Abstract
The innate immune system recognizes microbial pathogens via pattern recognition receptors. One such receptor, NOD2, via recognition of muramyl dipeptide (MDP), triggers a distinct network of innate immune responses, including the production of interleukin-32 (IL-32), which leads to the differentiation of monocytes into dendritic cells (DC). NOD2 has been implicated in the pathogenesis of human leprosy, yet it is not clear whether Mycobacterium leprae, which has a distinct MDP structure, can activate this pathway. We investigated the effect of MDP structure on the innate immune response, finding that infection of monocytes with M. leprae induces IL-32 and DC differentiation in a NOD2-dependent manner. The presence of the proximal l-Ala instead of Gly in the common configuration of the peptide side chain of M. leprae did not affect recognition by NOD2 or cytokine production. Furthermore, amidation of the d-Glu residue did not alter NOD2 activation. These data provide experimental evidence that NOD2 recognizes naturally occurring structural variants of MDP.
INTRODUCTION
The ability of the innate immune response to defend against microbial invaders involves germ line-encoded pattern recognition receptors (PRRs) which detect highly conserved pathogen-associated molecular patterns (PAMPs) of infectious agents. One such PRR, nucleotide-binding oligomerization domain 2 (NOD2), is a cytoplasmic receptor belonging to the NOD-like receptor family. NOD2 recognizes muramyl dipeptide (MDP), part of the peptidoglycan (PG) cell walls of Gram-positive and Gram-negative bacteria (1, 2).
We previously found that activation of NOD2, but not Toll-like receptor 2/1 (TLR2/1) or NOD1, induced the production of interleukin-32 (IL-32) in human monocytes. In addition, NOD2 activation induced the IL-32-dependent differentiation of monocytes into dendritic cells (DC). These IL-32-derived DC were distinguished from granulocyte-macrophage colony-stimulating factor (GM-CSF)-differentiated DC in having the capacity to cross-present exogenous antigen via major histocompatibility complex (MHC) class I to CD8+ T cells (3). Cross-presentation facilitates the induction of CD8+ cytotoxic T cell responses against intracellular pathogens that reside in the endosomal pathway. The biological relevance of this pathway was demonstrated in leprosy, in which NOD2, IL-32, and CD1+ DC all were more highly expressed in skin lesions from patients with the self-limited tuberculoid (T-lep) form versus the progressive lepromatous leprosy (L-lep) form. The relevance of NOD2 in the pathogenesis of leprosy is further suggested by the association of NOD2 gene polymorphisms with susceptibility to leprosy (4, 5).
MDP is the minimal essential structure of bacterial peptidoglycan required for its immunological effects, including the activity of Freund's complete adjuvant, which contains mycobacterial cell walls (6, 7). Typically, immunologic studies of NOD2 activation are performed using a synthetic MDP analogue, characterized by N-acetylmuramyl-l-alanyl-d-isoglutamine (8), which is present in most bacteria. The MDP in Mycobacterium spp. has several structurally distinct features, based on studies with the readily cultivable M. smegmatis and M. tuberculosis. MDP in these mycobacteria contains muramic acid residues that are N-glycolylated as well as N-acetylated (9, 10). The carboxyl functions of the peptide side chains are also partially modified by amidation, methylation, or an additional Gly residue (11, 12).
MDP from in vivo-derived and noncultivable M. leprae possesses the basic structural features of MDP from other mycobacteria but with the replacement of the proximal l-alanine by glycine in the peptide side chain and the lack of N-glycolylated muramic acid residues (10, 13). Therefore, the M. leprae peptidoglycan-derived MDP is structurally unique compared to other mycobacteria, as well as other pathogenic bacteria, yet its ability to activate the innate immune response is unknown. Given that structural modifications of MDP can alter biological activity (8, 14–17), we investigated the relationship of the structure of the M. leprae MDP with its ability to trigger immune activation of human monocytes.
MATERIALS AND METHODS
Bacteria and microbial ligands.
For activation of monocytes and HEK-NOD2 reporter cells, we used MDP (1 μg/ml; Invivogen, San Diego, CA), LL-MDP (1 μg/ml; Invivogen, San Diego, CA), live M. leprae (multiplicity of infection [MOI] of 10), and sonicated M. leprae (10 μg/ml). M. leprae was obtained from the footpad of nu/nu mice as described previously (18) and was provided by James L. Krahenbuhl of the National Hansen's Disease Programs, Health Resources Service Administration, Baton Rouge, LA. The M. leprae fractions (M. leprae sonicate, mAGP, and peptidoglycan) were obtained from armadillo spleen-derived M. leprae and generated as described elsewhere (13, 19, 20). All reagents were tested for endotoxin by LAL assay (Limulus amebocyte lysate; detection limit, <0.1 EU/ml; Lonza, Anaheim, CA).
Preparation and analysis of soluble muropeptide from M. leprae PG.
Peptidoglycan was isolated, solubilized, and fractionated by size exclusion chromatography on a Superdex peptide 10/300 GL column (Amersham Biosciences, Pittsburgh, PA) using the conditions described by Mahapatra et al. (10). The muropeptide-containing fractions were analyzed by liquid chromatography-mass spectrometry (LC-MS) using an Agilent 1200 series high-performance liquid chromatography (HPLC) system connected to a 6520 series accurate time-of-flight mass spectrometer (Q-TOF) by following conditions previously described (21). The positive ion-MS data were processed with Agilent MassHunter qualitative analysis software to identify potential compounds ions, followed by a search against a custom database containing calculated monoisotopic ion masses of possible uncross-linked and cross-linked muropeptides from M. leprae PG with a maximum molecular mass of 2 kDa to predict the structure of the compound ions. The amino acid compositions of the muropeptide fractionated by size exclusion chromatography were also analyzed by an EZ:faast GC-MS kit by following the manufacturer's instructions (Phenomenex, Torrance, CA). The concentrations of the muropeptides were normalized based on the abundance of diaminopimelic acid residues.
Enzymatic synthesis of M. leprae MDP analogues.
UDP-N-acetylmuramic acid (UDP-MurNAc) was synthesized by following methods described previously (22). UDP-N-acetylmuramyl-glycinyl–d-glutamate was synthesized from UDP-MurNac, Gly, and d-Glu using recombinant MurC and MurD enzymes of Escherichia coli. The reaction buffer and conditions used have been described previously (23, 24). UDP-N-acetylmuramyl-glycinyl-d-isoglutamine was synthesized in a similar reaction, except d-Glu was replaced with d-isoglutamine. The reaction mixtures were deproteinated and the nucleotide-linked MDPs were purified by ion-exchange chromatography by following the methods described previously (23). Nucleotides were removed by hydrolysis in 0.2 M trifluoroacetic acid at 60°C for 1 h, and the resulting MurNAC-glycinyl–d-glutamate or MurNAC-glycinyl-d–isoglutamine was dried under a stream of N2, resuspended in water, purified by size exclusion chromatography, and then analyzed by LC-MS as described above. The amino acid compositions of MDPs were analyzed by GC-MS as described above.
Monocyte purification.
We obtained whole blood from healthy donors (UCLA I.R.B. 11-001274) with informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll (GE Healthcare, Pittsburgh, PA) gradient centrifugation, and monocytes were further enriched using a Percoll density gradient (GE Healthcare, Pittsburgh, PA) and subsequent adherence in 1% fetal calf serum (FCS) for 2 h or were purified using an EasySep human monocyte enrichment kit without CD16 depletion (Stemcell Technologies, Vancouver, Canada). Monocyte purity was found to be >80% as measured by CD14 expression. Cells were cultured for 24 h in RPMI with 10% FCS (Omega Scientific, Tarzana, CA), penicillin (50 U/ml), streptomycin (50 μg/ml), and sodium pyruvate (1 mM).
Flow cytometry.
Cell surface expression of antigenic determinants was measured using epitope-specific antibodies, and cells were acquired and analyzed as described previously (25). For detection of CD1b, a monoclonal primary antibody (Bcd3.1; ATCC, Manassas, VA) was used, followed by an IgG1-specific secondary antibody (Invitrogen, Carlsbad, CA). Samples were acquired on an LSR II machine (BD, San Jose, CA) and analyzed using FlowJo software.
Cytokine ELISAs.
Secreted IL-32 protein in the supernatant was measured using an IL-32 sandwich enzyme-linked immunosorbent assay (ELISA) kit (SEL101; YbdY Biotech, South Korea) or a matched antibody pair (BioLegend, San Diego, CA). To measure secreted IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α), matched antibody pairs were used according to the manufacturer's recommendations (Biosource, San Diego, CA). For detection we used streptavidin-horseradish peroxidase (HRP) (1:1,000; Pierce, Rockford, IL) and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] HRP substrate mixture (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD), and plates were read at 405 nm.
Real-time qPCR.
Following stimulation of monocytes, RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), cDNA and quantitative PCR (qPCR) was performed as previously described (26). QuantiTect primers (Qiagen, Hilden, Germany) were used. The relative quantities of the gene tested per sample were calculated against h36B4 using the delta cycle threshold formula as previously described (27). The data were normalized by fold change to medium control samples.
HEK NOD2 reporter assay.
HEK-Blue hNOD2 reporter cells (Invivogen, San Diego, CA) were cultured in Dulbecco's modified Eagle's medium (DMEM), 4.5 g/liter glucose, 10% FCS (Omega Scientific, Tarzana, CA) with 50 U/ml penicillin, 50 μg/ml streptomycin, and 100 μg/ml normocin. For antibiotic selection, 30 μg/ml blasticidin and 100 μg/ml zeocin were added. HEK-Blue detection was achieved by adding 2.5 × 104 cells to a 20-μl sample, which was incubated at 37°C in 5% CO2 for 16 h. Secreted embryonic alkaline phosphatase (SEAP) was quantified using HEK-Blue detection (Invivogen, San Diego, CA) and measured by a spectrophotometer at 655 nm.
RESULTS
M. leprae infection of human monocytes induces IL-32 via NOD2.
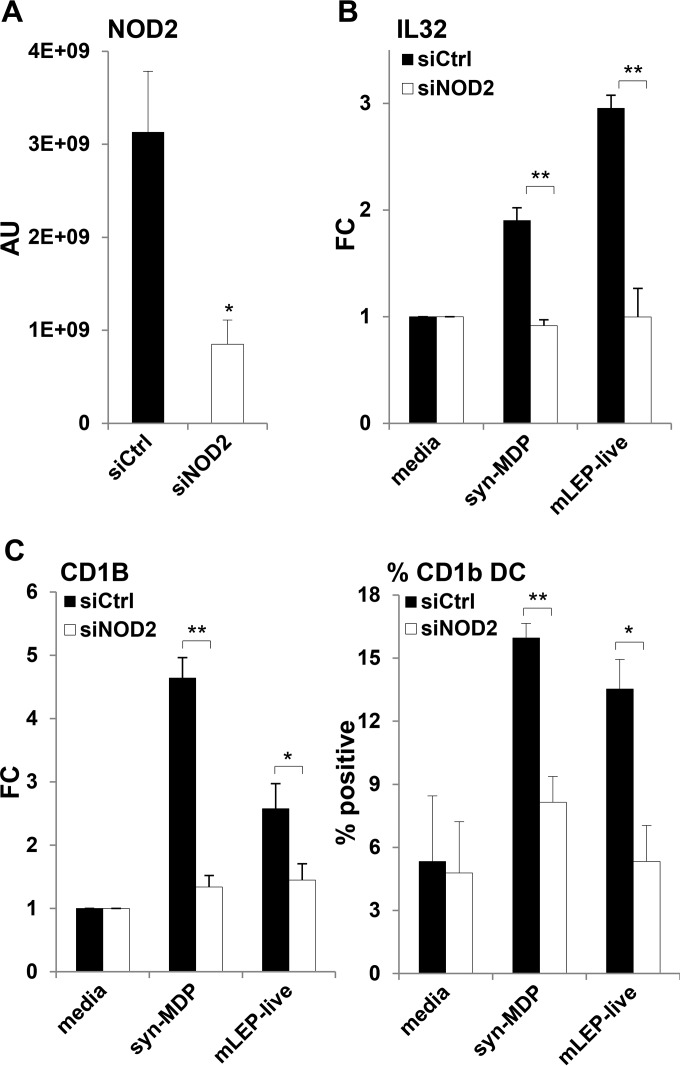
Although NOD2 single-nucleotide polymorphisms and NOD2 downstream immune responses are thought to contribute to the pathogenesis of leprosy, it is not clear whether M. leprae, which has a distinct MDP structure, activates the NOD2 pathway. To determine the role of NOD2 in leprosy infection, we silenced NOD2 gene expression in human monocytes using short interfering RNAs (siRNAs) (Fig. 1A) and measured the induction of IL-32 mRNA in response to infection with live M. leprae. IL-32 induction was measured, since it is specific to NOD2 versus TLR2/1 activation and is required to induce CD1b+ DC differentiation and cross-presentation, and its expression at the site of disease correlates with the self-limited versus progressive form of leprosy. Knockdown of NOD2 (siNOD2) almost completely blocked IL-32 induction in response to live M. leprae compared to the control (siCtrl)-treated cells (Fig. 1B). Similarly, knockdown of NOD2 blocked the response to the synthetic conventional MDP (N-acetylmuramyl-l-alanyl-d-isoglutamine, also called syn-MDP). At the same time, live M. leprae and syn-MDP induced expression of CD1b in monocytes, indicative of DC differentiation. Both live M. leprae and syn-MDP induced CD1b mRNA and protein expression, which was significantly reduced in the absence of NOD2 (Fig. 1C). These data indicate that NOD2 is crucial for the innate recognition of M. leprae in infected monocytes.
FIG 1.
NOD2L is a potent inducer of IL-32 and DC differentiation. siRNA knockdown of NOD2 in purified human monocytes significantly reduced NOD2 expression, shown as arbitrary units (AU) (A), blocked syn-MDP and live M. leprae induction of IL-32 mRNA, shown as mean fold change (FC) (B), and reduced the induction of CD1b+ DC, shown as the percentage of positive cells (C). Data are represented as means ± standard errors of the means (SEM) (n = 4). Statistical significance was calculated by two-tailed Student's t test. Asterisks indicate statistically significant differences: *, P < 0.05; **, P < 0.01.
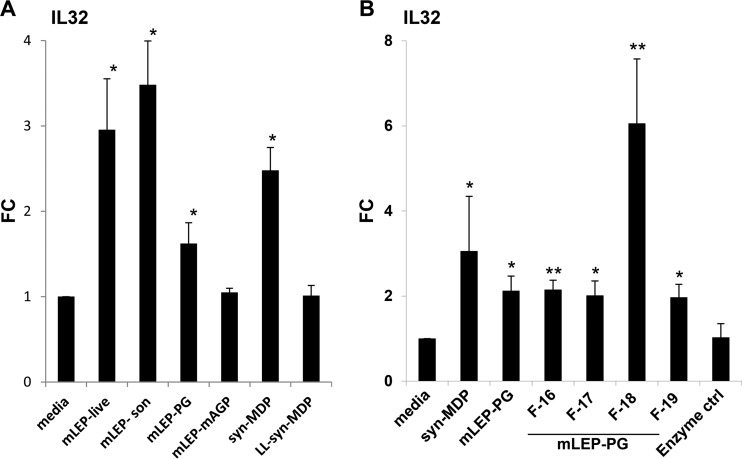
Identification of the M. leprae ligand(s) that induces IL-32.
To identify the M. leprae ligand(s) that regulates IL-32 expression, we cultured monocytes with live or sonicated bacilli, the M. leprae mycolyl-arabinogalactan-peptidoglycan (mAGP), the digested M. leprae peptidoglycan, or enriched fractions of muropeptides derived from M. leprae peptidoglycan. As reference controls, we compared the mycobacterial ligands to syn-MDP and inactive synthetic MDP (N-acetylmuramyl-l-alanyl-l-isoglutamine, or LL-syn-MDP) (Fig. 2A). The induction of IL-32 mRNA expression was significantly greater in cells treated with either live or sonicated M. leprae, the peptidoglycan fraction containing the muropeptides and syn-MDP, compared to the medium control (Fig. 2A). The M. leprae mAGP did not induce IL-32 expression. The mAGP complex is hydrophobic and insoluble and might not be accessible to host lytic enzymes that would release MDP, which is required for NOD2 activation. Additionally, M. leprae subcellular fractions, including cell wall core, cell wall protein, and cytosolic protein, which are expected to lack MDP, also did not induce IL-32 production (data not shown). The stereospecificity of NOD2 ligand recognition was confirmed in studies with LL-MDP which failed to induce IL-32.
FIG 2.
Induction of IL-32 by M. leprae. (A) Purified human monocytes were cultured with live M. leprae (MOI of 10) or 10 μg/ml of either sonicated bacilli (mLEP-son), digested M. leprae peptidoglycan (mLEP-PG), M. leprae mycolyl-arabinogalactan-peptidoglycan (mAGP), synthetic MDP (syn-MDP), or inactive synthetic MDP (LL-syn-MDP), and IL-32 gene expression was measured. (B) Four enriched fractions of muropeptides derived from mLEP-PG (mLEP-PG F-16, -17, -18, and -19) were tested for their ability to induce IL-32 expression and compared to digested M. leprae peptidoglycan (mLEP-PG) and synthetic MDP (syn-MDP). Data are represented as mean fold change (FC) compared to the medium control (ctrl) ± SEM (n = 6). Statistical significance was calculated by two-tailed Student's t test. Asterisks indicate statistically significant differences compared to media control: *, P < 0.05; **, P < 0.01.
The muropeptide fraction (M. leprae peptidoglycan) consisted of a heterogeneous mixture of muropeptides with potential modification sites on sugar and peptide residues. To identify the M. leprae ligand(s) within the M. leprae peptidoglycan preparation that was responsible for NOD2 activation, we further fractionated the digested M. leprae peptidoglycan by size exclusion chromatography (see Fig. S1 in the supplemental material) and tested these for their ability to induce IL-32 expression. The M. leprae peptidoglycan fraction 18 (F-18) was found to be the most potent, such that F-18 and the neighboring fractions were studied in greater detail. F-18 induced a 6-fold increase in IL-32 expression compared to the medium control (Fig. 2B). In comparison, F-16, F-17, and F-19 upregulated IL-32 expression by about 2-fold. The syn-MDP increased expression by 3-fold compared to that with medium alone.
F-18 of the M. leprae peptidoglycan digest and the two adjacent fractions (F-17 and F-19) were analyzed by LC-MS to determine their composition and relative quantity of muropeptides. Muropeptide composition was elucidated by interrogation of the MS data against a database of potential muropeptide structures and their calculated masses (Table 1). A comparison of the structures in the various M. leprae peptidoglycan fractions revealed that F-17 was comprised entirely of cross-linked muropeptides, while F-18 was a mixture of cross-linked and monomeric muropeptides and F-19 contained only monomeric muropeptides (Table 1). However, M. leprae peptidoglycan F-18 possessed a greater abundance of monomeric muropeptides in which the MurNAc residue was not modified to an anhydro form during enzymatic hydrolysis. Thus, this unmodified form of the monomeric muropeptide was likely the reason for the increased IL-32 induction by F-18.
TABLE 1.
Muropeptide composition of the fractions testeda
| Fraction no. and predicted muropeptide structure | Residue modification(s) |
Observed massd | Abundance (vol) | |
|---|---|---|---|---|
| Sugarb | Peptidec | |||
| 17 | ||||
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP) | Anhydro muramic acid | 4 Amidation | 1,668.7244 | 256,684 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Anhydro muramic acid | 4 Amidation | 1,810.7952 | 272,907 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP) | Deacetylated, anhydro muramic acid | 5 Amidation | 1,625.7257 | 370,152 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | NA | 4 Amidation | 1,757.768 | 448,216 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | NA | 4 Amidation | 1,757.7705 | 523,833 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | NA | 4 Amidation | 1,828.8073 | 653,560 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Anhydro muramic acid | 4 Amidation | 1,739.7597 | 804,009 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Anhydro muramic acid | 4 Amidation | 1,810.7961 | 888,780 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | NA | 4 Amidation | 1,828.8062 | 1,293,773 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Anhydro muramic acid | 4 Amidation | 1,739.7601 | 1,449,098 |
| 18 | ||||
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala)e | NA | 1 Amidation | 924.3924 | 39,795 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-Gly/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Deacetylated | 3 Amidation | 1,795.736f | 11,361 |
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP)e | Anhydro muramic acid | 1 Amidation | 835.3434 | 16,734 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Deacetylated | 3 Amidation, Gly | 1,866.7642f | 27,999 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | NA | 4 Amidation | 1,828.8115 | 17,990 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala) | Anhydro muramic acid | 4 Amidation | 1,739.763 | 17,117 |
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP)e | NA | 1 Amidation, Gly | 932.3628f | 15,261 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP) | Deacetylated | 5 Amidation, methylation | 1,657.7696 | 35,943 |
| Dimer (GlcNAc-MurNAc-Gly-d-Glu-DAP/GlcNAc-MurNAc-Gly-d-Glu-DAP) | NA | 2 Amidation, Gly | 1,745.723 | 15,281 |
| 19 | ||||
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala)e | Anhydro muramic acid | 2 Amidation, Gly, methylation | 976.4335 | 34,655 |
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP-d-Ala)e | Anhydro muramic acid | 1 Amidation | 906.3814 | 200,361 |
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP)e | Anhydro muramic acid | 2 Amidation, Gly, methylation | 905.3816 | 29,404 |
| Monomer (GlcNAc-MurNAc-Gly-d-Glu-DAP)e | NA | 1 Amidation | 932.3689f | 15,237 |
The muropeptide fraction obtained from M. leprae peptidoglycan was further fractionated by size exclusion chromatography. The dominant muropeptides from each fraction were identified using LC-MS analysis by interrogating the MS data against a database search. The predicted structures, sugar residue modifications, peptide residue modifications, observed mass, and abundance are indicated for fractions 17 to 19.
Anhydro muramic acid is a potential 1,6-anhydromuramic acid. Deacetylated indicates the loss of an N-acetyl group from one of the sugar residues. NA, not applicable.
Amidation, amidation of carboxylic acid of d-Glu and/or DAP; Gly, d-Glu or DAP residues modified by a glycine residue; methylation, methylation of free carboxylic acid groups of d-Glu or DAP. The number (1 to 5) indicates the number of carboxylic acid residues that are amidated.
All observed masses are H+ adducts unless otherwise noted.
Uncross-linked muropeptide.
Observed mass was a Na+ adduct.
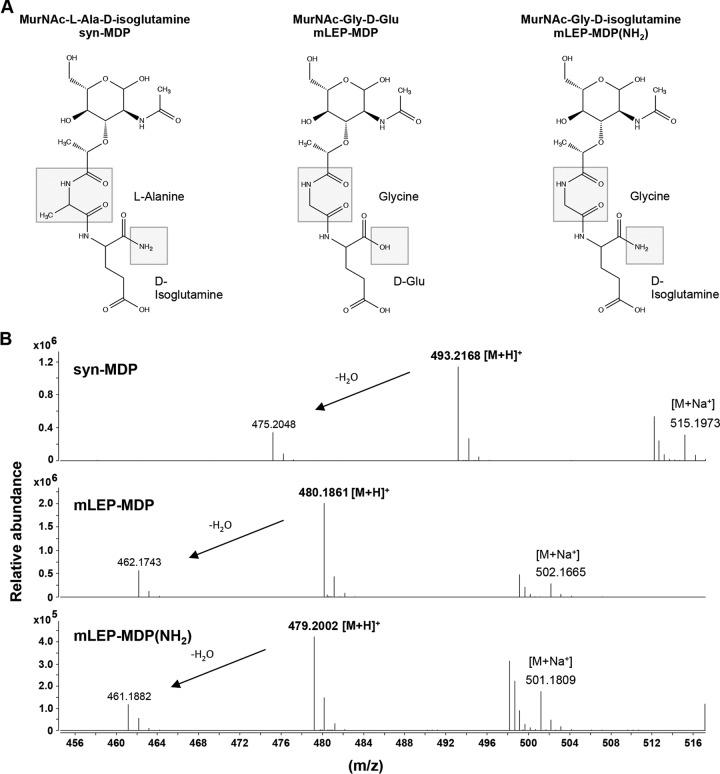
Innate immune responses to M. leprae MDP.
The structure of M. leprae peptidoglycan-derived muramyl dipeptide is unique, as the first amino acid residue of the tetrapeptide side chain is Gly instead of l-Ala and the d-Glu residues are not fully amidated. Thus, MDP naturally derived from M. leprae would contain a mixture of amidated and nonamidated N-acetylmuramyl-glycinyl-d-isoglutamine (Fig. 3A). To further test structure-function relationships of M. leprae MDP, we enzymatically synthesized the amidated and nonamidated form of MDP and confirmed the molecular structures by mass spectrometry (Fig. 3B; see also Fig. S1 in the supplemental material). The [M+H]+ molecular ion of m/z 493.2168 belonged to the syn-MDP standard. The [M+H]+ molecular ion of m/z 480.1861 yielded a calculated molecular formula of C18H29N3O12 that was consistent with the molecular formula of M. leprae MDP, and the [M+H]+ molecular ion of m/z 479.2002 represented amidated MDP of M. leprae MDP(NH2) with a calculated molecular formula of C18H30N4O11 (Fig. 3B; see also Fig. S1).
FIG 3.
Chemical structures of synthetic MDP and M. leprae MDP. (A) Structural differences between the different MDPs are highlighted (box). The first amino acid residue of M. leprae MDP is Gly instead of l-Ala, and the d-Glu residue is amidated [mLEP-MDP(NH2)] or nonamidated (mLEP-MDP). (B) mLEP-MDP(NH2) and mLEP-MDP were synthesized enzymatically, and the molecular structures were confirmed by mass spectrometry: [M+H]+ of 493.2168 m/z belongs to syn-MDP, [M+H]+ of 480.1861 m/z has a calculated molecular formula of C18H29N3O12, which is consistent with the molecular formula of mLEP-MDP, and [M+H]+ of 479.2002 m/z has a calculated molecular formula of C18H30N4O11, which is also the molecular formula of mLEP-MDP(NH2).
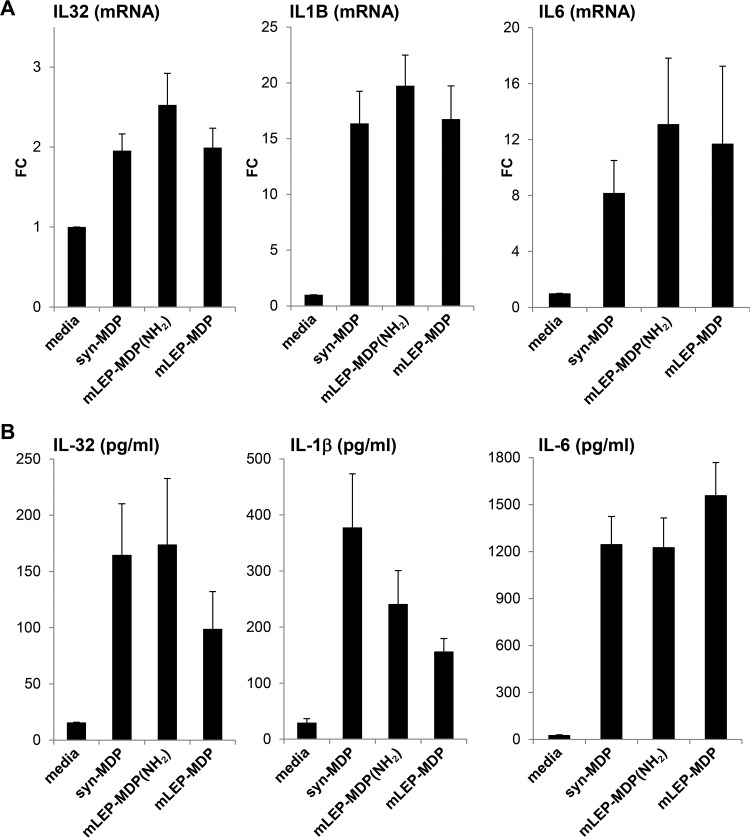
The cytokine induction in human monocytes was compared for these structurally distinct MDPs. The proinflammatory cytokines IL-32, IL-1β, and IL-6 were all significantly induced by syn-MDP and the amidated and nonamidated M. leprae MDP, as measured by mRNA expression (Fig. 4A) and protein secretion (Fig. 4B), indicating that the unique structure of the M. leprae MDP does not interfere with its ability to activate an innate immune response. The amidated form of the M. leprae MDP showed a trend, albeit not significant, to induce a stronger IL-32, IL-1B, and IL-6 mRNA response. IL-1B mRNA induction was 1.2-fold higher in monocytes stimulated with the amidated form of the M. leprae MDP than the nonamidated and synthetic form. However, the level of secreted IL-1β protein was 1.6-fold higher in monocytes cultured with the syn-MDP than the amidated M. leprae MDP and 2.4-fold higher than the nonamidated form of M. leprae MDP. The discrepancy between the induction of IL-1B mRNA and protein could be a consequence of differences in inflammasome activation, which requires further investigation of caspase/inflammasome components. To determine whether these compounds are comparably potent throughout a wide range of concentrations, we performed a dose titration and measured IL-32 induction. The compounds induced comparable amounts of IL-32 throughout a range of concentrations, with peak induction at 1 μg/ml (see Fig. S2 in the supplemental material).
FIG 4.
Cytokine response to structurally distinct MDPs. Purified human monocytes were activated by adding 1 μg/ml of syn-MDP, mLEP-MDP(NH2), or mLEP-MDP. After 24 h, IL-32, IL-1β, and IL-6 induction was measured by mRNA expression as fold change (FC) compared to the medium control (A) and protein secretion (B). Data are represented as means ± SEM (n ≥ 6).
M. leprae MDP activates NOD2 and induces human monocytes to differentiate into DC.
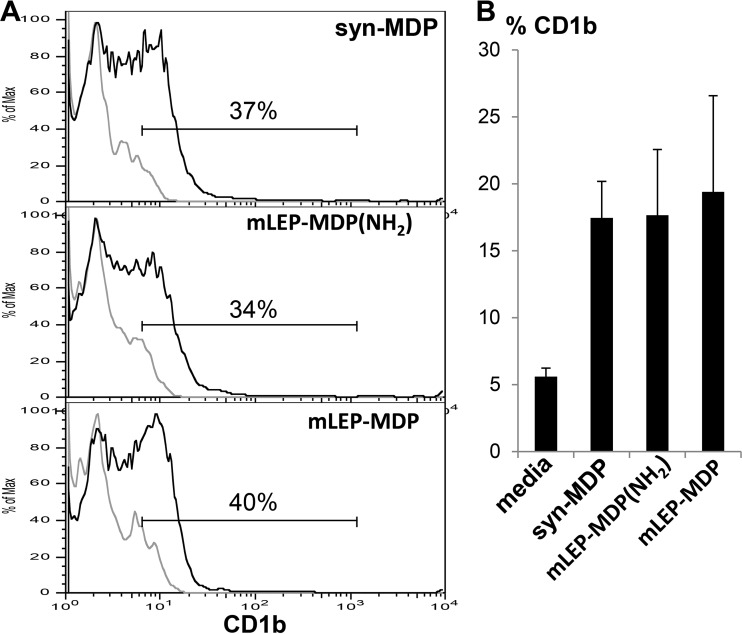
CD1b+ DC are potent antigen-presenting cells in induction of an adaptive T cell response in leprosy (28, 29). Here, we found that M. leprae MDP induces human monocytes to differentiate into CD1b+ DC with a magnitude similar to that of syn-MDP (Fig. 5). The frequency of CD1b+ DC at the site of disease correlates with clinical forms of the disease, i.e., greater in T-lep than in L-lep lesions (30). In addition, we showed that gene expression of both IL-32 and CD1B were significantly greater in T-lep than L-lep lesions (3). Therefore, the ability of M. leprae MDP to induce both IL-32 and CD1b+ DC is linked to host defense at the site of infection.
FIG 5.
Induction of DC differentiation by different MDPs. Purified human monocytes were activated using syn-MDP, mLEP-MDP(NH2), and mLEP-MDP, and DC differentiation was measured by flow cytometry for CD1b expression. A representative histogram (A) and the mean percentage of CD1b-positive cells ± SEM (B) are shown (n = 5).
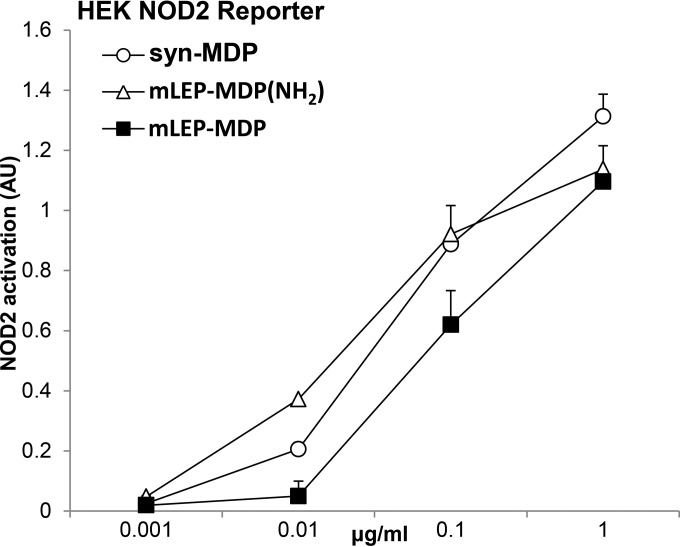
M. leprae MDP is recognized via NOD2.
To demonstrate that recognition of the M. leprae MDP was mediated by NOD2, we used a HEK NOD2 reporter cell line. This allows us to quantitatively assess NOD2 activation by structurally different compounds over a range of concentrations. At lower concentrations (0.01 and 0.1 μg/ml), the amidated form of M. leprae MDP was found to be a more potent activator of NOD2; however, this difference was not observed at higher concentrations (1 μg/ml) (Fig. 6). These data show that the M. leprae MDP is recognized by NOD2 in transfected HEK reporter cells.
FIG 6.
Quantification of NOD2 signaling induced by structurally distinct MDPs. HEK-NOD2 reporter cells were used to quantitatively assess NOD2 activation by stimulating cells with structurally different MDP compounds [syn-MDP, mLEP-MDP-(NH2), and mLEP-MDP] over a range of concentrations (0.001 to 1 μg/ml). Data are represented as mean arbitrary units (AU) ± SEM (n = 6).
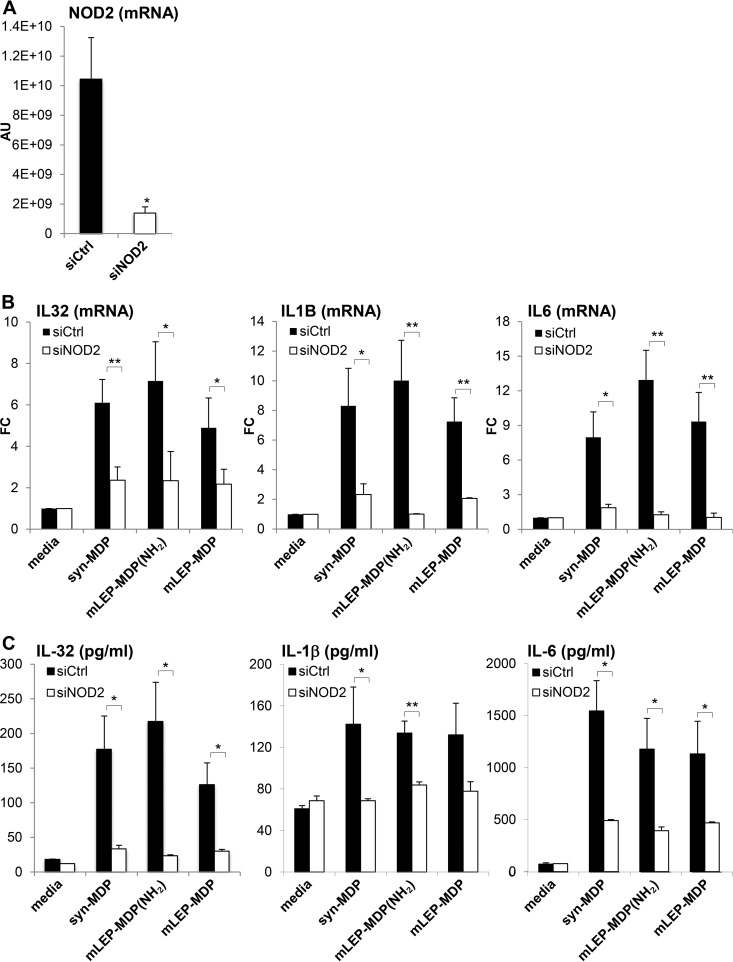
The role of NOD2 in M. leprae MDP-induced immune responses was tested by knockdown of NOD2 expression in monocytes using siRNAs with the subsequent measurement of cytokine responses. NOD2 mRNA expression was inhibited in monocytes transfected with siNOD2 by about 90% compared to that in siCtrl-transfected cells (Fig. 7A). The induction of IL-32, IL-1B, and IL-6 mRNAs (Fig. 7B) and protein (Fig. 7B) was significantly reduced by the knockdown of NOD2 mRNAs for all forms of MDP tested. Together, these data indicate that M. leprae MDP, despite its unique structure, activates human monocytes via NOD2 to trigger a range of innate immune responses with relevance to the pathogenesis of leprosy which are comparable to those induced by the MDP structures found in most other bacteria.
FIG 7.
siNOD2 abolished MDP-induced cytokine response. (A) Purified human monocytes were transfected with siNOD2 or siCtrl, and NOD2 gene expression was measured and is shown in arbitrary units (AU). siNOD2 knockdown monocytes were stimulated with 1 μg/ml of syn-MDP, mLEP-MDP(NH2), or mLEP-MDP and induction of IL-32, IL1B, and IL-6 mRNA, shown as fold change (FC) compared to the medium control (B), and protein expression was measured (C). Data are represented as means ± SEM (n = 4). Statistical significance was calculated by two-tailed Student's t test. Asterisks indicate statistically significant differences compared to the medium control: *, P < 0.05; **, P < 0.01.
DISCUSSION
The importance of NOD2 activation in immune responses has become evident since the identification of MDP as a key component of mycobacterial cell walls responsible for conferring adjuvant activity (6, 31) in inducing both B cell (32) and T cell (33) responses. NOD2 recognition of MDP triggers the induction of specific inflammatory responses to combat bacterial infection, including the production of IL-32, which in leprosy is linked to host defense against the pathogen M. leprae. Nevertheless, it was unknown whether the unique structure of the M. leprae MDP activates a NOD2 response. Here, we demonstrate that infection of monocytes with M. leprae induces the NOD2-dependent production of IL-32 as well as DC differentiation. The M. leprae MDP, which includes a replacement of l-Ala with Gly in the peptide side chain with or without amidation of the d-Glu residue, triggered IL-32 production and DC differentiation. These data provide evidence that host pattern receptors of the innate immune system can recognize naturally occurring structural variants of MDP, including the M. leprae MDP.
Investigation of the effect of the structure of the M. leprae MDP on innate immune responses was undertaken to understand the relationship between structure and function of this microbial ligand. Our strategy was to measure induction of IL-32 in monocytes, given that the M. leprae peptidoglycan fraction containing the muropeptides uniquely triggers IL-32. There are several modifications of the M. leprae MDP structure that could relate to bioactivity. First, the replacement of l-Ala with Gly in the peptide side chain of the M. leprae MDP did not alter NOD2 activation. Second, the presence or absence of an amide in the d-Glu residue in combination with Gly did not affect the innate immune response. Third, we were able to confirm that stereospecific alterations block NOD2 activity, as d-isoglutamine-to-l-isoglutamine alteration renders MDP inactive (8). Fourth, the muramic acid of M. leprae MDP is not N-glycolylated, as this bacterium does not contain a functional namH gene but appeared to be potent in stimulating cytokine responses and DC differentiation (34). Previously, study of the namH mutant of M. tuberculosis suggested that N-glycolylation of muramic acid was required to maximally induce TNF-α and IL-6 in macrophages; however, these particular cytokine responses required costimulation from LPS or trehalose dimycolate (15). Alternatively, other substitutions might compensate for the lack of N-glycolylation (14). We note that the muramic acid of M. leprae is N-acetylated, unlike other mycobacterial MDPs. In summary, the unique structure of the M. leprae MDP does not interfere with its ability to activate the innate immunity in vitro. Based on our studies indicating that live M. leprae induces monocytes to release IL-32 and to differentiate into CD1b+ DC, and that NOD2 downstream immune responses, including IL-32, are expressed at the site of leprosy infection, it is reasonable to speculate that the unique structure of M. leprae MDP does not significantly alter immune activation in vivo. In addition to inducing IL-32, M. leprae MDP stimulated monocyte release of IL-1β and IL-6, as well as of TNF-α and IL-12p40 (data not shown), and induced DC differentiation.
The ability of the M. leprae MDP to induce proinflammatory cytokines as well as DC differentiation most likely contributes to host defense, given that NOD2, IL-32, and CD1+ DC are more frequent in the self-limited T-lep versus progressive L-lep lesions (3). Consistent with this hypothesis, single-nucleotide polymorphisms in the NOD2 gene have implicated this PRR as the key innate immune receptor that contributes to host defense in leprosy (4, 5). A number of studies have implicated NOD2 directly in other mycobacterial diseases. In tuberculosis (TB), nonsynonymous variants of NOD2 were shown to be associated with active disease in a cohort of patients from Houston (35). Furthermore, detection of M. tuberculosis and M. paratuberculosis in human and murine macrophages is NOD2 dependent (36, 37), and NOD2 is important for the cytokine response and NO production in mouse macrophages infected with M. tuberculosis (38, 39). In a mouse model of M. tuberculosis infection NOD2 deficiency did not affect the early phase of infection and bacterial burden (39), but the pulmonary bacterial burden was increased and survival decreased in NOD2-deficient mice (38). In addition to leprosy, human variants of NOD2 have been associated with susceptibility to Crohn's disease (4, 5, 40, 41). It is interesting that Crohn's disease is a chronic granulomatous disorder of the gut with similarities to Johnne's disease, a mycobacterially induced granulomatous disorder in cows. However, an association of Crohn's disease with mycobacterial infection remains controversial. It should be noted that despite years of intensive research, the mechanism by which single-nucleotide polymorphisms (SNPs) in NOD2 lead to the enhanced inflammation associated with Crohn's disease remains enigmatic (4, 5, 42).
It is unclear why M. leprae has evolved to possess a structural variant of MDP, except to possibly escape immune detection (10). However, our data indicate that NOD2 recognizes these naturally occurring structural variants of MDP to mount an effective host response. Future studies using patient monocytes and/or transfected cell lines are required to determine whether mutations in NOD2 lead to altered responses against certain structural variants of MDP, contributing to the pathogenesis of leprosy infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (NIH R01s AI022553, AR040312, and AI047868) and the Swiss National Science Foundation (SNF, SSMBS, PASMP3-123256).
We are grateful to Stephan Krutzik for scientific discussions.
Author contributions: R.L.M. and M.S. designed the experiments and did the majority of the writing; R.L.M., M.S., J.T.B., and S.M. interpreted the data; M.S., P.L., and A.W.C. performed biological experiments; S.M. prepared native and synthetic MDP analogues and performed mass spectrometry; H.J.K. isolated M. leprae fractions and produced recombinant enzymes; J.T.B., P.J.B., and S.M. contributed to the overall study design.
We have no competing financial interests to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00334-16.
REFERENCES
- 1.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. 2007. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem 282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 2.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 3.Schenk M, Krutzik SR, Sieling PA, Lee DJ, Teles RM, Ochoa MT, Komisopoulou E, Sarno EN, Rea TH, Graeber TG, Kim S, Cheng G, Modlin RL. 2012. NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med 18:555–563. doi: 10.1038/nm.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HZ, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ. 2009. Genomewide association study of leprosy. N Engl J Med 361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 5.Berrington WR, Macdonald M, Khadge S, Sapkota BR, Janer M, Hagge DA, Kaplan G, Hawn TR. 2010. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J Infect Dis 201:1422–1435. doi: 10.1086/651559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellouz F, Adam A, Ciorbaru R, Lederer E. 1974. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun 59:1317–1325. doi: 10.1016/0006-291X(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 7.Adam A, Ellouz F, Ciorbaru R, Petit JF, Lederer E. 1975. Peptidoglycan adjuvants: minimal structure required for activity. Z Immunitatsforsch Exp Klin Immunol 149:341–348. [PubMed] [Google Scholar]
- 8.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem 278:5509–5512. [DOI] [PubMed] [Google Scholar]
- 9.Lederer E, Adam A, Ciorbaru R, Petit JF, Wietzerbin J. 1975. Cell walls of Mycobacteria and related organisms; chemistry and immunostimulant properties. Mol Cell Biochem 7:87–104. doi: 10.1007/BF01792076. [DOI] [PubMed] [Google Scholar]
- 10.Mahapatra S, Crick DC, McNeil MR, Brennan PJ. 2008. Unique structural features of the peptidoglycan of Mycobacterium leprae. J Bacteriol 190:655–661. doi: 10.1128/JB.00982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotani S, Yanagida I, Kato K, Matsuda T. 1970. Studies on peptides, glycopetides and antigenic polysaccharide-glycopeptide complexes isolated from an L-11 enzyme lysate of cell walls of Mycobacterium-tuberculosis strain H37rv. Biken J 13:249–275. [PubMed] [Google Scholar]
- 12.Mahapatra S, Yagi T, Belisle JT, Espinosa BJ, Hill PJ, McNeil MR, Brennan PJ, Crick DC. 2005. Mycobacterial lipid II is composed of a complex mixture of modified muramyl and peptide moieties linked to decaprenyl phosphate. J Bacteriol 187:2747–2757. doi: 10.1128/JB.187.8.2747-2757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper P, Kandler O, Darbre A. 1987. Peptidoglycan and arabinogalactan of Mycobacterium leprae. J Gen Microbiol 133(Part 5):1187–1194. [DOI] [PubMed] [Google Scholar]
- 14.Chen KT, Huang DY, Chiu CH, Lin WW, Liang PH, Cheng WC. 2015. Synthesis of diverse N-substituted muramyl dipeptide derivatives and their use in a study of human NOD2 stimulation activity. Chemistry 21:11984–11988. doi: 10.1002/chem.201501557. [DOI] [PubMed] [Google Scholar]
- 15.Coulombe F, Divangahi M, Veyrier F, de Leseleuc L, Gleason JL, Yang YB, Kelliher MA, Pandey AK, Sassetti CM, Reed MB, Behr MA. 2009. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med 206:1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotani S, Watanabe Y, Shimono T, Harada K, Shiba T. 1976. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J 19:9–13. [PubMed] [Google Scholar]
- 17.Maeda K, Koga T, Sakamoto S, Onoue K, Kotani S, Kusumoto S, Shiba T, Sumiyoshi A. 1980. Structural requirement of synthetic N-acetylmuramyl dipeptides for induction of experimental allergic encephalomyelitis in the rat. Microbiol Immunol 24:771–776. doi: 10.1111/j.1348-0421.1980.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri R, Randhawa B, Krahenbuhl J. 2005. Application of a viability-staining method for Mycobacterium leprae derived from the athymic (nu/nu) mouse foot pad. J Med Microbiol 54:235–242. doi: 10.1099/jmm.0.45700-0. [DOI] [PubMed] [Google Scholar]
- 19.Marques MA, Chitale S, Brennan PJ, Pessolani MC. 1998. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae. Infect Immun 66:2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschfield GR, McNeil M, Brennan PJ. 1990. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol 172:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahapatra S, Piechota C, Gil F, Ma Y, Huang H, Scherman MS, Jones V, Pavelka MS Jr, Moniz-Pereira J, Pimentel M, McNeil MR, Crick DC. 2013. Mycobacteriophage Ms6 LysA: a peptidoglycan amidase and a useful analytical tool. Appl Environ Microbiol 79:768–773. doi: 10.1128/AEM.02263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahapatra S, Crick DC, Brennan PJ. 2000. Comparison of the UDP-N-acetylmuramate:L-alanine ligase enzymes from Mycobacterium tuberculosis and Mycobacterium leprae. J Bacteriol 182:6827–6830. doi: 10.1128/JB.182.23.6827-6830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yagi T, Mahapatra S, Mikuova K, Crick DC, Brennan PJ. 2003. Polymerization of mycobacterial Arabinogalactan and ligation to peptidoglycan. J Biol Chem 278:26497–26504. [DOI] [PubMed] [Google Scholar]
- 24.Reddy SG, Waddell ST, Kuo DW, Wong KK, Pompliano DL. 1999. Preparative enzymatic synthesis and characterization of the cytoplasmic intermediates of murein biosynthesis. J Am Chem Soc 121:1175–1178. doi: 10.1021/ja983850b. [DOI] [Google Scholar]
- 25.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda YM, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. 2003. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med 9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 26.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 27.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 28.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Soriano T, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL. 1995. CD1-restricted T cell recognition of microbial lipoglycans. Science 269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 29.Krutzik SR, Tan B, Li HY, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. 2005. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieling PA, Jullien D, Dahlem M, Tedder TF, Rea TH, Modlin RL, Porcelli SA. 1999. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J Immunol 162:1851–1858. [PubMed] [Google Scholar]
- 31.Adam A, Ciorbaru R, Ellouz F, Petit JF, Lederer E. 1974. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun 56:561–567. doi: 10.1016/0006-291X(74)90640-8. [DOI] [PubMed] [Google Scholar]
- 32.Specter S, Cimprich R, Friedman H, Chedid L. 1978. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J Immunol 120:487–491. [PubMed] [Google Scholar]
- 33.Sugimoto M, Germain RN, Chedid L, Benacerraf B. 1978. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant, N-acetyl muramyl-l-alanyl-d-isoglutamine (MDP). J Immunol 120:980–982. [PubMed] [Google Scholar]
- 34.Raymond JB, Mahapatra S, Crick DC, Pavelka MS Jr. 2005. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem 280:326–333. doi: 10.1074/jbc.M411006200. [DOI] [PubMed] [Google Scholar]
- 35.Austin CM, Ma X, Graviss EA. 2008. Common nonsynonymous polymorphisms in the NOD2 gene are associated with resistance or susceptibility to tuberculosis disease in African Americans. J Infect Dis 197:1713–1716. doi: 10.1086/588384. [DOI] [PubMed] [Google Scholar]
- 36.Ferwerda G, Girardin SE, Kullberg BJ, Le BL, de Jong DJ, Langenberg DM, van CR, Adema GJ, Ottenhoff TH, Van der Meer JW, Netea MG. 2005. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1:279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferwerda G, Kullberg BJ, de Jong DJ, Girardin SE, Langenberg DM, van Crevel R, Ottenhoff TH, Van der Meer JW, Netea MG. 2007. Mycobacterium paratuberculosis is recognized by Toll-like receptors and NOD2. J Leukoc Biol 82:1011–1018. doi: 10.1189/jlb.0307147. [DOI] [PubMed] [Google Scholar]
- 38.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. 2008. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol 181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 39.Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S. 2007. Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect Immun 75:5127–5134. doi: 10.1128/IAI.00458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 41.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 42.Ting JP, Duncan JA, Lei Y. 2010. How the noninflammasome NLRs function in the innate immune system. Science 327:286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.