Abstract
Nocardia species, particularly Nocardia brasiliensis, are etiologic agents of mycetoma, a chronic subcutaneous infection. Until now, little has been known about the pathogenic mechanisms involved in nocardial infection. Traditionally, subculture in rich media has been a simple way to induce attenuation. In this work, we report the changes in virulence toward mice and in genomic constitution of N. brasiliensis produced after 200 continuous subcultures in brain heart infusion (BHI) medium (P-200 strain). The ability of the N. brasiliensis P-200 strain to produce experimental infection was tested using BALB/c mice. P-200 was also used to immunize mice to determine whether it could induce resistance against a challenge with a nonsubcultured isolate (P-0). Comparative proteomic analysis between N. brasiliensis P-0 and P-200 was performed by two-dimensional (2-D) electrophoresis, and the genome sequence was obtained through Roche 454 sequence analysis. Virulence in BALB/c mice was completely lost, and BALB/c mice immunized with P-200 bacterial cells were resistant to mycetoma production by the nonsubcultured strain. Whole-genome sequence analysis revealed that P-200 lost a total of 262,913 bp distributed in 19 deleted regions, involving a total of 213 open reading frames (ORFs). The deleted genes included those encoding bacterial virulence factors, e.g., catalase, nitrate reductase enzymes, and a group of mammalian cell entry (MCE) family proteins, which may explain the loss of virulence of the isolate. Thus, completely attenuated N. brasiliensis was obtained after 200 passages in BHI medium, and putative Nocardia virulence genes were identified for the first time.
INTRODUCTION
Mycetoma is a chronic subcutaneous infection reported mostly in countries with tropical and subtropical weather (1); it is characterized by tumefaction of the anatomical site affected (particularly the upper and lower limbs), the production of subcutaneous abscesses, and fistulae. It is a deforming and stigmatizing disease caused by fungi or soil actinobacteria. The etiologic agent is inoculated through the skin via minor trauma with wood splinters or thorns contaminated with soil or organic matter (1). In Mexico, the most commonly isolated agents are Nocardia brasiliensis and Actinomadura madurae. The first agent causes approximately 70% of cases in the country and more than 90% of cases in the state of Nuevo Leon (1).
N. brasiliensis is a species of Gram-positive, partially acid-fast filamented bacilli that belongs to the Corynebacterineae suborder, a group of bacteria characterized by a type IV cell wall (which possesses an arabinogalactan cell wall polysaccharide) and by the presence of abundant mycolic acids and trehalose-derived lipids (2). Little is known about the pathogenic properties of Nocardia spp.; several molecules have been reported among the putative virulence factors, including superoxide dismutase (SOD) and catalase, enzymes that may decrease the ability of phagocytes to destroy bacteria by O2-derived mechanisms (3). An intact N. brasiliensis cell wall also appears to be important to avoid intracellular destruction either by polymorphonuclear leukocytes or by rabbit alveolar or human-derived THP-1 macrophages. The removal of its outer layer appears to decrease the virulence of N. brasiliensis (4).
Recently, the complete genome sequence of N. brasiliensis strain HUJEG-1 became available (5). Bioinformatic analysis revealed the presence of an extensive synthetic machinery for lipid compounds, nonribosomal protein synthases (NRPS), hydrolases, lipases, and proteases that might be important for nocardial virulence. In this work, we induced attenuation of N. brasiliensis HUJEG-1 by subculturing it 200 times in brain heart infusion (BHI) medium, and we determined putative virulence-associated genes by using whole-genome sequence analysis.
MATERIALS AND METHODS
Subculture method.
N. brasiliensis HUJEG-1 (ATCC 700358), which has been utilized in previous assays (6, 7), was used for these experiments. Bacterial cultures obtained from mouse lesions were kept frozen at −70°C in 20% skim milk and represented the parental strain (P-0). From these stocks, bacteria were grown on Sabouraud agar at 30°C for 4 to 7 days, and a single colony was then placed in a 7-ml sterile Eveljham-Potter device. We added 2.5 ml of sterile saline, ground the bacterial mass to obtain a homogeneous suspension, and adjusted the turbidity to a McFarland standard of 1. We inoculated a 125-ml Erlenmeyer flask containing 33 ml of previously sterilized liquid BHI medium with 0.1 ml of this suspension. We then incubated the culture with constant agitation at 110 rpm and 37°C. After 72 h, the bacterial mass was harvested by centrifugation at 2,500 rpm for 3 min, washed, and ground as described above. A new Erlenmeyer flask was inoculated with 0.1 ml of this suspension. These steps were repeated until 200 subcultures (P-200) had been reached. Samples were taken every 10 passages (including P-0) and kept frozen at −70°C. The entire process took approximately 6 years to complete.
Experimental mycetoma in a murine model.
Cultures were obtained from aliquots of the P-200 and P-0 strains, which were stored in a deep freezer. The inoculums were prepared using a previously published technique (8) and adjusted to 20 mg (wet weight) of N. brasiliensis in 50 μl of saline solution. Female 8- to 12-week-old BALB/c mice were injected with 50 μl of nocardial suspension in the right footpad, and the development of lesions was scored from 0 (no inflammatory changes) to 4+ (extension of the lesions beyond the ankle of the animal, with extensive production of inflammation and abscesses), as previously described (8). The thickness of each lesion was measured with calipers every week for 12 weeks.
The study was approved by the Comité Local de Investigación en Salud 1906, Centro de Investigación Biomédica del Noreste, IMSS. The animal handling was performed according to the NORMA Oficial Mexicana NOM-062-ZOO-1999 (Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio [Technical specifications for the production, care and handling of laboratory animals]).
Induction of infection resistance in a murine model.
To examine whether infection with subcultured N. brasiliensis produced a state of immune resistance, a group of animals (n = 20) were inoculated in the right footpad with N. brasiliensis that had been subcultured 200 times (P-200). After 12 weeks, the left footpad was inoculated with nonsubcultured bacteria (P-0). As a control, we inoculated a group of animals of the same age (n = 20) with the nonsubcultured isolate in the right footpad. In all cases, the development of lesions was scored and measured as described above.
Proteomic analysis.
Both N. brasiliensis strains (P-0 and P-200) were cultured on RPMI 1640 medium with agitation for 2 weeks at 37°C and 110 rpm. The supernatant or culture filtrate protein (CFP) was collected by centrifugation and concentrated by lyophilization. To obtain the intracellular proteins, a 3-day culture in BHI medium was disrupted with a fast-prep system using zirconia beads. The debris was eliminated by centrifugation, and the supernatant was dialyzed against phosphate-buffered saline (PBS). In both cases, the proteins were quantified by the Bradford method. Approximately 100 μg of each antigen was analyzed by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (2-D SDS-PAGE). We initially used a pH range of 1 to 10 and later narrowed it to 4 to 7. The gels were stained with Coomassie blue R-250. Spots missing among the P-200 antigens were cut out of the P-0 gels by use of a scalpel, placed in an Eppendorf tube, covered with 100 μl of MilliQ water, and sent to Applied Biomics (Hayward, CA) for amino acid sequencing. The resulting sequences were analyzed using the BLAST program at the NCBI website.
Whole-genome sequencing.
A suspension of N. brasiliensis HUJEG-1 subjected to 200 passages in BHI (P-200) was plated on a BHI agar plate to obtain separated colonies. After incubation for 6 days at 37°C, a colony was picked, a suspension in saline was prepared, and the procedure was repeated 4 more times to try to avoid DNA heterogeneity in the sample. The DNA was extracted from the last clone and subjected to mass sequencing using a Roche/454 GS (FLX Titanium) sequencing platform (8-kb library). The Roche/454 GS reads were assembled using Newbler 2.5.3 software (Roche Diagnostics, Branford, CT). The obtained contigs were compared to those already published for P-0 (GenBank accession number NC_018681.1) by using Sequencher software (Gene Codes, CA), and the presence of genetic changes, single nucleotide polymorphisms (SNPs), deletions, and duplications was scored.
Accession number(s).
The data from this whole-genome shotgun project have been deposited at DDBJ/EMBL/GenBank under accession number LRRM00000000. The version described in this paper is version LRRM01000000.
RESULTS
Biological changes produced by continuous passaging.
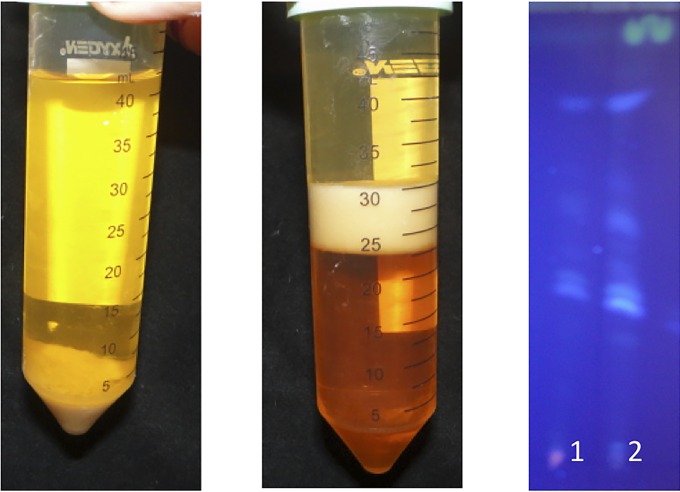
Bacteria were subcultured every 48 to 72 h, with constant agitation, in BHI medium; after 200 passages, changes in the macroscopic morphology were observed (Fig. 1). Instead of the bacteria growing as a tight cumulus of entangled filaments, a more disperse suspension was observed. Additionally, when the bacteria were stained with Kinyoun stain, P-200 cells showed lighter staining than P-0 cells, and when the nocardial mass was suspended in chloroform-methanol, differences in cell density were observed (CHCl3 density = 1.48 g/ml; CH3OH density = 0.791 g/ml). For P-0, the bacterial mass was observed at the bottom after centrifugation; in contrast, the P-200 culture separated the two solvents after centrifugation because it possessed an intermediate density (Fig. 2). Thin-layer chromatography (TLC) analysis of the chloroform-methanol extracts of P-200 and P-0 showed a marked decrease in the acyl-glycerol fraction in P-0 (Fig. 2).
FIG 1.
Biological changes observed in N. brasiliensis after 200 passages in BHI medium. (Left) Growth of P-0 in BHI medium as small microcolonies of bacteria. (Middle) After 200 passages, the isolate grew as a homogenous suspension. (Right) Kinyoun staining of P-200, showing a negative reaction.
FIG 2.
Changes in cell wall composition of N. brasiliensis P-200. (Left) Suspension of P-0 in chloroform-methanol. The bacterial cell mass stayed in the bottom of the tube. (Middle) Suspension of P-200 in the same solvent mix. In this case, the bacterial mass was located between both solvents. (Right) TLC analysis of chloroform-methanol extracts of P-0 (lane 1) and P-200 (lane 2).
Virulence of N. brasiliensis P-200.
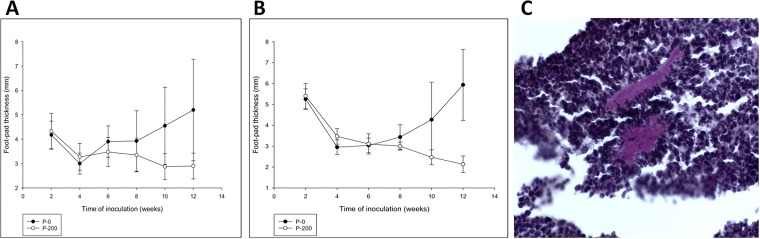
Virulence was tested in BALB/c mice. When mice were injected with the parental strain, P-0, 90% of the animals developed lesions after 12 weeks of infection (Fig. 3A). In contrast, only 10% of the animals infected with P-200 developed small lesions at this time, and after 2 weeks, the lesions completely disappeared. Fifteen animals from the P-200 strain-infected group, with absolutely no lesions, were challenged in the contralateral footpad with P-0 (Fig. 3B). Fifteen weeks after inoculation, all mice, except one which developed a 1+ lesion, presented no lesions (not shown). We analyzed the lesions histologically 5 weeks after infection with P-0, and we observed the presence of a strong mononuclear infiltrate in the lesions, along with the presence of granules in several stages of apparent destruction (Fig. 3C).
FIG 3.
Virulence assay of P-0 and P-200 in BALB/c mice. (A) Female animals were inoculated with either P-0 or P-200, and the footpad thickness was measured every week. (B) Twelve weeks later, the animals inoculated with P-200 were challenged with P-0. As a control, a naive group of animals of the same age were inoculated with P-0. (C) Histological findings in a footpad biopsy specimen from a mouse inoculated with P-200 and reinoculated with P-0, showing polymorphonuclear leukocytes destroying the Nocardia granules.
Genomic mass sequencing of P-200.
The genome sequence of the P-200 strain was determined using a Roche/454 GS (FLX Titanium) sequencing platform (8-kb library). A total of 44,023 reads were obtained, providing approximately 24× genome coverage. The Roche/454 GS reads were assembled into 70 scaffolds by use of Newbler 2.5.3 software (Roche Diagnostics, Branford, CT).
Comparing the sequences of the N. brasiliensis HUJEG-1 P-0 (accession number NC_018681.1) and P-200 scaffolds revealed the presence of 17 deleted regions (Table 1), with sizes ranging from 8 to 216,368 bp, for a total of 262,913 bp affecting 213 genes, including 107 exclusive to N. brasiliensis.
TABLE 1.
Deleted regions in N. brasiliensis P-200a
| Region no. | Deleted region |
Gene | Protein | Corresponding region in reference sequence |
Organism(s) with ortholog | NCBI COG | ||
|---|---|---|---|---|---|---|---|---|
| Start position | End position | Start position | End position | |||||
| 1 | 541011 | 541445 | 03I_002340 | Clp protease | 540929 | 543247 | Nocardia cyriacigeorgica, Nocardia farcinica, Rhodococcus jostii | COG0542 |
| 2 | 1449785 | 1450050 | Intergenic region | |||||
| 3 | 1453274 | 1453310 | Intergenic region | |||||
| 4 | 1501900 | 1533608 | 03I_006545 | Putative drug resistance transporter | 1500162 | 1502150 | N. cyriacigeorgica, N. farcinica | COG0477 |
| 03I_006550 | PadR family transcriptional regulator | 1502234 | 1502887 | N. cyriacigeorgica, N. farcinica | COG1695 | |||
| 03I_006555 | Monooxygenase, FAD-binding protein | 1503068 | 1504273 | N. cyriacigeorgica, N. farcinica | COG0654 | |||
| 03I_006560 | ATP-binding transport protein NatA | 1504341 | 1505108 | N. brasiliensis | COG1131 | |||
| 03I_006565 | Hypothetical protein | 1505093 | 1506658 | N. brasiliensis | ||||
| 03I_006570 | LysR family transcriptional regulator | 1506719 | 1507639 | N. brasiliensis | COG0583 | |||
| 03I_006575 | XRE family transcriptional regulator | 1507644 | 1508447 | N. cyriacigeorgica, R. jostii | COG1396 | |||
| 03I_006580 | Hypothetical protein | 1508578 | 1509024 | N. cyriacigeorgica | ||||
| 03I_006585 | Hypothetical protein | 1509060 | 1509791 | N. cyriacigeorgica, R. jostii | ||||
| 03I_006590 | Haloalkane dehalogenase | 1510380 | 1511252 | N. cyriacigeorgica | COG0596 | |||
| 03I_006595 | Putative RNA polymerase ECF-type sigma factor | 1511337 | 1512200 | N. cyriacigeorgica | COG1595 | |||
| 03I_006600 | C4-dicarboxylate transporter/malic acid transport protein | 1512157 | 1513347 | N. cyriacigeorgica, R. jostii | COG1275 | |||
| 03I_006605 | LysR family transcriptional regulator | 1513418 | 1514326 | N. cyriacigeorgica, R. jostii | COG0583 | |||
| 03I_006610 | Phosphate uptake regulator PhoU | 1514345 | 1515028 | N. cyriacigeorgica, N. farcinica | COG0704 | |||
| 03I_006615 | Transglutaminase domain-containing protein | 1515153 | 1515956 | R. jostii | COG1305 | |||
| 03I_006620 | Na-Ca exchanger/integrin-beta4 | 1515963 | 1516991 | N. brasiliensis | ||||
| 03I_006625 | Pyridoxamine 5′-phosphate oxidase-related FMN-binding protein | 1518688 | 1519095 | N. cyriacigeorgica | COG3467 | |||
| 03I_006630 | Transporter | 1519200 | 1520300 | N. farcinica, R. jostii | COG0387 | |||
| 03I_006635 | YrbE family protein | 1520752 | 1520752 | N. cyriacigeorgica, N. farcinica | COG0767 | |||
| 03I_006640 | YrbE family protein | 1521601 | 1522467 | N. cyriacigeorgica, N. farcinica | COG0767 | |||
| 03I_006645 | Mce family protein | 1522498 | 1522498 | N. cyriacigeorgica, N. farcinica | COG3008 | |||
| 03I_006650 | Mce family protein | 1523496 | 1524497 | N. cyriacigeorgica, N. farcinica | COG1463 | |||
| 03I_006655 | Mce family protein | 1524485 | 1525519 | N. cyriacigeorgica, N. farcinica | COG1463 | |||
| 03I_006660 | Mce family protein | 1525489 | 1526604 | N. cyriacigeorgica, N. farcinica | COG1463 | |||
| 03I_006665 | Mce family protein | 1526601 | 1526601 | N. cyriacigeorgica, N. farcinica | COG0282 | |||
| 03I_006670 | Mce family protein | 1527728 | 1527728 | N. cyriacigeorgica, N. farcinica | COG1463 | |||
| 03I_006675 | Hypothetical protein | 1528699 | 1529298 | N. cyriacigeorgica, N. farcinica | ||||
| 03I_006680 | Hypothetical protein | 1529433 | 1529433 | N. brasiliensis | ||||
| 03I_006685 | Aminoglycoside O-phosphotransferase | 1530067 | 1530951 | N. cyriacigeorgica, N. farcinica, R. jostii | COG3570 | |||
| 03I_006690 | Short-chain dehydrogenase/reductase SDR | 1530978 | 1530978 | N. brasiliensis | COG1028 | |||
| 03I_006695 | Signal transduction histidine kinase | 1532065 | 1533246 | N. brasiliensis | COG4585 | |||
| 03I_006700 | Two-component LuxR family transcriptional regulator | 1533243 | 1533890 | N. brasiliensis | COG2197 | |||
| 5 | 2271950 | 2277016 | 03I_Or42277 | 16S rRNA | 2271959 | 2273488 | ||
| 6 | 2372465 | 2372842 | 03I_010455 | Glyoxylate carboligase | 2372219 | 2373994 | COG3960 | |
| 7 | 2578134 | 2578295 | Intergenic region | |||||
| 8 | 2933006 | 2933045 | Intergenic region | |||||
| 9 | 3597423 | 3597782 | 03I_016110 | DNA-directed RNA polymerase subunit beta (RpoB) | 3596307 | 3599822 | N. cyriacigeorgica, N. farcinica, R. jostii | |
| 10 | 3901876 | 3903739 | 03I_Or42279 | 16S rRNA | 3902217 | 3903746 | ||
| 11 | 3904013 | 3907228 | Intergenic region | |||||
| 12 | 4429801 | 4429809 | Intergenic region | |||||
| 13 | 4795932 | 5012300 | 03I_021225 | Gamma-glutamyltransferase | 4795342 | 4797141 | N. brasiliensis | COG0405 |
| 03I_021230 | Methyltransferase | 4797178 | 4798242 | N. brasiliensis | COG0500 | |||
| 03I_021235 | Cytochrome P-450 | 4798239 | 4799441 | R. jostii | COG2124 | |||
| 03I_021240 | Alcohol dehydrogenase GroES domain-containing protein | 4799438 | 4800493 | N. brasiliensis | COG1064 | |||
| 03I_021245 | Aldehyde dehydrogenase | 4800513 | 4802000 | N. farcinica, R. jostii | COG1012 | |||
| 03I_021250 | Putative amidotransferase | 4802029 | 4803891 | R. jostii | COG0367 | |||
| 03I_021255 | ABC transporter | 4803936 | 4806227 | N. cyriacigeorgica, N. farcinica, R. jostii | COG0178 | |||
| 03I_021260 | Short-chain fatty acid MFS superfamily protein | 4806415 | 4807854 | R. jostii | COG2031 | |||
| 03I_021265 | Hypothetical protein | 4807919 | 4808521 | N. brasiliensis | ||||
| 03I_021270 | Hypothetical protein | 4808582 | 4809127 | N. farcinica | ||||
| 03I_021275 | Hypothetical protein | 4809244 | 4810527 | N. brasiliensis | ||||
| 03I_021280 | Hypothetical protein | 4810655 | 4811164 | N. farcinica | COG2259 | |||
| 03I_021285 | Hypothetical protein | 4811244 | 4812002 | N. farcinica, R. jostii | COG3384 | |||
| 03I_021290 | MarR family transcriptional regulator | 4812093 | 4812554 | N. farcinica, R. jostii | COG1846 | |||
| 03I_021295 | NADP-dependent oxidoreductase domain-containing protein | 4812632 | 4813675 | R. jostii | COG0667 | |||
| 03I_021300 | Abortive infection protein | 4813887 | 4814675 | N. brasiliensis | COG1266 | |||
| 03I_021305 | Dioxygenase | 4814780 | 4815688 | N. farcinica | COG2175 | |||
| 03I_021310 | Transporter | 4815883 | 4816527 | N. farcinica | COG1174 | |||
| 03I_021315 | ABC transporter ATP-binding protein | 4816524 | 4817804 | N. farcinica | COG1125 | |||
| 03I_021320 | ABC transporter permease | 4817801 | 4818538 | N. farcinica | COG1174 | |||
| 03I_021325 | Transporter permease | 4818535 | 4819515 | N. farcinica | COG1732 | |||
| 03I_021330 | Hypothetical protein | 4819550 | 4820365 | N. farcinica, R. jostii | ||||
| 03I_021335 | Hypothetical protein | 4820488 | 4821048 | N. brasiliensis | ||||
| 03I_021340 | Nonribosomal peptide synthetase | 4821049 | 4841793 | N. cyriacigeorgica, N. farcinica, R. jostii | COG1020 | |||
| 03I_021345 | Hypothetical protein | 4841793 | 4857035 | N. cyriacigeorgica, N. farcinica, R. jostii | COG1020 | |||
| 03I_021350 | SARP family transcriptional regulator | 4857537 | 4860785 | N. brasiliensis | COG0745 | |||
| 03I_021355 | Hypothetical protein | 4860727 | 4861287 | N. brasiliensis | ||||
| 03I_021360 | Hypothetical protein | 4861313 | 4862404 | N. brasiliensis | COG0451 | |||
| 03I_021365 | Nonribosomal peptide synthetase | 4862392 | 4863786 | N. brasiliensis | COG1020 | |||
| 03I_021370 | Acetylornithine deacetylase or succinyl-diaminopimelate desuccinylase | 4863783 | 4864988 | R. jostii | COG0624 | |||
| 03I_021375 | UbiE/COQ5 family methyltransferase | 4865045 | 4865869 | N. brasiliensis | COG0500 | |||
| 03I_021380 | Alcohol dehydrogenase | 4866058 | 4867254 | N. farcinica, R. jostii | COG1454 | |||
| 03I_021385 | Sensor histidine kinase | 4867244 | 4868566 | N. brasiliensis | ||||
| 03I_021390 | d-3-Phosphoglycerate dehydrogenase | 4868598 | 4869608 | R. jostii | COG0111 | |||
| 03I_021395 | Phosphoenolpyruvate phosphomutase | 4869662 | 4870588 | N. brasiliensis | COG2513 | |||
| 03I_021400 | Phosphonopyruvate decarboxylase | 4870585 | 4871736 | N. brasiliensis | COG4032 | |||
| 03I_021405 | Aldehyde dehydrogenase | 4871726 | 4873117 | N. brasiliensis | COG1012 | |||
| 03I_021410 | Short-chain dehydrogenase/reductase SDR | 4873151 | 4873954 | N. brasiliensis | COG1028 | |||
| 03I_021415 | Polyketide synthase | 4873956 | 4874171 | N. brasiliensis | COG3321 | |||
| 03I_021420 | AMP-dependent synthetase and ligase | 4874171 | 4875613 | N. brasiliensis | COG0318 | |||
| 03I_021425 | Class III aminotransferase | 4875616 | 4876941 | N. brasiliensis | COG0160 | |||
| 03I_021430 | Hypothetical protein | 4876976 | 4877728 | N. brasiliensis | COG0842 | |||
| 03I_021435 | ABC transporter-like protein | 4877725 | 4878678 | N. brasiliensis | COG1131 | |||
| 03I_021440 | Penicillin-binding protein | 4878669 | 4879817 | N. brasiliensis, R. jostii | COG1680 | |||
| 03I_021445 | Pantoate–beta-alanine ligase PanC | 4879877 | 4880716 | N. brasiliensis | COG0414 | |||
| 03I_021450 | LuxR family transcriptional regulator | 4880906 | 4881586 | N. brasiliensis | COG2197 | |||
| 03I_021455 | Integral membrane sensor signal transduction histidine kinase | 4881576 | 4882817 | N. brasiliensis | COG4585 | |||
| 03I_021460 | Hypothetical protein | 4882983 | 4883552 | N. brasiliensis | ||||
| 03I_021465 | Hypothetical protein | 4883543 | 4883869 | N. brasiliensis | ||||
| 03I_021470 | Hypothetical protein | 4883931 | 4884668 | N. brasiliensis, N. farcinica, R. jostii | COG3393 | |||
| 03I_021475 | LuxR family transcriptional regulator | 4884766 | 4887378 | N. brasiliensis, R. jostii | COG1066 | |||
| 03I_021480 | Hypothetical protein | 4887544 | 4887990 | N. brasiliensis, R. jostii | ||||
| 03I_021485 | Hypothetical protein | 4888075 | 4888605 | N. brasiliensis | ||||
| 03I_021490 | Hypothetical protein | 4888830 | 4889687 | N. brasiliensis, N. farcinica | COG0693 | |||
| 03I_021495 | HTH-type transcriptional regulator GlxA | 4889733 | 4890743 | N. brasiliensis, N. cyriacigeorgica | COG4977 | |||
| 03I_021500 | Exonuclease SbcC | 4890883 | 4891464 | N. brasiliensis, N. farcinica, R. jostii | ||||
| 03I_021505 | Helix-turn-helix domain-containing protein | 4891566 | 4892396 | N. brasiliensis, N. cyriacigeorgica | COG2207 | |||
| 03I_021510 | Hypothetical protein | 4892421 | 4892924 | N. brasiliensis, N. cyriacigeorgica | ||||
| 03I_021515 | Hypothetical protein | 4893061 | 4893711 | N. brasiliensis | ||||
| 03I_021520 | Putative transcriptional regulator TetR | 4893718 | 4894305 | N. brasiliensis, N. farcinica | COG1309 | |||
| 03I_021525 | Phytanoyl-coenzyme A (CoA) dioxygenase (PhyH) family protein | 4894403 | 4895257 | N. brasiliensis, N. farcinica, R. jostii | COG5285 | |||
| 03I_021530 | Hypothetical protein | 4895383 | 4896189 | N. brasiliensis | ||||
| 03I_021535 | Peptidase M14 carboxypeptidase | 4896301 | 4897695 | N. brasiliensis | COG2866 | |||
| 03I_021540 | Glutamate-cysteine ligase GCS2 | 4897981 | 4899078 | N. brasiliensis | COG2170 | |||
| 03I_021545 | Hypothetical protein | 4899169 | 4899396 | N. brasiliensis | ||||
| 03I_021550 | Short-chain dehydrogenase | 4899479 | 4900318 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG1028 | |||
| 03I_021555 | FAD-binding monooxygenase | 4900447 | 4902174 | N. brasiliensis | ||||
| 03I_021560 | Luciferase | 4902171 | 4903256 | N. brasiliensis | COG2141 | |||
| 03I_021565 | Pyridoxal-5′-phosphate-dependent protein subunit beta | 4903272 | 4904462 | N. brasiliensis | COG0498 | |||
| 03I_021570 | Opine dehydrogenase | 4904459 | 4905574 | N. brasiliensis, N. farcinica | ||||
| 03I_021575 | Acyl-CoA reductase | 4905577 | 4907022 | N. brasiliensis | ||||
| 03I_021580 | Hypothetical protein | 4907010 | 4908308 | N. brasiliensis | ||||
| 03I_021585 | Ferredoxin | 4908610 | 4908825 | N. brasiliensis | COG1141 | |||
| 03I_021590 | LuxE bioluminescence protein | 4908850 | 4909983 | N. brasiliensis | COG1541 | |||
| 03I_021595 | Acyltransferase LuxD | 4910022 | 4910987 | N. brasiliensis | ||||
| 03I_021600 | Sodium/hydrogen exchanger | 4910945 | 4912375 | N. brasiliensis | COG0475 | |||
| 03I_021605 | Fluorinating enzyme | 4912446 | 4913309 | N. brasiliensis | COG1912 | |||
| 03I_021610 | S-Adenosyl-l-homocysteine hydrolase | 4913437 | 4914891 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG0499 | |||
| 03I_021615 | Methylthioadenosine phosphorylase | 4915122 | 4916000 | N. brasiliensis | COG0005 | |||
| 03I_021620 | Adenine phosphoribosyltransferase | 4916036 | 4916611 | N. brasiliensis | COG0503 | |||
| 03I_021625 | Translation initiation factor, aIF-2BI family protein | 4916581 | 4917585 | N. brasiliensis | COG0182 | |||
| 03I_021630 | Serine hydroxymethyltransferase GlyA | 4917783 | 4919630 | N. brasiliensis, R. jostii | COG0112 | |||
| 03I_021635 | DNA-binding regulatory protein | 4919638 | 4920213 | N. brasiliensis | COG1396 | |||
| 03I_021640 | Hypothetical protein | 4920272 | 4921222 | N. brasiliensis | COG0697 | |||
| 03I_021645 | Two-component system response regulator | 4921827 | 4922435 | N. brasiliensis | COG2197 | |||
| 03I_021650 | Universal stress protein UspA-like protein | 4922436 | 4922879 | N. brasiliensis | COG0589 | |||
| 03I_021655 | Hypothetical protein | 4922918 | 4923145 | N. brasiliensis | ||||
| 03I_021660 | Hypothetical protein | 4923186 | 4923701 | N. brasiliensis | COG2606 | |||
| 03I_021665 | OsmC-like protein | 4923894 | 4924364 | N. brasiliensis, R. jostii | COG1764 | |||
| 03I_021670 | Peptidase M15A | 4924375 | 4926096 | N. brasiliensis | COG3108 | |||
| 03I_021675 | Peptidase M15A | 4926115 | 4928226 | N. brasiliensis | ||||
| 03I_021680 | Hypothetical protein | 4928280 | 4929614 | N. brasiliensis | ||||
| 03I_021685 | Hypothetical protein | 4929628 | 4930167 | N. brasiliensis | ||||
| 03I_021690 | Hypothetical protein | 4930190 | 4931440 | N. brasiliensis | ||||
| 03I_021695 | Hypothetical protein | 4931440 | 4933926 | N. brasiliensis | ||||
| 03I_021700 | Hypothetical protein | 4933923 | 4934150 | N. brasiliensis | ||||
| 03I_021705 | SARP family transcriptional regulator | 4934354 | 4935067 | N. brasiliensis | COG3629 | |||
| 03I_021710 | Hypothetical protein | 4934996 | 4936714 | N. brasiliensis | ||||
| 03I_021715 | SARP family transcriptional regulator | 4936750 | 4937550 | N. brasiliensis | COG3629 | |||
| 03I_021720 | Alpha/beta-fold hydrolase | 4937701 | 4938486 | N. brasiliensis | COG0596 | |||
| 03I_021725 | Oxidoreductase | 4938581 | 4939534 | N. brasiliensis | COG0604 | |||
| 03I_021730 | TetR family transcriptional regulator | 4939638 | 4940246 | N. brasiliensis | COG1309 | |||
| 03I_021735 | FAD-linked oxidase domain-containing protein | 4940308 | 4941654 | N. brasiliensis | COG0277 | |||
| 03I_021740 | Hypothetical protein | 4941757 | 4942299 | N. brasiliensis, N. cyriacigeorgica, R. jostii | COG5485 | |||
| 03I_021745 | 4-Carboxymuconolactone decarboxylase | 4942296 | 4942679 | N. brasiliensis | COG0599 | |||
| 03I_021750 | 3-Oxoadipate enol-lactonase | 4942676 | 4943452 | N. brasiliensis, R. jostii | COG0596 | |||
| 03I_021755 | 3-Carboxy-cis,cis-muconate cycloisomerase | 4943449 | 4944795 | N. brasiliensis, R. jostii | COG0015 | |||
| 03I_021760 | Protocatechuate 3,4-dioxygenase alpha subunit | 4944788 | 4945312 | N. brasiliensis, R. jostii | COG3485 | |||
| 03I_021765 | Protocatechuate 3,4-dioxygenase beta subunit | 4945305 | 4946045 | N. brasiliensis, R. jostii | COG3485 | |||
| 03I_021780 | CoA transferase B subunit | 4947234 | 4948013 | N. brasiliensis, N. farcinica, R. jostii | COG2057 | |||
| 03I_021785 | CoA transferase A subunit | 4948010 | 4948825 | N. brasiliensis, N. farcinica, R. jostii | COG1788 | |||
| 03I_021790 | 4-Hydroxybenzoate 3-monooxygenase | 4948828 | 4950030 | N. brasiliensis, R. jostii | COG0654 | |||
| 03I_021795 | Transmembrane transport protein | 4950027 | 4951259 | N. brasiliensis, R. jostii | COG0477 | |||
| 03I_021800 | LysR family transcriptional regulator | 4951362 | 4952264 | N. brasiliensis, R. jostii | COG0583 | |||
| 03I_021805 | Glycosidase | 4952276 | 4954621 | N. brasiliensis, R. jostii | COG1554 | |||
| 03I_021810 | Hydrolase | 4954618 | 4955349 | N. brasiliensis, R. jostii | COG0637 | |||
| 03I_021825 | Hypothetical protein | 4956335 | 4957054 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | COG2129 | |||
| 03I_021830 | DsbA oxidoreductase | 4957051 | 4957644 | N. brasiliensis | ||||
| 03I_021835 | Transcriptional regulator | 4957793 | 4958872 | N. brasiliensis | COG2207 | |||
| 03I_021840 | Cell surface protein | 4959086 | 4959961 | N. brasiliensis | ||||
| 03I_021845 | Hypothetical protein | 4960040 | 4960426 | N. brasiliensis | ||||
| 03I_021850 | Putative cysteine synthase | 4960527 | 4961639 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | COG0031 | |||
| 03I_021855 | Putative MFS transporter | 4961636 | 4962907 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | COG0477 | |||
| 03I_021860 | Amino acid binding protein | 4962998 | 4964092 | N. brasiliensis, R. jostii | ||||
| 03I_021865 | Sigma factor | 4964772 | 4965512 | N. brasiliensis | COG1191 | |||
| 03I_021870 | Hypothetical protein | 4965603 | 4965887 | N. brasiliensis | ||||
| 03I_021875 | AraC family transcriptional regulator | 4965976 | 4966569 | N. brasiliensis | COG2207 | |||
| 03I_021880 | Hypothetical protein | 4966735 | 4966926 | N. brasiliensis | ||||
| 03I_021885 | 5,10-Methylenetetrahydrofolate reductase | 4967383 | 4968366 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG0685 | |||
| 03I_021890 | Hypothetical protein | 4968457 | 4968840 | N. brasiliensis, N. farcinica, R. jostii | ||||
| 03I_021895 | Glyoxalase/bleomycin resistance protein/dioxygenase | 4968853 | 4969248 | N. brasiliensis | COG0346 | |||
| 03I_021900 | Thimet oligopeptidase | 4969268 | 4971199 | N. brasiliensis | COG0339 | |||
| 03I_021905 | PadR-like family transcriptional regulator | 4971239 | 4971832 | N. brasiliensis | COG1695 | |||
| 03I_021910 | Ferredoxin | 4971845 | 4973587 | N. brasiliensis, R. jostii | COG1018 | |||
| 03I_021915 | Hypothetical protein | 4973826 | 4974689 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | COG0596 | |||
| 03I_021920 | Hypothetical protein | 4974798 | 4975796 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | ||||
| 03I_021925 | Methyltransferase | 4975793 | 4976491 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG2890 | |||
| 03I_021930 | Hypothetical protein | 4976529 | 4976678 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | COG3369 | |||
| 03I_021935 | Oxidoreductase | 4976757 | 4978205 | N. brasiliensis, N. cyriacigeorgica | COG0665 | |||
| 03I_021940 | Hypothetical protein | 4978223 | 4978432 | N. brasiliensis | ||||
| 03I_021945 | Catalase | 4978659 | 4980830 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG0753 | |||
| 03I_021950 | Cytochrome P450 monooxygenase | 4980837 | 4982192 | N. brasiliensis | COG2124 | |||
| 03I_021955 | DNA ligase | 4982302 | 4984293 | N. brasiliensis, N. farcinica | COG0272 | |||
| 03I_021960 | Propionyl-CoA carboxylase beta chain (PCCase) (propanoyl-CoA:carbon dioxide ligase) | 4984482 | 4986032 | N. brasiliensis, N. cyriacigeorgica, N. farcinica, R. jostii | COG4799 | |||
| 03I_021965 | Hypothetical protein | 4986188 | 4986607 | N. brasiliensis | ||||
| 03I_021970 | Hypothetical protein | 4986612 | 4987046 | N. brasiliensis | ||||
| 03I_021975 | Serine/threonine protein kinase | 4987060 | 4988448 | N. brasiliensis | COG0515 | |||
| 03I_021980 | Hypothetical protein | 4988845 | 4990197 | N. brasiliensis, R. jostii | ||||
| 03I_021985 | Type 11 methyltransferase | 4990248 | 4991027 | N. brasiliensis | COG0500 | |||
| 03I_021990 | Hypothetical protein | 4991125 | 4991433 | N. brasiliensis | ||||
| 03I_021995 | Hypothetical protein | 4991467 | 4993941 | N. brasiliensis, R. jostii | ||||
| 03I_022000 | DNA-binding protein | 4994209 | 4995144 | N. brasiliensis | COG1396 | |||
| 03I_022005 | Monooxygenase FAD-binding protein | 4995520 | 4997040 | N. brasiliensis | COG0654 | |||
| 03I_022010 | Hypothetical protein | 4997078 | 4997308 | N. brasiliensis | ||||
| 03I_022015 | LysR family transcriptional regulator | 4997443 | 4998345 | N. brasiliensis | COG0583 | |||
| 03I_022020 | Cobalt ABC transporter ATPase | 4998379 | 4999098 | N. brasiliensis, R. jostii | COG1122 | |||
| 03I_022025 | ABC transporter permease | 4999095 | 4999856 | N. brasiliensis, R. jostii | COG0619 | |||
| 03I_022030 | Cobalamin (vitamin B12) biosynthesis CbiM protein | 4999857 | 5000921 | N. brasiliensis, R. jostii | COG0310 | |||
| 03I_022035 | ArsR family transcriptional regulator | 5001072 | 5001416 | N. brasiliensis | COG0640 | |||
| 03I_022040 | Hypothetical protein | 5001435 | 5002505 | N. brasiliensis | COG3236 | |||
| 03I_022045 | Cupin | 5002682 | 5003389 | N. brasiliensis, R. jostii | COG2140 | |||
| 03I_022050 | RNA polymerase factor sigma 70 | 5003373 | 5004428 | N. brasiliensis | COG1595 | |||
| 03I_022055 | NADPH:quinone reductase and related Zn-dependent oxidoreductase | 5004425 | 5005423 | N. brasiliensis | COG0604 | |||
| 03I_022060 | Hypothetical protein | 5005604 | 5006257 | N. brasiliensis, N. cyriacigeorgica, N. farcinica | ||||
| 03I_022065 | Putative AraC family transcriptional regulator | 5006364 | 5007230 | N. brasiliensis, N. cyriacigeorgica | COG2207 | |||
| 03I_022070 | Hydrolase | 5007230 | 5008423 | N. brasiliensis | COG1680 | |||
| 03I_022075 | Hypothetical protein | 5008639 | 5009004 | N. brasiliensis | COG0346 | |||
| 03I_022080 | Hypothetical protein | 5009015 | 5009701 | N. brasiliensis | COG5479 | |||
| 03I_022085 | MarR family transcriptional regulator | 5009886 | 5010323 | N. brasiliensis, N. cyriacigeorgica, R. jostii | COG1846 | |||
| 03I_022090 | Hypothetical protein | 5010400 | 5011047 | N. brasiliensis, N. cyriacigeorgica | ||||
| 03I_022095 | Hypothetical protein | 5011268 | 5011873 | N. brasiliensis | ||||
| 03I_022100 | Putative membrane porin | 5011922 | 5012581 | N. brasiliensis | COG0094 | |||
| 14 | 5025398 | 5027080 | 03I_022175 | Nitrate reductase Z subunit beta (NarY) | 5024840 | 5026579 | N. cyriacigeorgica, N. farcinica | COG1140 |
| 15 | 5029253 | 5029670 | 03I_022190 | Nitrate reductase Z subunit alpha (NarZ) | 5029170 | 5030117 | COG5013 | |
| 16 | 5393100 | 5393780 | 03I_023565 | Putative peptidase | 5392840 | 5393805 | N. brasiliensis | COG3590 |
| 17 | 5867835 | 5868021 | Intergenic region | |||||
The data were prepared by aligning the contigs obtained for P-200 with the reference sequence (accession number NC_18681.1) by using Sequencer software. The orthologous genes were determined by using BLAST searches.
On analyzing the clusters of orthologous groups of proteins (COGs) of the deleted genes, we observed 156 different NCBI COGs. The most frequent COGs, with four genes each, were COG583 (transcriptional regulators), COG596 (predicted hydrolases or acyltransferases), COG1463 (ABC-type transport system involved in resistance to organic solvents, periplasmic component [secondary metabolite biosynthesis, transport, and catabolism]), and COG2207 (AraC-type DNA-binding domain-containing proteins). There were six other COGs associated with three genes, and the rest were associated with one or two genes.
In Table 1, we list the hypothetical open reading frames (ORFs) in the deleted DNA regions. The putatively important lost virulence genes included genes encoding a catalase, an SOD, several proteases and peptidases, and a mammalian cell entry (MCE) operon. Other single-nucleotide indels are summarized in Table 2.
TABLE 2.
Single-nucleotide indels
| Indel no. | Position of indel | Original sequencea | Nucleotide changea | Protein designation | Gene | ORF location |
|---|---|---|---|---|---|---|
| 1 | 19559 | : | G | Intergenic region | ||
| 2 | 516378 | C | : | Hypothetical protein | O3I_002245 | 515626–516600 |
| 3 | 645912 | G | : | Intergenic region | ||
| 4 | 1451834 | : | A | Intergenic region | ||
| 5 | 2086472 | : | G | Intergenic region | ||
| 6 | 2277031 | G | : | Intergenic region | ||
| 7 | 3631248 | : | C | Intergenic region | ||
| 8 | 4429802 | C | : | Intergenic region | ||
| 9 | 4429803 | C | : | Intergenic region | ||
| 10 | 4429804 | G | : | Intergenic region | ||
| 11 | 4429805 | C | : | Intergenic region | ||
| 12 | 4429806 | A | : | Intergenic region | ||
| 13 | 4429807 | G | : | Intergenic region | ||
| 14 | 4429808 | C | : | Intergenic region | ||
| 15 | 6723711 | : | C | Hypothetical protein | O3I_029575 | 6723369–6723722 |
| 16 | 6777743 | : | C | Hypothetical protein | O3I_029815 | 6777516–6777797 |
| 17 | 7492119 | G | : | Intergenic region | ||
| 18 | 8590058 | : | G | Intergenic region | ||
| 19 | 8590058 | : | C | Intergenic region |
:, no nucleotide at indicated position.
A comparison analysis revealed 36 SNPs (Table 3), including 5 synonymous SNPs, 15 nonsynonymous SNPs, and 16 SNPs located in intergenic regions (Table 3). Some putative affected proteins involved in virulence included several peptidases and certain enzymes involved in cell wall peptidoglycan synthesis, such as O3I_042080, a peptidoglycan lipid II flippase and an ortholog of MurJ of Escherichia coli. This orthologous protein is an important enzyme in peptidoglycan translocation to the bacterial periplasm.
TABLE 3.
Locations of SNPs in the N. brasiliensis P-200 genomea
| SNP no. | Location | Original codon (amino acid) | Nucleotide change (amino acid) | Gene | Protein designation | Location |
|---|---|---|---|---|---|---|
| 1 | 263186 | CGC (R) | CTC (L) | O3I_001145 | Hypothetical protein | 261412–263706 |
| 2 | 307213 | CAG (Q) | AAG (R) | O3I_001340 | Hypothetical protein | 305875–307494 |
| 3 | 307214 | CAG (Q) | AGG (R) | O3I_001340 | Hypothetical protein | 305875–307494 |
| 4 | 415131 | GCG (A) | ACG (T) | O3I_001810 | Putative trypsin-like serine protease | 414578–415738 |
| 5 | 442835 | ATG (M) | GTG (V) | O3I_001920 | DNA topoisomerase I subunit omega | 441014–443857 |
| 6 | 939436 | GCG (A) | GCA (A) | O3I_004090 | Putative nonribosomal peptide synthetase (modular protein) | 925811–942625 |
| 7 | 1022704 | GCT (A) | GCC (A) | O3I_004395 | Putative peptidase | 1021063–1023102 |
| 8 | 1022963 | GCG (A) | GTG (V) | O3I_004395 | Putative peptidase | 1021063–1023102 |
| 9 | 1214362 | CGG (R) | TGG (W) | O3I_005190 | Homoserine O-acetyltransferase | 1213901–1215061 |
| 10 | 1240877 | GAA (E) | GAT (D) | O3I_005320 | d-Alanyl-d-alanine carboxypeptidase | 1240385–1241674 |
| 11 | 1429121 | GCC (A) | GTC (V) | O3I_006200 | Homoserine kinase | 1428787–1429752 |
| 12 | 1451830 | Intergenic region | ||||
| 13 | 1451835 | Intergenic region | ||||
| 14 | 1451836 | Intergenic region | ||||
| 15 | 2082888 | GTC (V) | TTC (F) | O3I_009135 | O-Dimethylpuromycin-O-methyltransferase | 2082487–2083548 |
| 16 | 2277017 | Intergenic region | ||||
| 17 | 3631212 | Intergenic region | ||||
| 18 | 3631314 | Intergenic region | ||||
| 19 | 3631353 | Intergenic region | ||||
| 20 | 3631428 | Intergenic region | ||||
| 21 | 3631431 | Intergenic region | ||||
| 22 | 3656905 | GTC (V) | GTG (V) | O3I_016400 | FAD-dependent oxidoreductase | 3656411–3657805 |
| 23 | 3864067 | TCC (S) | TTC (F) | O3I_017285 | Acyl-CoA dehydrogenase | 3863076–3864872 |
| 24 | 5105062 | Intergenic region | ||||
| 25 | 6684580 | Intergenic region | ||||
| 26 | 6684588 | Intergenic region | ||||
| 27 | 6777353 | Intergenic region | ||||
| 28 | 7492092 | Intergenic region | ||||
| 29 | 7492093 | Intergenic region | ||||
| 30 | 7794000 | GGC (G) | GGG (G) | O3I_034520 | Membrane-bound C5 sterol desaturase Erg3 | 7793862–7794752 |
| 31 | 7924188 | Intergenic region | ||||
| 32 | 7997064 | GCG (A) | GCA (A) | O3I_035455 | Hypothetical protein | 7996777–7997214 |
| 33 | 8020777 | GAC (D) | AAC (N) | O3I_035540 | Alpha-ketoglutarate decarboxylase | 8019496–8023245 |
| 34 | 8296987 | GAA (E) | TAA (stop) | O3I_036955 | Transcriptional regulator | 8296886–8297533 |
| 35 | 9157861 | TGG (W) | TGA (stop) | O3I_040880 | Hypothetical protein | 9157676–9158764 |
| 36 | 9419279 | AGT (S) | GGT (G) | O3I_042080 | Peptidoglycan lipid II flippase | 9415559–9419329 |
Nucleotide positions were determined by aligning P-200 contig sequences against the N. brasiliensis reference sequence (accession no. NC_18681.1).
Other genetic changes observed in P-200 included short nucleotide sequence duplications. At nucleotide 7,459,445, we observed 12 duplications of the sequence 5′-TGGCCGGGC-3′, interrupting gene 03I-032985 (from nucleotides 7,459,087 to 7,460,010), which encodes a hypothetical protein. BLAST analysis of the protein sequence encoded by this ORF showed homology to an orthologous protein sequence present in several N. brasiliensis strains but not in other Nocardia species. It also showed homology to a streptomyces RNA polymerase sigma factor. Another duplication of 77 nucleotides occurred at position 6,504,536, in an intergenic zone (not shown).
Proteomic analysis of P-200.
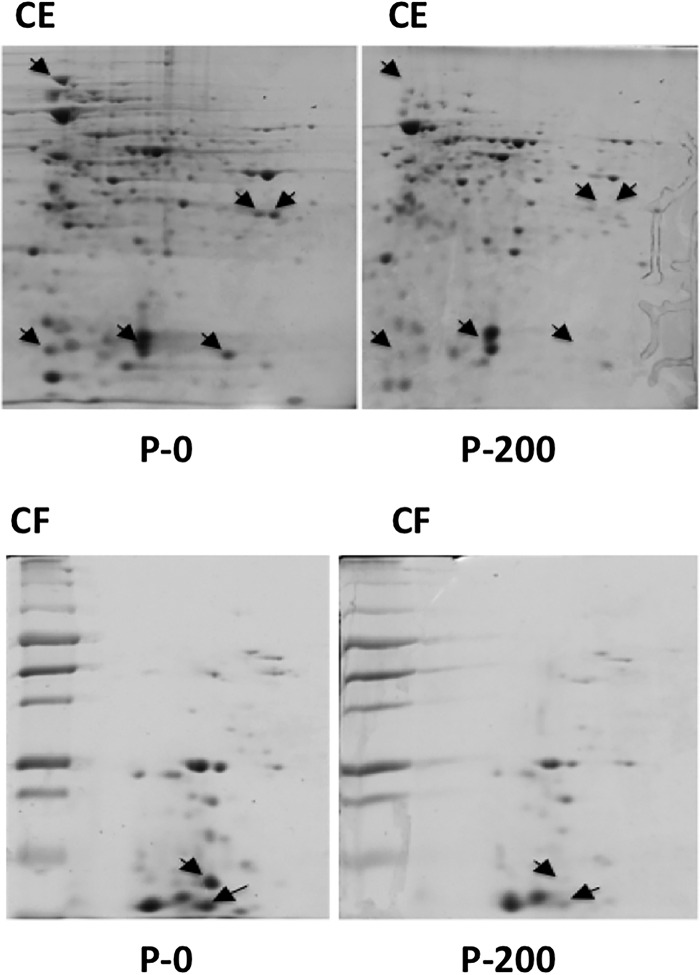
To study the putative proteomic changes derived from the genetic changes, we analyzed the protein content of a cellular extract (CE) obtained by breakage of nocardial cells by use of a fast-prep system, and we also analyzed proteins secreted into the medium (culture filtrate proteins [CFPs]). The extracts were analyzed by SDS-PAGE with a 12% gel and/or by 2-D electrophoresis using a pH gradient of 4 to 7 (Bio-Rad) and a 12% SDS-PAGE gel. The CE of P-0 showed abundant dots in the pH range of 4 to 7. Comparison with the P-200 CE dot pattern showed that some protein dots were missing for P-200 (Fig. 4), including one for a low-molecular-weight protein. Since low-molecular-weight proteins have been associated with attenuation of Mycobacterium bovis BCG, we performed an amino acid sequence analysis of this dot. The results showed that this protein corresponds to a hypothetical protein (encoded by gene 03I_038645 in the N. brasiliensis genome [accession number NC_018681.1]) with a calculated molecular weight of 15,308. BLAST analysis with the whole-protein sequence showed high conservation among other Nocardia species, although it was not associated with any protein of known function. The 2-D analysis of the P-0 CFPs showed a smaller number of protein dots; two low-molecular-weight proteins were clearly lost (Fig. 4). Amino acid sequence analysis followed by BLAST searching identified one of them as the 10-kDa cochaperonin GroES. This protein, together with GroEL or HSP65, is important for the proper folding of many proteins; its deletion in Mycobacterium tuberculosis results in a nonviable clone (9). The GroES gene was complete in P-200; the product of the CDS coding for the GroES protein in M. tuberculosis produces several spots (10). Therefore, it is possible that this lost dot represents an isoform of the protein.
FIG 4.
Analysis by 2-D gel electrophoresis of cell extracts (CE) and culture filtrates (CF) of Nocardia brasiliensis. A pH gradient of 4 to 7 was used for isoelectric focusing, and a 12% gel was used for the SDS-PAGE analysis. Clear differences in the spot patterns are indicated by arrows.
DISCUSSION
Continuous passaging has been used to attenuate microorganisms for a long time. In 1885, Pasteur and Roux attenuated rabies virus by subculturing a sample from wild rabies in rabbit brains (11, 12). Years later, Calmette and Guérin subcultivated a virulent isolate of Mycobacterium bovis to obtain an avirulent bacterium that is still used as a vaccine (bacillus Calmette-Guérin [BCG]) (13). However, in most of these cases, the molecular events underlying the attenuation were unknown at the time that the vaccines were developed. Molecular biology techniques, such as mass sequencing, are powerful tools by which to determine the nature of these biological changes. In the case of BCG, the loss of specific genes after approximately 230 continuous subcultures, leading to a loss of virulence, is already known (14); however, the original isolate was lost, thus making a comparative analysis impossible. Even as few as 15 to 20 subcultures can result in lost biological properties, such as virulence, in the case of fungi, protozoa, or viruses (15–17). The most extreme case of continuous passaging was reported for E. coli after 6,000 daily passages, equivalent to 40,000 generations (18). Few changes were observed before 20,000 generations; however, after that point, mutations accumulated rapidly in subsequent generations, resulting in a total loss of 1.2% of the genome, with 627 SNPs and 26 deletion or insertion changes after 40,000 generations. Most of the deletions were related to insertion sequences and were less than 25,000 bp long. A large inversion (1,493,854 bp) occurred as early as 5,000 passages. We previously reported a decrease in virulence of N. brasiliensis after fewer than 130 passages (27); however, the loss of virulence was not complete, and the genomes of the subcultured strains were not obtained. In this work with P-200, we observed that N. brasiliensis lost a very large DNA fragment (262,913 bp) and that 36 SNPs occurred in a smaller number of duplications. Although in the case of N. brasiliensis the number of generations per subculture was not calculated (because it grows as entangled filaments rather than in cell suspensions as E. coli does), it took 6,000 subcultures to achieve 40,000 generations for E. coli, and therefore 200 subcultures may correspond to approximately 1,333 generations.
In a similar number of subcultures (270 passages), Salmonella enterica lost approximately 224,873 bp after 1,500 generations (19), which is more comparable to our case, in which 262,913 bp were lost after 200 subcultures. However, in the case of S. enterica, we do not know whether other biological changes associated with subculturing occurred.
A bovine isolate of M. bovis was used by Calmette and Guérin to produce an attenuated bacterial strain after 230 subcultures (13); the genes associated with virulence were identified by comparing the genome of BCG against the genome map of M. tuberculosis, with subsequent knockout analysis (14). Although M. tuberculosis and M. bovis are similar species, they are not identical and have important biological differences (20). The attenuated strain M. bovis BCG has been used as a vaccine since its production in 1921 (21); however, protection levels vary greatly (22). A possible explanation for the low level of protection by BCG is overpassaging (23). Because Calmette and Guérin did not have the tools to determine the point at which virulence was lost, it is possible that the bacteria underwent genomic changes (and the loss of important antigens) before Calmette and Guérin decided that virulence was completely attenuated. In our work, we kept samples obtained every 5 to 10 passages and can thus determine in future assays the times when the genetic changes occurred.
The large deletion in P-200 is located at nucleotide position 4,795,932. In comparing the genome of N. brasiliensis with those of other Nocardia species, we observed higher synteny in the first 2,000 Mb before and after the oriC site (dnaJ gene). Independently of genome size, bacteria try to protect the DNA around oriC, avoiding a loss of DNA material and the presence of transposons or insertion sequences that may introduce changes in this important region; this zone has been termed the nucleocore of the genome. It appears that during growth in a rich medium, such as BHI medium, N. brasiliensis can lose a considerable amount of DNA material outside the nucleocore. N. brasiliensis is a species of soil bacteria with a genome size more similar to those of other soil bacteria, such as Actinomadura, Saccharopolyspora, and Streptomyces (approximately 8 to 10 Mb) (24, 25), than to those of human pathogens (approximately 4 Mb). This large chromosome allows soil inhabitants to encode pathways necessary for growth by use of simple compounds as carbon and nitrogen sources, including even aromatic compounds, by use of enzymes such as protocatechuate or homogentisate oxidase (26). In prolonged culture in rich media, it seems that these enzymes are dispensable.
In addition to the loss of DNA, other biological changes occurred. The bacteria grew more rapidly, and they grew in the form of a bacterial suspension instead of the typical entangled filaments. Furthermore, the bacterial cell density decreased, possibly because of biochemical changes in the cell wall. In the host (mice), all these changes were reflected by an inability to produce subcutaneous lesions. With this new genomic information, we can now track the specific gene or genes responsible for Nocardia pathogenesis.
ACKNOWLEDGMENT
This work fulfills in part the requirements for a doctoral degree for Carolina Gonzalez-Carrillo.
REFERENCES
- 1.López-Martínez R, Méndez-Tovar LJ, Bonifaz A, Arenas R, Mayorga J, Welsh O, Vera-Cabrera L, Padilla-Desgarennes MC, Contreras Pérez C, Chávez G, Estrada R, Hernández-Hernández F, Manzano-Gayosso P. 2013. Update on the epidemiology of mycetoma in Mexico. A review of 3933 cases. Gac Med Mex 149:586–592. [PubMed] [Google Scholar]
- 2.Brown-Elliott BA, Conville P, Wallace RJ Jr. 2006. Current status of nocardia taxonomy and recommended identification methods. Clin Microbiol Newsl 37:25–32. [Google Scholar]
- 3.Beaman BL, Black CM, Doughty F, Beaman L. 1985. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun 47:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trevino-Villarreal JH, Vera-Cabrera L, Valero-Guillén PL, Salinas-Carmona MC. 2012. Nocardia brasiliensis cell wall lipids modulate macrophage and dendritic responses that favor development of experimental actinomycetoma in BALB/c mice. Infect Immun 80:3587–3601. doi: 10.1128/IAI.00446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vera-Cabrera L, Ortiz-Lopez R, Elizondo-Gonzalez R, Ocampo-Candiani J. 2013. Complete genome sequence analysis of Nocardia brasiliensis HUJEG-1 reveals a saprobic lifestyle and the genes needed for human pathogenesis. PLoS One 8:e65425. doi: 10.1371/journal.pone.0065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meester I, Rosas-Taraco AG, Salinas-Carmona MC. 2014. Nocardia brasiliensis induces formation of foamy macrophages and dendritic cells in vitro and in vivo. PLoS One 9:e100064. doi: 10.1371/journal.pone.0100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salinas-Carmona MC, Rosas-Taraco AG, Welsh O. 2012. Systemic increased immune response to Nocardia brasiliensis co-exists with local immunosuppressive microenvironment. Antonie Van Leeuwenhoek 102:473–480. doi: 10.1007/s10482-012-9779-y. [DOI] [PubMed] [Google Scholar]
- 8.González-Martínez NA, Lozano-Garza HG, Castro-Garza J, De Osio-Cortez A, Vargas-Villarreal J, Cavazos-Rocha N, Ocampo-Candiani J, Makarov V, Cole ST, Vera-Cabrera L. 2015. In vivo activity of the benzothiazinones PBTZ169 and BTZ043 against Nocardia brasiliensis. PLoS Negl Trop Dis 9:e0004022. doi: 10.1371/journal.pntd.0004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollenkopf HJ, Jungblut PR, Raupach B, Mattow J, Lamer S, Zimny-Arndt U, Schaible UE, Kaufmann SH. 1999. A dynamic two-dimensional polyacrylamide gel electrophoresis database: the mycobacterial proteome via Internet. Electrophoresis 20:2172–2180. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Geison GL. 1978. Pasteur's work on rabies: reexamining the ethical issues. Hastings Cent Rep 8:26–33. [PubMed] [Google Scholar]
- 12.Pasteur L. 1885. Méthode pour prévenir la rage après morsure. Comptes Rendus Acad Sci 101:765–774. [Google Scholar]
- 13.Calmette A, Guérin C. 1920. Nouvelles recherches expérimentales sur la vaccination des bovidés contre la tuberculose. Ann Inst Pasteur 34:553–560. [Google Scholar]
- 14.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 15.Shah FA, Allen N, Wright CJ, Butt TM. 2007. Repeated in vitro subculturing alters spore surface properties and virulence of Metarhizium anisopliae. FEMS Microbiol Lett 276:60–66. doi: 10.1111/j.1574-6968.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 16.Songe MM, Thoen E, Evensen Ø, Skaar I. 2014. In vitro passages impact on virulence of Saprolegnia parasitica to Atlantic salmon, Salmo salar L. parr. J Fish Dis 37:825–834. doi: 10.1111/jfd.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druelle J, Sellin CI, Waku-Kouomou D, Horvat B, Wild FT. 2008. Wild type measles virus attenuation independent of type I IFN. Virol J 5:22. doi: 10.1186/1743-422X-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrick JE, Yu DS, Yoon SH, Jeong H, Oh TK, Schneider D, Lenski RE, Kim JF. 2009. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 461:1243–1247. doi: 10.1038/nature08480. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson AI, Koskiniemi S, Eriksson S, Kugelberg E, Hinton JC, Andersson DI. 2005. Bacterial genome size reduction by experimental evolution. Proc Natl Acad Sci U S A 102:12112–12116. doi: 10.1073/pnas.0503654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina E, Ryan L, LaCourse R, North RJ. 2006. Superior virulence of Mycobacterium bovis over Mycobacterium tuberculosis (Mtb) for Mtb-resistant and Mtb-susceptible mice is manifest as an ability to cause extrapulmonary disease. Tuberculosis (Edinb) 86:20–27. doi: 10.1016/j.tube.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 2004. BCG vaccine. WHO position paper. Wkly Epidemiol Rec 79:27–38. [PubMed] [Google Scholar]
- 22.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, Snell L, Mangtani P, Adetifa I, Lalvani A, Abubakar I. 2014. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behr MA, Small PM. 1997. Has BCG attenuated to impotence? Nature 389:133–134. [DOI] [PubMed] [Google Scholar]
- 24.Vera-Cabrera L, Ortiz-Lopez R, Elizondo-González R, Campos-Rivera MP, Gallardo-Rocha A, Molina-Torres CA, Ocampo-Candiani J. 2014. Draft genome sequence of Actinomadura madurae LIID-AJ290, isolated from a human mycetoma case. Genome Announc 2:e00201-14. doi: 10.1128/genomeA.00201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliynyk M, Samborskyy M, Lester JB, Mironenko T, Scott N, Dickens S, Haydock SF, Leadlay PF. 2007. Complete genome sequence of the erythromycin-producing bacterium Saccharopolyspora erythraea NRRL23338. Nat Biotechnol 25:447–453. doi: 10.1038/nbt1297. [DOI] [PubMed] [Google Scholar]
- 26.Zeinali M, Vossoughi M, Ardestani SK. 2008. Degradation of phenanthrene and anthracene by Nocardia otitidiscaviarum strain TSH1, a moderately thermophilic bacterium. J Appl Microbiol 105:398–406. doi: 10.1111/j.1365-2672.2008.03753.x. [DOI] [PubMed] [Google Scholar]
- 27.Almaguer-Chávez JA, Welsh O, Lozano-Garza HG, Said-Fernández S, Romero-Díaz VJ, Ocampo-Candiani J, Vera-Cabrera L. 2011. Decrease of virulence for BALB/c mice produced by continuous subculturing of Nocardia brasiliensis. BMC Infect Dis 11:290. doi: 10.1186/1471-2334-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]