age persisted in those not receiving and those receiving radiation (Fig. 1b and 1c).
Fig. 1.
Proportion recurrence-free, by age at surgery, in (a) entire population, (b) in cohort not receiving radiation, and (c) in cohort receiving radiation.
Our population included only women undergoing BCS. To exclude the possibility that the observed relationship between age and recurrence risk is due to treatment selection bias toward BCS among young women, we examined the proportion of patients undergoing BCS and mastectomy by age (Supplemental Fig. 2). The proportion of women undergoing BCS was correlated with age, with younger women more frequently undergoing mastectomy, thereby demonstrating that the observed association of young age and higher risk of recurrence is not due to underutilization of mastectomy in younger women.
Multivariable analysis
Multivariable analysis of 2,634 patients with complete data was performed, adjusting for presentation, family history, necrosis, number of excisions, margin status, adjuvant RT, adjuvant endocrine therapy, and treatment period. Grade was not significantly associated with age (p=0.4), nor with recurrence on either univariate (p=0.4) or multivariable (p=0.9) analysis; therefore it was not included in the final multivariable model (Table 2).
TABLE 2.
Multivariable Cox models for recurrence, for entire population, and those without and with radiation
| Entire population (n=2634)
|
No-radiation cohort (n=1163)
|
Radiation cohort (n=1471)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Events | HR | p value | n | Events | HR | p value | n | Events | HR | p value | |
| Age | ||||||||||||
| <40 | 119 | 29 | 1 | -- | 46 | 15 | 1 | -- | 59 | 14 | 1 | -- |
| 40 – 49 | 625 | 99 | 0.82 | 0.36 | 247 | 52 | 0.83 | 0.54 | 378 | 47 | 0.89 | 0.71 |
| 50 – 59 | 791 | 75 | 0.46 | 0.0005 | 305 | 38 | 0.45 | 0.01 | 486 | 37 | 0.52 | 0.04 |
| 60 – 69 | 641 | 64 | 0.50 | 0.003 | 263 | 48 | 0.69 | 0.21 | 378 | 16 | 0.32 | 0.002 |
| 70 – 79 | 362 | 44 | 0.56 | 0.02 | 220 | 37 | 0.64 | 0.16 | 142 | 7 | 0.37 | 0.04 |
| ≥80 | 96 | 5 | 0.21 | 0.0015 | 82 | 5 | 0.24 | 0.006 | 14 | 0 | ||
|
| ||||||||||||
| Presentation | 0.008 | 0.02 | 0.25 | |||||||||
| Clinical | 302 | 59 | 1 | 150 | 38 | 1 | 152 | 21 | 1 | |||
| Radiological | 2332 | 257 | 0.67 | 1013 | 157 | 0.65 | 1319 | 100 | 0.75 | |||
|
| ||||||||||||
| Family History | 0.02 | 0.03 | 0.21 | |||||||||
| No | 1614 | 180 | 1 | 713 | 109 | 1 | 901 | 71 | 1 | |||
| Yes | 1020 | 136 | 1.31 | 450 | 86 | 1.37 | 570 | 50 | 1.26 | |||
|
| ||||||||||||
| Necrosis | 0.006 | 0.004 | 0.85 | |||||||||
| Absent | 941 | 105 | 1 | 595 | 79 | 1 | 346 | 26 | 1 | |||
| Present | 1693 | 211 | 1.42 | 568 | 116 | 1.54 | 1125 | 95 | 1.04 | |||
|
| ||||||||||||
| Number of excisions | 0.01 | 0.002 | 0.52 | |||||||||
| ≤2 | 2433 | 284 | 1 | 1121 | 179 | 1 | 1312 | 105 | 1 | |||
| ≥3 | 201 | 32 | 1.64 | 42 | 16 | 2.33 | 159 | 16 | 1.19 | |||
|
| ||||||||||||
| Margin status | 0.01 | 0.0004 | 0.82 | |||||||||
| Close/positive | 522 | 81 | 1.39 | 202 | 50 | 1.81 | 320 | 31 | 0.95 | |||
| Negative | 2112 | 235 | 1 | 961 | 145 | 1 | 1151 | 90 | 1 | |||
|
| ||||||||||||
| Radiation therapy | <0.0001 | |||||||||||
| Yes | 1163 | 195 | 0.42 | NA | NA | |||||||
| No | 1471 | 121 | 1 | |||||||||
|
| ||||||||||||
| Endocrine therapy | <0.0001 | 0.006 | 0.001 | |||||||||
| Yes | 587 | 39 | 0.50 | 189 | 20 | 0.52 | 398 | 19 | 0.44 | |||
| No | 2047 | 277 | 1 | 974 | 175 | 1 | 1073 | 102 | 1 | |||
|
| ||||||||||||
| Year of surgery | 0.005 | 0.001 | 0.91 | |||||||||
| ≤1998 | 567 | 145 | 1 | 331 | 100 | 1 | 236 | 45 | 1 | |||
| ≥1999 | 2067 | 171 | 0.7 | 832 | 95 | 0.61 | 1235 | 76 | 0.98 | |||
After adjustment for 8 clinicopathologic and treatment variables, risk of recurrence remained significantly lower in older age groups, with decreasing HRs with increasing age category (HRs with <40y as reference: 40–49y [0.82, p=0.36], 50–59y [0.46, p=0.0005], 60–69y [0.50, p=0.003], 70–79y [0.56, p=0.02], ≥80y [0.21, p=0.0015]) (Table 2).
Separate multivariable analyses of the 1163 women treated without RT and the 1471 who received RT were performed. Age was significantly associated with recurrence risk in both groups, but the effect was most evident in women receiving radiation (Table 2).
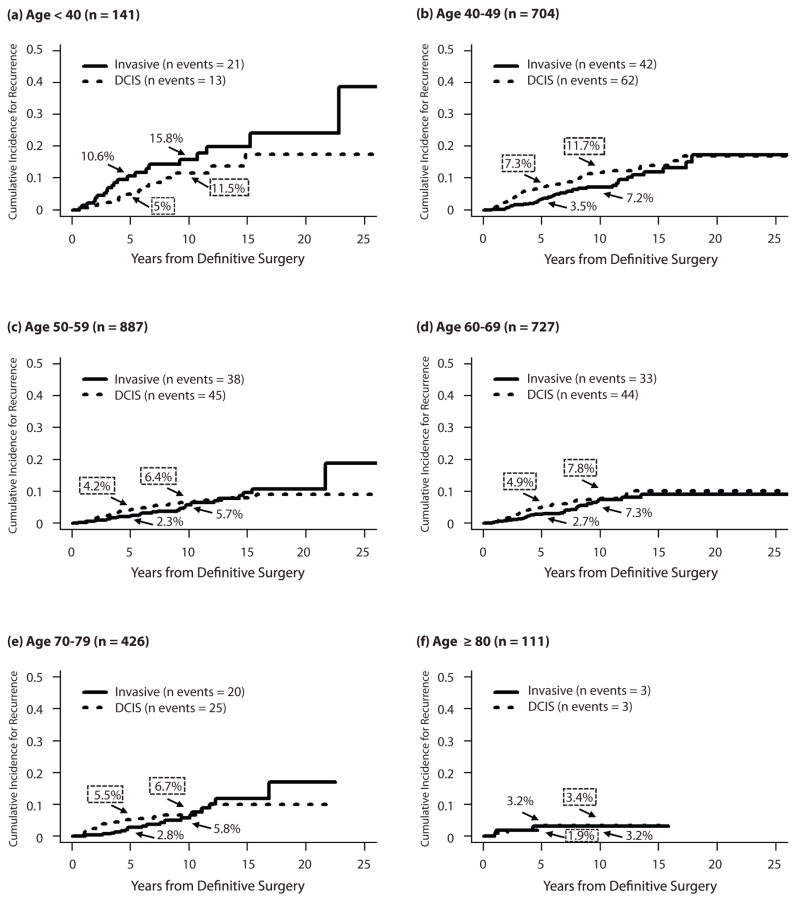
Invasive versus in situ recurrences
Because the implications of invasive and in situ recurrence differ, we examined them as competing risks according to age (Fig. 2). Women <40y were empirically at higher risk for invasive recurrence than DCIS recurrence (10-year invasive vs. DCIS risk: 15.8% vs. 11.5%). In contrast, in all other age groups the risk of DCIS recurrence was at least as high as the risk of invasive recurrence. 10-year risk estimates for invasive recurrence were 15.8% vs. 6.5% for those <40 vs. ≥40y.
Fig. 2.
Competing risk curves for invasive vs. DCIS recurrence by age, for women age (a) <40y, (b) 40–49y, (c) 50–59y, (d) 60–69y, (e) 70–79y, and (f) ≥80y.
We also built a competing risk multivariable model to adjust for other clinicopathologic factors which vary by age or are associated with recurrence (Table 3). The effect of age on risk of invasive recurrence was greater than the effect of age on overall recurrence or in situ recurrence. For both invasive and in situ recurrences, the use of radiation was associated with an approximate 50% reduction in the risk of recurrence (p≤0.00004).
TABLE 3.
Competing risk multivariable model for invasive versus in situ recurrence
| Invasive recurrence
|
In situ recurrence
|
|||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Age | ||||
| <40 | 1 | 1 | ||
| 40 – 49 | 0.48 | 0.01 | 1.33 | 0.4 |
| 50 – 59 | 0.33 | 0.0002 | 0.69 | 0.3 |
| 60 – 69 | 0.29 | 0.00008 | 0.88 | 0.7 |
| 70 – 79 | 0.36 | 0.003 | 0.81 | 0.6 |
| ≥80 | 0.14 | 0.01 | 0.24 | 0.06 |
|
| ||||
| Presentation | 0.8 | 0.02 | ||
| Clinical | 1.07 | 1.61 | ||
| Radiological | 1 | 1 | ||
|
| ||||
| Family History | 0.3 | 0.04 | ||
| No | 1 | 1 | ||
| Yes | 1.21 | 1.38 | ||
|
| ||||
| Necrosis | 0.2 | 0.02 | ||
| Absent | 1 | 1 | ||
| Present | 1.27 | 1.5 | ||
|
| ||||
| Number of excisions | 0.8 | 0.007 | ||
| ≤2 | 1 | 1 | ||
| ≥3 | 1.1 | 1.92 | ||
|
| ||||
| Margin status | 0.2 | 0.1 | ||
| Close/positive | 1.31 | 1.33 | ||
| Negative | 1 | 1 | ||
|
| ||||
| Radiation therapy | 0.00004 | <0.00001 | ||
| Yes | 0.43 | 0.45 | ||
| No | 1 | 1 | ||
|
| ||||
| Endocrine therapy | 0.02 | 0.003 | ||
| Yes | 0.53 | 0.49 | ||
| No | 1 | 1 | ||
|
| ||||
| Year of surgery | 0.9 | 0.0005 | ||
| ≤1998 | 1 | 1 | ||
| ≥1999 | 1.03 | 0.56 | ||
Age and risk of distant disease
19 (0.6%) patients ultimately developed distant disease (without contralateral invasive breast cancer). Women <40y accounted for 21% of these but constituted only 4.7% of our population. This suggests that young women may be at higher risk of developing distant disease, which is consistent with the finding that younger women were at particularly high risk for invasive recurrence. Yet, even among the youngest women <40y, only 2.8% developed distant metastases.
DISCUSSION
It has been recognized for almost two decades that age is associated with risk of recurrence for DCIS treated with BCS. In 1999, Van Zee et al. found that 6-year recurrence rates varied by age (47% [<40y], 14% [40–69y], 11% [≥70y], p=0.05), and that the differences persisted when stratified by radiation use.12 In 2000, Vicini et al. also reported that young age was a risk factor for recurrence among women who underwent BCS and RT; 10-year recurrence rates were 26% in women <45 vs. 8.6% in women ≥45y (p=0.03).13 Cutuli et al. found that young age was associated with higher 7-year recurrence rates, but only in those treated with RT (29% [≤40y], 13% [41–60y], 8% [≥61y], p<0.001).14
As results of the randomized studies of adjuvant RT or tamoxifen for DCIS became available, these findings were confirmed with prospective data. In 2001, Bijker et al. reported EORTC 10853 results at median follow-up of 5.4y; age ≤40 compared to >40 was associated with a two-fold increased risk of recurrence (HR=2.14, p=0.02) on multivariable analysis.16 With 15.7 years of follow-up, Donker et al. confirmed these findings with women ≤40y having a higher risk of recurrence than those >40y (HR=1.94, p=0.009).9 While NSABP B-17 found no difference in recurrence rates between age groups (≤49, 50–59, ≥60y) at a median follow-up of 7.5 years,17 in NSABP B-24 older women were found to have a lower risk of recurrence at a median follow-up of 6.2 years (relative risk for ≥50 vs. ≤49y = 0.43 [95% CI 0.31–0.59]).18 In a combined analysis of B-17 and B-24 with long-term follow-up (16.8 and 13.6 years, respectively), age was a significant risk factor for both invasive and DCIS recurrence.8 In that analysis, with ≥65y as the reference, younger women had a higher risk of invasive recurrence (HRs: 2.1 [<45y], 1.8 [45–54y], 1.5 [55–64y], p=0.003) and a higher risk of DCIS recurrence (HRs: 2.9 [<45y], 1.8 [45–54y], 1.7 [55–64y], p<0.001).
However, based on this literature it is challenging to fully understand the relationship of age and recurrence risk because age categories have been variably defined and the extremes of age are under-represented. Both retrospective and prospective randomized trials have used widely varying age categorization schemes. In the randomized trials, the youngest cohort was defined as ≤40 (similar to our dataset) in the EORTC 10853 trial9; in contrast it was defined as <52y in the SweDCIS study.7 The definition of the oldest age category has ranged from >40y to ≥65y.8, 9 In addition, screening patterns limited the evaluation of the extremes of age; the SweDCIS and UK/ANZ trials primarily recruited from a screen-detected population ≥50y. Overall, few younger women were included in the trials: SweDCIS, 35 women <40y; EORTC 10853, 51 women ≤40y; UK/ANZ, 160 women <50y; and NSABP B-17, 138 women <45y. There were also relatively few older patients included: UK/ANZ, 181 women ≥65y; NSABP B-17, 159 women ≥65y, SweDCIS, 180 women ≥67y, and EORTC 10853, only 11 women >70y.19
We sought to examine the relationship of age and recurrence across the full spectrum of age. Because of the large size of our series and the lack of exclusions, we were able to systematically describe recurrence rates by decade of age, thereby reflecting the actual age distribution of patients presenting with DCIS and undergoing BCS. Women <40 and ≥70y constituted 4.7% and 18% of our population, respectively.
Our data demonstrate not only that the risk of recurrence after BCS for women with DCIS significantly decreases with age, but that it appears to do so in a non-linear fashion. Those in the youngest group (<40y) are at particularly high risk, while the eldest (≥80y) are at particularly low risk. These findings persisted when the cohorts that did not and did receive radiation were analyzed separately.
Numerous clinicopathologic factors have been found to influence the risk of recurrence of DCIS and many of them vary by age, thereby potentially confounding the association of age and recurrence. Because our population is large, well-characterized, and has significant follow-up, we were able to adjust for many factors that vary with age at diagnosis, thereby enabling us to assess the independent effect of age on recurrence. The relationship between age and recurrence risk persisted after controlling for other factors with multivariable analysis.
The overview of the randomized trials of radiation for DCIS noted that RT resulted in a larger proportional reduction in risk for women ≥50y as compared to women <50y (HR 0.38 vs. 0.69, p=0.0004).6 Updated results from the SweDCIS and UK/ANZ trials confirmed that there are age-related differences in the responsiveness to RT with smaller proportional and absolute risk reduction from RT in younger women.7, 10 We found that after stratification for RT use, the hazard ratio for recurrence decreased with age in both RT and no RT cohorts, with the effect being more marked in the RT cohort; this observation is consistent with the finding of a greater benefit of RT with age.
The long-term outcome following an invasive recurrence is worse than after an in situ recurrence.7–9 Data from EORTC 10853, SweDCIS, and pooled data from NSABP B-17 and B-24 trials showed that young age was associated with higher risk for an invasive recurrence.7–9 In our population, recurrences in women <40y were more likely invasive than DCIS, in contrast to all other age groups in whom DCIS recurrences were at least as frequent as invasive recurrence (Fig. 2). Further, we found that the relationship between age and invasive recurrence risk was much stronger than that between age and DCIS recurrence. As compared to women with ages ≥80 or 50–79, women <40y had a 7-fold or 3-fold higher risk of developing an invasive recurrence, respectively.
Narod et al. recently reported SEER data showing that women with DCIS had a 3.3% 20-year breast cancer-specific mortality (including those who developed contralateral breast cancer), with women <35y having a higher risk of breast cancer-specific mortality than older women (HR=2.58, p<0.001).11 Our population also had an excellent distant-disease free survival, with only 19 (0.6%) women, without contralateral invasive cancer, ultimately developing distant metastasis. While the number of distant events in our population is too small to perform a formal analysis, our finding that women <40y are at particularly high risk for invasive recurrence and are over-represented among those who did ultimately develop distant disease (4 of 19), is consistent with the observation of higher mortality among young women with DCIS. It is important to note that there were only 3 cases (0.1%) of distant metastases in the absence of invasive local recurrence or contralateral invasive breast cancer, supporting the importance of maintaining local control in these patients.
The association between age and risk of recurrence should be incorporated into a thorough discussion with a woman regarding the various treatment options, including the relative importance to that individual woman of the various risks and benefits of each option. Given that there is no evidence of a survival advantage with or without RT, or with BCS or mastectomy, and given that the quality of life implications—both the psychological and the physical—of various treatment options will vary in different women, we believe that the optimal approach is to provide risk estimates that are as accurate as possible. We have previously developed a nomogram20 (available at www.nomograms.org), now validated in at least 4 different independent populations,21–24 that incorporates age and provides a risk estimate for an individual woman. A careful discussion with the patient can then help her weigh the risks and benefits and their relative value for her, so that she can make her best decision.
CONCLUSIONS
Risk of recurrence following BCS for DCIS decreases with age; this effect is particularly strong at the extremes of age, and persists even after controlling for other clinicopathologic and treatment factors. This finding remained true in cohorts that were treated with and without RT. The oldest women are at low risk of recurrence; the youngest have a higher overall and especially invasive recurrence rate, although mortality remains low. These findings should inform discussions in patients presenting with DCIS and be incorporated into risk/benefit considerations for surgical planning and adjuvant treatments.
Supplementary Material
Distribution of cases of DCIS treated with breast-conservation by age. The number and percentage of the population in each age group is shown.
Number and proportion of cases of DCIS by age and treatment, for women treated 1995–2010. Numbers overlying each section of the solid bars reflect the number of cases in each age group that underwent each treatment option. Vertical axis shows proportion of each age group that underwent each treatment option.
SYNOPSIS.
Following breast-conserving surgery for DCIS, women ≥80y are at low risk for any recurrence, while those <40y are at higher risk of recurrence, especially invasive recurrence, even after adjustment for other clinicopathologic variables and use of adjuvant therapies.
Acknowledgments
This study was presented as a podium presentation at the 69th Society of Surgical Oncology Annual Cancer Symposium, Boston, MA, March 2–5, 2016, and funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748. The authors have no conflicts of interest to declare.
Footnotes
DISCLOSURES: This study was presented as a podium presentation at the 69th Society of Surgical Oncology Annual Cancer Symposium, Boston, MA, March 2–5, 2016, and funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748. The authors have no conflicts of interest to declare.
References
- 1.Allison KH, Abraham LA, Weaver DL, et al. Trends in breast biopsy pathology diagnoses among women undergoing mammography in the United States: a report from the Breast Cancer Surveillance Consortium. Cancer. 2015;121(9):1369–78. doi: 10.1002/cncr.29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–7. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 4.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. 2010;17(10):2554–62. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 5.Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer. 1999;85(3):616–28. [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative G. Correa C, McGale P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–77. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS Trial. J Clin Oncol. 2014;32(32):3613–8. doi: 10.1200/JCO.2014.56.2595. [DOI] [PubMed] [Google Scholar]
- 8.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–88. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31(32):4054–9. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12(1):21–9. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1(7):888–96. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 12.Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999;86(9):1757–67. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Clin Oncol. 2000;18(2):296–306. doi: 10.1200/JCO.2000.18.2.296. [DOI] [PubMed] [Google Scholar]
- 14.Cutuli B, Cohen-Solal-le Nir C, de Lafontan B, et al. Breast-conserving therapy for ductal carcinoma in situ of the breast: the French Cancer Centers’ experience. Int J Radiat Oncol Biol Phys. 2002;53(4):868–79. doi: 10.1016/s0360-3016(02)02834-1. [DOI] [PubMed] [Google Scholar]
- 15.Subhedar P, Olcese C, Patil S, Morrow M, Van Zee KJ. Decreasing Recurrence Rates for Ductal Carcinoma In Situ: Analysis of 2996 Women Treated with Breast-Conserving Surgery Over 30 Years. Ann Surg Oncol. 2015;22(10):3273–81. doi: 10.1245/s10434-015-4740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19(8):2263–71. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16(2):441–52. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 19.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355(9203):528–33. doi: 10.1016/s0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 20.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010;28(23):3762–9. doi: 10.1200/JCO.2009.26.8847. [DOI] [PubMed] [Google Scholar]
- 21.Collins LC, Achacoso N, Haque R, et al. Risk Prediction for Local Breast Cancer Recurrence Among Women with DCIS Treated in a Community Practice: A Nested, Case-Control Study. Ann Surg Oncol. 2015;22(Suppl 3):502–8. doi: 10.1245/s10434-015-4641-x. [DOI] [PubMed] [Google Scholar]
- 22.Sweldens C, Peeters S, van Limbergen E, et al. Local relapse after breast-conserving therapy for ductal carcinoma in situ: a European single-center experience and external validation of the Memorial Sloan-Kettering Cancer Center DCIS nomogram. Cancer J. 2014;20(1):1–7. doi: 10.1097/PPO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Li H, Tan PH, et al. Validation of a nomogram in the prediction of local recurrence risks after conserving surgery for Asian women with ductal carcinoma in situ of the breast. Clin Oncol (R Coll Radiol) 2014;26(11):684–91. doi: 10.1016/j.clon.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Yi M, Meric-Bernstam F, Kuerer HM, et al. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30(6):600–7. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of cases of DCIS treated with breast-conservation by age. The number and percentage of the population in each age group is shown.
Number and proportion of cases of DCIS by age and treatment, for women treated 1995–2010. Numbers overlying each section of the solid bars reflect the number of cases in each age group that underwent each treatment option. Vertical axis shows proportion of each age group that underwent each treatment option.