Abstract
Cystic fibrosis (CF) is characterized by an excessive neutrophilic inflammatory response within the airway as a result of defective cystic fibrosis transmembrane receptor (CFTR) expression and function. Interleukin-17A induces airway neutrophilia and mucin production associated with Pseudomonas aeruginosa colonization, which is associated with the pathophysiology of cystic fibrosis. The objectives of this study were to use the preclinical murine model of cystic fibrosis lung infection and inflammation to investigate the role of IL-17 in CF lung pathophysiology and explore therapeutic intervention with a focus on IL-17. Cftr-deficient mice (CF mice) and wild-type mice (WT mice) infected with P. aeruginosa had robust IL-17 production early in the infection associated with a persistent elevated inflammatory response. Intratracheal administration of IL-17 provoked a neutrophilic response in the airways of WT and CF animals which was similar to that observed with P. aeruginosa infection. The neutralization of IL-17 prior to infection significantly improved the outcomes in the CF mice, suggesting that IL-17 may be a therapeutic target. We demonstrate in this report that the pathophysiological contribution of IL-17 may be due to the induction of chemokines from the epithelium which is augmented by a deficiency of Cftr and ongoing inflammation. These studies demonstrate the in vivo contribution of IL-17 in cystic fibrosis lung disease and the therapeutic validity of attenuating IL-17 activity in cystic fibrosis.
INTRODUCTION
Cystic fibrosis (CF) lung disease is characterized by an exaggerated inflammatory response associated with the robust infiltration of neutrophils within the airways (1, 2). The etiology of this excessive inflammation remains to be elucidated. Many cytokines and small molecules have been shown to recruit neutrophils into the airway of individuals with CF, including interleukin-8 (IL-8) and leukotriene (LT) B4 (3–7). IL-17 has traditionally been identified to be a product of activated T lymphocytes and is critically important in the host lung response to infections caused by Gram-negative bacteria, including Pseudomonas aeruginosa (8, 9). Experiments with IL-17 receptor knockout (KO) mice have demonstrated an increased susceptibility to Gram-negative bacterial pneumonia due to excessive neutrophil recruitment into the airways (10–13) and the upregulation of airway mucins, potentially contributing to inefficient mucociliary clearance (5, 14).
Increased concentrations of IL-17 have been associated with a wide range of inflammatory diseases, including rheumatoid arthritis (15, 16), inflammatory bowel disease (17), diabetes (18, 19), cancer (20), and allergic asthma (21, 22). The utilization of neutralizing IL-17A antibodies in a mouse model of allergic asthma (23, 24) and lipopolysaccharide-induced inflammation (9, 25) demonstrated decreased airway neutrophilia, implicating IL-17 as a potential therapeutic target in asthma. IL-17 has also been associated with P. aeruginosa infections, linking it to not just the pathology of chronic inflammation but also inflammation secondary to bacterial infections (13, 24, 26–31). In recent studies, we have also identified IL-17 to be pathophysiological in individuals with CF during infection, correlating with the severity of lung function and proinflammatory cytokines (32).
In this study, we confirmed that Cftr-deficient mice (CF mice) have an altered response to P. aeruginosa infection, demonstrated by early neutrophil accumulation and enhanced IL-17 production compared to that in the wild-type (WT) mouse controls. We further show that the preclinical neutralization of IL-17 markedly reduced neutrophil recruitment in the murine model and decreased inflammation and infection, suggesting that IL-17 is a potential therapeutic target in patients with CF. The studies highlighted in this report demonstrate the role of IL-17 in the early lung inflammatory response to P. aeruginosa infection in patients with CF. The study also demonstrates how the in vivo murine model of CF lung infection and inflammation provides a useful tool to explore the therapeutic development of anti-inflammatory therapies.
MATERIALS AND METHODS
Animals.
Gut-corrected Cftr-KO mice, Cftrtm1Unc-TgN(FABP-hCFTR) mice (CF mice), C57/B6.129P2-Cftrtm1Un−/− mice (CF KO [CF−/−] mice), and WT C57/B6.129P2-Cftrtm1Unc mice (CF+/+ mice) were made available by the CF Animal Core at Case Western Reserve University. The fatty acid binding protein (FABP) CF mice are deficient in the CF transmembrane receptor (CFTR), minimizing the effects of gastrointestinal (GI) obstruction, which would make the large in vivo studies that are outlined in this report difficult (33). B6.129S7-Rag1tm1Mom recombination activating gene 1 (Rag1)-deficient mice and B6.1292P2 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The procedures were approved by Case Western Reserve University's Institutional Animal Care and Use Committee (IACUC approval number 2014-0093).
Infection models.
We utilized the well-characterized P. aeruginosa (mucoid clinical isolate M5715)-embedded agarose bead model (27, 28) to create chronic pulmonary infections. Mice were inoculated via the transtracheal route with 50 μl of P. aeruginosa agar beads (dose range, 1.1 × 104 to 6.9 × 105 CFU/mouse) as previously described (34, 35). Sterile beads were utilized in a subset of animals to control for any potential response to the agarose beads. To evaluate the acute response to P. aeruginosa, mice were inoculated with a suspension of free P. aeruginosa (not embedded into agarose beads) at a dose of 3.8 × 107 to 4.9 × 107 CFU/mouse (36–38). All mice were weighed daily and monitored for clinical signs and gross lung pathology on the basis of the standardized and highly reproducible animal assessment systems outlined in Table 1 (33). Bronchoalveolar lavage (BAL) fluid was obtained from all animals unless the lungs were being preserved for histology. For histology, explanted lungs were gently perfused through the left ventricle and trachea with 4% paraformaldehyde, sectioned, and stained with hematoxylin and eosin (H&E). Serum was obtained from all mice and evaluated for cytokine concentrations. The BAL fluid was evaluated for the total white cell count and differential, cytokine concentrations, and bacteriology. After BAL, the lungs were homogenized to determine the remaining levels of P. aeruginosa infection without knowledge of the sample source using a standardized scoring system, as follows: 0, no bacteria; 1, few bacterial colonies; 2, multiple bacterial colonies; 3, enough bacterial colonies that some were beginning to become confluent; 4, bacterial colonies that were too numerous to count. The comparisons were corrected for the dilution factor.
TABLE 1.
Clinical and gross lung pathology scores
| Score | Clinical signs or gross lung appearance |
|---|---|
| Clinical scores | |
| 0 | Healthy appearance, normal activity |
| 1 | Slightly scruffy or scruffy appearance |
| 2 | Slightly scruffy and slightly dehydrated or scruffy and slightly dehydrated |
| 3 | Scruffy, dehydrated, and decreased activity |
| 4 | Scruffy, dehydrated, and decreased activity or hunched back and painful and slow gait |
| 5 | Moribund or dead |
| Gross lung pathology scores | |
| 0 | Within normal limits |
| 1 | Slightly darker red than normal |
| 2 | Darker red than normal, a few nodules |
| 3 | Several nodules, <25% consolidation of the lobe |
| 4 | Numerous nodules, 25%–50% consolidation of the lobe |
| 5 | Numerous nodules, 50%–75% consolidation of the lobe |
| 6 | >75% consolidation of the lobe |
IL-17 inflammatory response. (i) Administration of recombinant IL-17.
Mice received 3 μg/25 μl of recombinant IL-17 (R&D Systems) or saline as a sham treatment by intranasal administration. Animals were euthanized at 6 or 24 h after IL-17 administration to determine the lung inflammatory cell infiltrate and cytokine response.
(ii) Neutralization of IL-17 activity.
Two hundred micrograms of mouse anti-IL-17 monoclonal antibody (R&D Systems, Minneapolis, MN) or 200 μg of mouse IgG in 200 μl of phosphate-buffered saline (control) was injected subcutaneously into CF KO mice 3 days prior to endobronchial infection with P. aeruginosa-containing agar beads. Mice were sacrificed at 3 days after infection, and BAL was performed for measurement of cell counts and cytokines as described previously (28, 29). For histology, a separate set of mice was euthanized, and their lungs were perfused with 4% paraformaldehyde without undergoing BAL in order to preserve the lung structure. The lungs were sectioned and stained with H&E, evaluated by light microscopy, and scored by an observer unaware of the tissue source.
In vitro monitoring of IL-17 activity on inflammation.
Human bronchial epithelial (16HBEo-) cells transfected with the CFTR-specific nucleotide sequence from positions 1 to 131 in either the sense direction (16HBE-S cells, non-CF phenotype) or the antisense direction (16HBE-AS cells, CF phenotype) were utilized to study the effect of IL-17 in a CF environment (39, 40). Cells were treated in quadruplicate with IL-17 at the following concentrations: 1, 10, 100, and 1,000 ng/ml. Positive controls consisted of cells treated with tumor necrosis factor alpha (TNF-α) and IL-1β (100 ng/ml), and negative controls consisted of cells in Dulbecco's modified Eagle medium (DMEM). Cells were incubated for 4 h at 37°C in 5% CO2. Quantitative protein analysis was performed on the cells, and supernatants were analyzed for IL-8 and IL-6. LA-4 cells (mouse airway epithelial cells; ATCC CCL-196) and A549 cells (human lung airway epithelial cells; ATCC CCL-185) were also used to determine the combinational effects of inflammation on the IL-17 response and chemokine production and the consistency of the effect on human versus murine cell sources.
In vitro stimulation of mouse T cells.
T lymphocytes were negatively selected from mouse spleen and thymus using a magnetically activated cell sorting total T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Following the determination of viability, cells were plated in anti-mouse CD3-coated T-cell activation microplates (BD Pharmingen, San Diego, CA) at 2 × 105 cells/well. T cells were then stimulated with phytohemagglutinin (PHA; 1 mg/ml), anti-CD3 (1 μg/ml) plus anti-CD28 (5 μg/ml), various concentrations of IL-23 (1, 30, 100, and 1,000 ng/ml), or RPMI 1640 (negative control). Supernatants were collected at 24 and 48 h to measure the amount of IL-17 secreted by use of the Luminex technology (see the next section).
Cytokine analysis.
Murine TNF-α, IL-6, macrophage inflammatory protein 2 (MIP-2), keratinocyte-derived chemokine (KC), IL-23, and IL-17 were analyzed using the Luminex technology and reagents (R&D Systems, Minneapolis, MN) or a Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN). The lower-end sensitivity and specificity of the assays were 11 ± 7 pg/ml, depending on the cytokine of analysis. Human IL-6 and IL-8 were analyzed using the Luminex technology following the manufacturer's specifications (R&D Systems, Minneapolis, MN), with the sensitivity of the assays being 9 ± 5 pg/ml.
IL-17 array analysis.
Whole lungs from CF and WT mice were harvested 24 h after intranasal inoculation with P. aeruginosa to capture the acute response to IL-17. Whole-lung homogenates were processed for mRNA followed by cDNA analysis and validation of ubiquitous GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene expression using reverse transcription-PCR. The cDNA was evaluated for IL-17 pathway-specific responses using an IL-17 superarray (catalog number PAMM-073A; SA Biosciences, Valencia, CA) following the manufacturer's protocol.
Statistical analysis.
WT mice (n = 23) and CF mice (n = 25) were utilized for the agarose bead studies, with subsets of mice being harvested at specific time points for various analyses. WT mice (n = 19) and CF mice (n = 17) were utilized for the acute infection studies. In the intranasal challenge experiments, there were at least 6 mice in each group at each time point. The sample size for the experiments on day 2 comparing Rag1−/− mice with WT mice was 13 for both groups. The sample size for both experiments utilizing intranasal inoculation of free P. aeruginosa in at least 3 different sets of studies was 6 for both groups (WT and CF mice). The concentrations of inflammatory cells and cytokines were analyzed either by analysis of variance (ANOVA) with data transformed to the natural log to approximate the normal distribution or via a nonparametric test for data not normally distributed. Differences were considered significant if P was ≤0.05 after the Bonferroni correction. IL-17-to-P. aeruginosa cell count ratios were compared using the Wilcoxon rank test or the Fisher exact test. The results of in vitro experiments involving naive T lymphocytes, neutrophils, and airway epithelial cell lines were compared using ANOVA with Tukey's test. In the array analysis, the statistical software package SigmaPlot (version 2.03), SAS (version 9.1; SAS Institute, Cary, NC), or GraphPad Prism was utilized. Data are represented as the means ± standard errors of the means (SEMs) of the raw data. Statistical significance was defined by a P value of ≤0.05.
RESULTS
IL-17 in P. aeruginosa-infected CF mice.
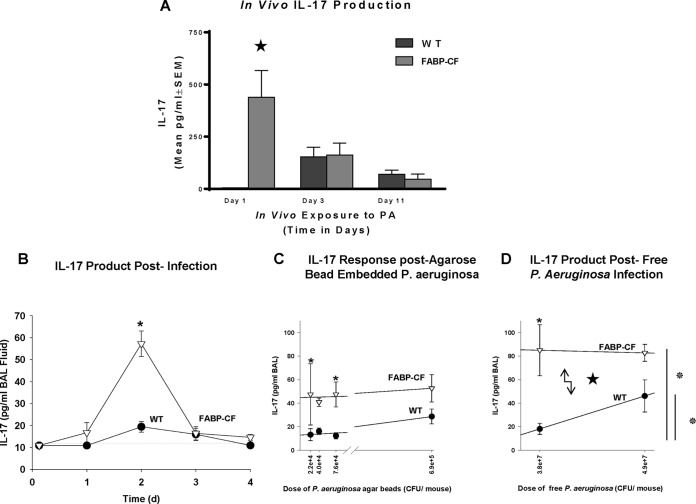
The production of IL-17 in response to chronic P. aeruginosa infection (1 × 105 CFU) impregnated on agarose beads is shown in Fig. 1. The BAL fluid concentrations of IL-17 in the CF animals were detectable on days 1 (P ≤ 0.05), 3 (P ≤ 0.05), and 10 (P = 0.07) and were higher than those for uninfected CF control animals (Fig. 1A). WT animals had no detectable IL-17 at 24 h. However, IL-17 concentrations went up on days 3 (P ≤ 0.05) and 10 (P ≤ 0.07) compared to the concentration in the uninfected WT mouse controls. CF mice had significantly dramatically more IL-17 at 24 h than the WT animals (P ≤ 0.05), and the levels became comparable to those in the controls by days 3 and 10. Sterile beads were used as controls for a potential foreign body response to the agarose beads and produced no detectable IL-17 response in either the CF or WT mice at any time point (data not shown). Since the highest concentration of IL-17 was observed at 24 h and subsequently decreased to control levels by day 3, we evaluated the IL-17 response daily from the time of infection to day 4 (Fig. 1B). CF mice had increased concentrations of IL-17 by day 1 and the levels peaked by day 2 (Fig. 1B; P ≤ 0.05), but the levels returned to levels comparable to the concentrations in WT mice by day 3, suggesting a unique acute IL-17-specific response to P. aeruginosa (1 × 105 CFU) infection in CF mice very early upon exposure to the pathogen. When the dose of P. aeruginosa was increased to 7 × 105 CFU, the WT mice had a robust IL-17 response; however, the IL-17 level in WT mice still did not reach the level seen in CF mice (Fig. 1C; P ≤ 0.05), suggesting a difference in the sensitivity to P. aeruginosa exposure between the WT and CF mice. To ensure no contribution of the agarose beads in the IL-17 response, we also acutely infected WT and CF mice with P. aeruginosa not embedded in agarose beads but in a soluble free inoculum. In these studies, we observed that the IL-17 response in the CF mice was still significantly increased at all time points (Fig. 1D; P ≤ 0.05) compared to that in WT mice. The WT mice demonstrated elevated IL-17 levels at the higher doses of P. aeruginosa, supporting the observation that CF mice have an augmented IL-17 response to acute P. aeruginosa infection compared to infected WT mice.
FIG 1.
(A) The BAL fluid IL-17 level was measured on days 1, 3, and 10 postinoculation with P. aeruginosa (PA)-impregnated agarose beads. *, P ≤ 0.05. The IL-17 level was significantly elevated in Cftr-deficient animals at 24 h postinfection (*, P ≤ 0.05) but was not different between WT and Cftr-deficient animals at 3 or 10 days. (B) Concentration of IL-17 in BAL fluid obtained from CF and WT mice after intratracheal inoculation with P. aeruginosa agar beads at 3 h and days 1 through 4. CF mice (n ≥ 6) had more IL-17 on day 1 (*, P = 0.07) and on day 2 (*, P ≤ 0.05) than noninfected CF and WT mouse controls (n ≥ 6) at the baseline. d, day. (C) Mean BAL fluid IL-17 levels obtained on day 2 after inoculation from CF mice (n = 7) and WT mice (n = 7) are plotted against the number of P. aeruginosa CFU. The BAL fluid IL-17 level was significantly higher in CF mice than uninfected CF mouse controls at the baseline (*, P ≤ 0.05) and WT mice infected with the same dose of P. aeruginosa (*, P ≤ 0.05). At a higher dose of P. aeruginosa, the BAL fluid IL-17 concentration increased in the WT mice but not to a level as high as that in the BAL fluid of CF animals. (D) Both CF mice (n = 6) and WT mice (n = 6) into which freely suspended P. aeruginosa (not embedded in agarose beads) was inoculated intranasally had increased levels of IL-17 production in response to an increase in the dose of P. aeruginosa, with CF mice consistently having higher levels of IL-17 (*, P ≤ 0.05). Both CF and WT mice challenged with 4.9 × 107 CFU/mouse had elevated levels of IL-17, with CF mice having higher levels than WT mice, while the infected WT mice had concentrations significantly elevated relative to those in the uninfected WT mouse controls. Values represent the mean ± SEM of the IL-17 concentration.
Inflammatory response in CFTR-deficient mice.
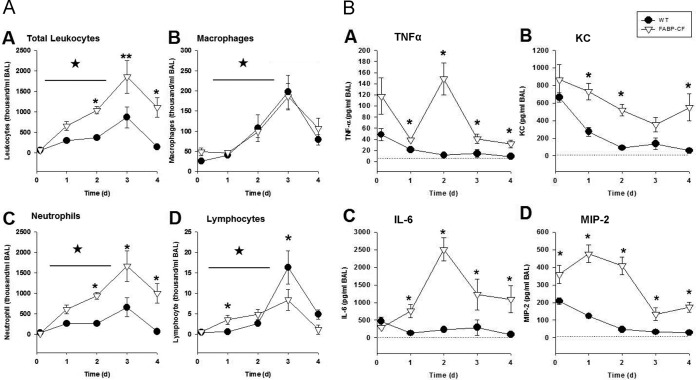
Since CFTR-deficient mice had an altered acute response to P. aeruginosa infection, as evidenced by the overproduction of IL-17, we evaluated how this might correlate with the change in inflammation. We evaluated the total white cell count and differential (Fig. 2A) and the cytokine concentrations (Fig. 2B) in BAL fluid on days 1 through 4 postinfection using P. aeruginosa-embedded agarose beads. As in previous studies, the CF mice displayed a prolonged and exacerbated inflammatory response compared to WT mice, with the CF mice having increased white cell counts (Fig. 2A.A; P ≤ 0.05) and increased absolute numbers of macrophages (Fig. 2A.B; P ≤ 0.05), neutrophils (Fig. 2A.C; P ≤ 0.05), and lymphocytes (Fig. 2A.D; P ≤ 0.05), consistent with our previous observations (27). By day 2, the CF mice had a significant increase in total leukocytes (P ≤ 0.05), neutrophils (P ≤ 0.05), and lymphocytes (starting at day 1). WT mice did not demonstrate a significant change in the cellular response until day 3. Throughout the studies, the CF animals consistently had elevated total leukocyte concentrations (Fig. 2A.A; P ≤ 0.05) which phenotypically correlated with elevated neutrophil counts (Fig. 2A.C, P ≤ 0.05) relative to the total leukocyte concentrations in the WT animals (Fig. 2A.A). While the white cell differential in WT mice demonstrated elevated neutrophil counts (Fig. 2A.C; P < 0.05) and increased numbers of macrophages by day 3 compared to those at the baseline (Fig. 2A.B and Fig. 2A.C; P ≤ 0.05), the numbers of macrophages were not significantly different between the two groups at any of the time points (Fig. 2A.B). The lymphocyte responses between the WT and CF mouse models were very different. CF mice had elevated lymphocyte counts on days 1 (P ≤ 0.05) and 2 (P = 0.06) compared to those in WT mice. However, WT mice had a significant and reproducible peak in lymphocyte recruitment at day 3 (P ≤ 0.05) that was significantly elevated compared to that in CF mice. The levels of lymphocytes in WT mice continued to be higher than those in CF mice out to day 4, although the magnitude of the lymphocyte peak in WT mice started to wane (P ≤ 0.05).
FIG 2.
(A) BAL fluid cell counts and differentials were obtained from CF and WT mice after intratracheal inoculation with P. aeruginosa agar beads at 3 h and days 1 through 4. Values represent the means ± SEMs of the total and absolute cell counts. (A.A) The CF mice had elevated leukocyte counts at all time points relative to WT animals (*, P ≤ 0.05), and both groups of animals had elevated leukocyte counts relative to uninfected controls (*, P ≤ 0.05). (A.B) Both CF and WT animals had similar macrophage numbers, with the difference from the numbers at the baseline reaching significance by day 2 (*, P ≤ 0.05) and the difference being sustained to day 4 (*, P ≤ 0.05). (A.C) Neutrophil counts were increased in the CF mice relative to those at the baseline (*, P ≤ 0.05) and in the WT controls (*, P ≤ 0.05) at the same time points. (A.D) Lymphocyte counts were elevated in CF mice on day 1 (*, P ≤ 0.05) and day 2 (*, P = 0.08), but by day 3, WT mice had more lymphocytes (*, P ≤ 0.05), and this was sustained to day 4 (*, P ≤ 0.05). (B) Cytokines (IL-6, TNF-α, KC, MIP-2) were obtained from the BAL fluid of CF mice and WT mice after intratracheal inoculation with P. aeruginosa agar beads at 3 h and days 1 through 4. Values represent the mean ± SEM concentrations of TNF-α (B.A), KC (B.B), IL-6 (B.C), and MIP-2 (B.D). IL-6 and TNF-α levels were significantly higher in CF mice than in WT mice on days 1 through 4 (*, P ≤ 0.05). The KC level was significantly higher in CF mice than in WT mice on days 1, 2, and 4 (*, P ≤ 0.05). The MIP-2 level was significantly higher in the CF mice than in WT mice at all time points (*, P ≤ 0.05).
To determine what might contribute to the changes in the lung white cell differential in the two models, we evaluated the BAL fluid from the mice for the presence of the proinflammatory cytokines TNF-α (Fig. 2B.A), KC (Fig. 2B.B), IL-6 (Fig. 2B.C), and MIP-2 (Fig. 2B.D) from the same samples used to identify the differentials (Fig. 2A). BAL fluid from both WT and CF mice demonstrated significantly elevated concentrations of TNF-α, KC, IL-6, and MIP-2 at the start of infection (P ≤ 0.05 for all cytokines evaluated), but concentrations were the highest in CF animals (P ≤ 0.05 for all cytokines evaluated) (27). The time course of the cytokine response showed that while TNF-α and IL-6 levels peaked at day 2, there was a decrease by day 4 (Fig. 2B.A and Fig. 2B.C). This is similar to the IL-17 response shown in Fig. 1B. The levels of the neutrophil chemokines KC and MIP-2 remained elevated in CF mice compared to levels in WT mice throughout the study (P ≤ 0.05 for all comparisons), with KC levels continuing to escalate in the CF mice (Fig. 2B.B and Fig. 2B.D), consistent with the increase in neutrophil counts (Fig. 2B.B).
Intratracheal instillation of IL-17 results in neutrophil recruitment in mice.
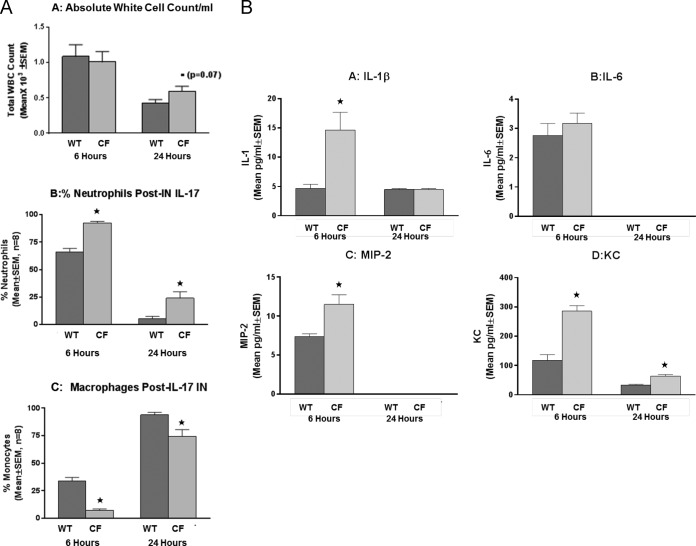
To determine the significance of IL-17 in the early phase of the inflammatory response to P. aeruginosa, CF and WT mice received 100 ng/50 μl of recombinant IL-17 and then underwent BAL at 6 or 24 h after IL-17 administration, and the BAL fluid was evaluated for inflammatory cell recruitment (Fig. 3A) and cytokine concentrations (Fig. 3B). CF animals challenged with IL-17 had higher white cell counts than the controls (Fig. 3A.A; P = 0.07), with a significant increase in the absolute numbers of neutrophils being found in both WT and CF animals at 6 h (198 ± 10 cells/ml and 277 ± 4 cells/ml for WT and CF mice, respectively; P ≤ 0.05) and 24 h (16 ± 7 cells/ml and 72 ± 18 cells/ml for WT and CF mice, respectively; P ≤ 0.05) relative to the numbers in non-IL-17-treated mice at the baseline. The CF animals consistently had greater numbers of neutrophils than WT mice (Fig. 3A.B; P ≤ 0.05), regardless of the time point, while they had significantly smaller numbers of macrophages (Fig. 3A.C; P ≤ 0.05) at both 6 and 24 h. The cytokine profiles in response to the IL-17 challenge were also different between the CF and the WT mice. In the WT animals, IL-17 treatment resulted in an increase in BAL fluid IL-1β (Fig. 3B.A), IL-6 (Fig. 3B.B), MIP-2 (Fig. 3B.C), and KC (Fig. 3B.D) levels at 6 h (P ≤ 0.05 for all four cytokines), and the levels returned to the baseline levels by 24 h. CF animals, in contrast, demonstrated elevated concentrations of IL-1β (Fig. 3B.A), IL-6 (Fig. 3B.B), MIP-2 (Fig. 3B.C), and KC (Fig. 3B.D) relative to those at the baseline (P ≤ 0.05 for all four cytokines), and KC levels remained elevated at 24 h (P ≤ 0.05). TNF-α was not induced with IL-17 in either the CF or the WT mice (data not shown).
FIG 3.
Recombinant IL-17 (3 μg/25 μl) was given intranasally (IN) to both WT and Cftr-deficient animals. (A) Mice were sacrificed at 6 and 24 h, and BAL fluid was evaluated for white blood cell (WBC) counts and the relative numbers of neutrophils and macrophages. (A.A) There was no difference in the total white cell counts between the WT (dark bars) and CF (light bars) animals at 6 h, with a slight increase being noted in the CF mice at 24 h (*, P = 0.07). (A.B) Neutrophil counts were increased in both the CF and WT mice at 6 h (*, P ≤ 0.05), and the increase was sustained and higher at 6 and 24 h in CF mice (*, P ≤ 0.05). (A.C) BAL fluid macrophage counts increased at 24 h in both groups compared to those at 6 h after IL-17 administration (*, P ≤ 0.05). CF animals had fewer macrophages than WT mice at 24 h (P ≤ 0.05). (B) Inoculation with IL-17 induced the cytokines IL-1β, IL-6, MIP-2, and KC. Cftr-deficient animals had significantly more IL-1β (B.A), MIP-2 (B.C), and KC (B.D) than the WT control at 6 h (*, P ≤ 0.05, n = 8), with CF mouse KC concentrations remaining elevated at 24 h (*, P ≤ 0.05). (B.B) IL-6 levels were not changed by IL-17 treatment.
To monitor IL-17 in vivo, we measured BAL fluid IL-17 concentrations in animals with and without exposure to recombinant IL-17 (3 μg). At 6 h, both WT and CF animals had significantly more IL-17 than the administered dose (8,000 ± 3,750 pg/ml and 12,500 ± 4,536 pg/ml for WT and CF mice, respectively), which implicates an IL-17 autocrine response. By 24 h, the response to IL-17 was less, with WT and CF mice having 194 ± 63 and 756 ± 169 pg/ml, respectively (P ≤ 0.05 relative to the levels at 6 h), and with the CF mice consistently having greater concentrations of IL-17 than the control mice (P ≤ 0.05).
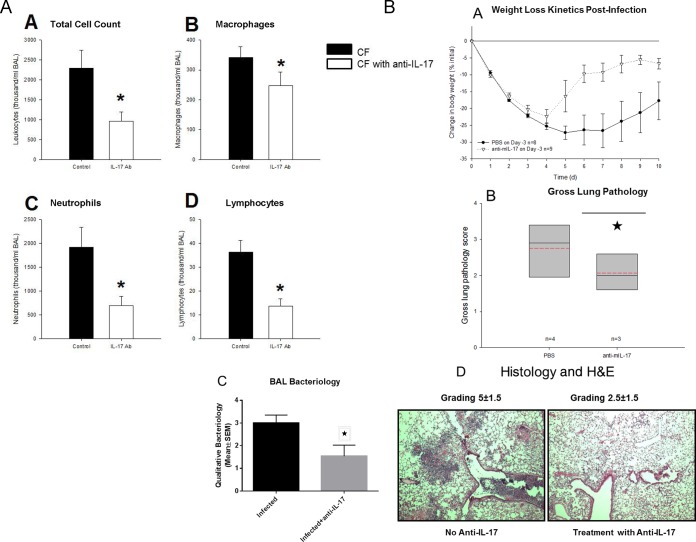
Anti-IL-17A antibody suppresses P. aeruginosa-induced inflammation in CF mice.
Since the IL-17 response was very dramatic in the acute P. aeruginosa infection, we neutralized IL-17 prior to infection in the CF and WT mice to determine the impact of IL-17 on the response to infection. CF and WT mice were given either neutralizing IL-17A antibody or anti-mouse IgG antibody (control) subcutaneously 3 days prior to P. aeruginosa inoculation using the agarose bead model to sustain chronic infection. On day 3, BAL fluid was collected for determination of the total white cell count and differential and cytokine levels (Fig. 4A) and histopathology and constitutive responses (Fig. 4B). Total BAL fluid levels of leukocytes (Fig. 4A.A), macrophages (Fig. 4A.B), neutrophils (Fig. 4A.C), and lymphocytes (Fig. 4A.D) were significantly lower in mice that received IL-17A antibody pretreatment (Fig. 4A; P ≤ 0.05 for each comparison). The levels of proinflammatory cytokines (KC, MIP-2, TNF-α, and IL-6) also tended to be lower in the group that received anti-IL-17A antibody, although the differences did not reach statistical significance (data not shown). Treatment of the CF mice with the anti-IL-17A antibody also improved the responses to infection, as demonstrated by less weight loss (Fig. 4B.A; P ≤ 0.05), an improved clinical score (data not shown; P = 0.07), and less lung inflammation (Fig. 4B.B; P ≤ 0.05). Further, the treatment with anti-IL-17 neutralizing antibodies decreased the intensity of infection in the lungs (Fig. 4B.C; P ≤ 0.05), with improved lung histology secondary to P. aeruginosa infection being seen (Fig. 4B.D, which shows representative images for 1 of 5 mice; for scoring, P ≤ 0.05).
FIG 4.
BAL fluid differentials were evaluated in Cftr-deficient mice receiving neutralizing anti-IL-17 monoclonal antibody or control nonspecific Ig monoclonal antibody prior to infection. (A) Mice were administered 200 μl anti-IL-17 (white bars, n = 23) or control nonspecific Ig monoclonal antibody (black bars, n = 17) prior to infection with a P. aeruginosa agarose bead preparation. The neutralization of IL-17 resulted in a decrease in total leukocyte (A.A), macrophage (A.B), neutrophil (A.C), and lymphocyte (A.D) counts. Ab, antibody. *, P ≤ 0.05 for all cell groups. (B) Treatment of the CF animals with anti-IL-17A monoclonal antibody improved the responses to infection, including weight loss (B.A) (P ≤ 0.05) and lung pathology (B.B) (*, P ≤ 0.05). Treatment of CF animals with anti-IL-17 resulted in decreased infection (B.C) (*, P ≤ 0.05) and inflammation (B.D) (P ≤ 0.05). PBS, phosphate-buffered saline; mIL-17, mouse IL-17.
Role of lymphocytes in IL-17 response to P. aeruginosa in CF mice.
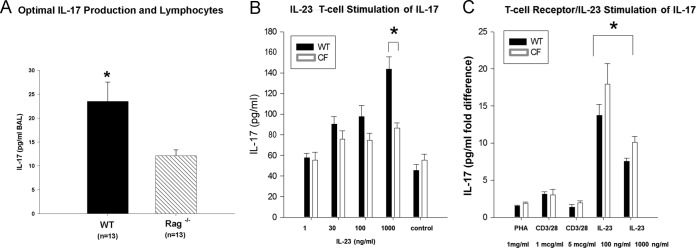
Because T-cell IL-17 is thought to be dependent on IL-23, we measured IL-23 levels in BAL fluid. The CF mice had increased BAL fluid IL-23 levels at day 2 compared to WT animals (476 ± 151 pg/ml IL-23 versus 189 ± 53 pg/ml IL-23, respectively; P ≤ 0.05). However, the increase in the amount of IL-23 was short-lived, with no statistically significant difference being found by day 3 (324 ± 115 pg/ml IL-23 versus 316 ± 57 pg/ml IL-23 in CF and WT mice, respectively), thus demonstrating the lack of sustainability of the response. To further explore the role of T cells in the IL-17 response to in vivo infection with P. aeruginosa, Rag1−/− mice (n = 13) and WT mice (n = 13) were infected using the agarose bead model. Mice were sacrificed at 2 days postinfection, and then BAL fluid was collected to monitor IL-17 concentrations. BAL fluid from the WT mice had significantly elevated concentrations of IL-17 relative to BAL fluid from the Rag1−/− mice (Fig. 5A; P ≤ 0.05), which still had detectable IL-17. Flow cytometry of the cells recruited from the BAL fluid of WT and CF mice demonstrated that WT mice had 74% ± 4% CD4+ T cells, with only 2% ± 0.3% of cells staining positive for IL-17, while CF mice had a total of 87% ± 3% CD4+ T cells, with only 3% ± 1.5% of cells staining positive for IL-17, a finding which is inconsistent with the BAL fluid concentrations of IL-17. These studies demonstrated the role of T cells in the intensity of the IL-17 response to P. aeruginosa infection, since WT mice had more IL-17 than Rag1−/− mice, but also implicate another source of IL-17, since the Rag1−/− mice had detectable levels of the cytokine, the focus of other studies in our laboratories (32).
FIG 5.
(A) The IL-17 level was measured in BAL fluid from WT mice (n = 13) and Rag1−/− mice (n = 13) 2 days after intratracheal challenge with P. aeruginosa agar beads at a dose sufficient to generate an IL-17 response in the WT mice. Rag1−/− mice had detectable IL-17, whereas WT mice did not (mean value ± SEM; *, P ≤ 0.05). (B) To determine IL-17 responsiveness in CF mice, CF and WT mouse T lymphocytes were treated in vitro with IL-23 at different concentrations. Values represent the mean ± SEM of the fold difference in the amount of IL-17 over that for the negative control. There was no significant difference in the level of IL-17 production from WT or CF mouse lymphocytes after stimulation at 24 h, except at the 1,000-ng/ml concentration of IL-23 (*, P ≤ 0.05). (C) Results of treatment with PHA at 1 mg/ml, anti-CD3 at 1 μg/ml plus anti-CD28 at 1 μg/ml, anti-CD3 antibody at 1 μg/ml plus anti-CD28 antibody at 5 μg/ml, IL-23 at 100 ng/ml, and IL-23 at 1,000 ng/ml at 48 h. Both CF and WT mouse lymphocytes had a significantly (*, P ≤ 0.05) higher response to nanogram-per-milliliter concentrations of IL-23 than to microgram-per-milliliter or milligram-per-milliliter concentrations of the other stimuli.
To determine whether there may be a defect in the capacity of CF mouse T cells to respond to IL-23, the primary mechanism associated with IL-17 production from lymphocytes, T lymphocytes isolated from the spleens of naive CF and WT mice were stimulated with different concentrations of IL-23 (1, 30, 100, and 1,000 ng/ml) for 24 and 48 h. There were no significant differences in T-cell response to IL-23 between CF and WT mice except with IL-23 at 1,000 ng/ml (Fig. 5B, data obtained at 24 h; P ≤ 0.05). To explore the sustainability of the IL-17 response to IL-23, T cells were cultured for 48 h with IL-23 and compared to nonspecifically activated T cells (PHA) and activation through the T-cell receptor (anti-CD3/CD28). Lymphocytes from both the WT and CF animals had 2- to 10-fold greater IL-17 responses to stimulation with IL-23 than to stimulation with PHA or CD3/CD28 (Fig. 5C, data obtained at 48 h). These data are consistent with IL-23 inducing naive T lymphocytes toward the production of IL-17 (Th17 cells) (20, 35), suggesting that although CF and WT mouse T cells are capable of making IL-17 in response to IL-23, this is not the primary source of IL-17 at 24 h postinfection in the CF murine model of lung infection and inflammation. These data suggest a time-sensitive switch to Th17 activity, which is upregulated in response to IL-23, consistent with the findings reported in the literature (6, 12, 22).
IL-17 stimulates the production of IL-6 and IL-8 in airway epithelial cells.
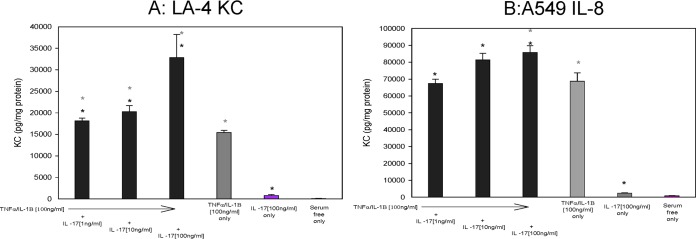
To determine the potential pathophysiological role of IL-17 in the airways that is consistent with our murine model and that might translate into a biomarker for disease, we evaluated the impact of recombinant IL-17 on mouse airway epithelial cells (ATCC CCL-196; LA-4 cells) and human airway epithelial cells (ATCC CCL-185; A549 cells). These two cell lines were grown to confluence in monolayers and treated with a proinflammatory cocktail; TNF-α–IL-1β (100 ng/ml) was used to simulate cytokine-mediated inflammation and was used with and without the addition of various concentrations of IL-17 (1 to 100 ng/ml). Stimulation with TNF-α–IL-1β (P ≤ 0.05) but not IL-17 by itself increased the secretion of KC (Fig. 6A) by the murine epithelial cells. Further, when the IL-17 was combined with the TNF-α–IL-1β, there was an enhanced effect on KC secretion (P ≤ 0.05). MIP-2 levels were also enhanced by IL-17 and augmented by the addition of TNF-α–IL-1β with kinetics similar to those of the LA-4 cells, emphasizing the impact of the IL-17 on chemokine production (data not shown; P ≤ 0.05). Importantly, the results of the experiments with the human cell line mimicked those of the studies with the murine cell line. TNF-α–IL-1β stimulation of A549 cells induced IL-8 (a human neutrophil chemokine) and IL-6 (data not shown) compared to their levels in control cells at the baseline, similar to the findings for the mouse LA-4 cell line. IL-17 treatment enhanced IL-8 chemokine production over that achieved with TNF-α–IL-1β alone (Fig. 6B; P ≤ 0.05), implicating a role for IL-17 in inflammation.
FIG 6.
Immortalized mouse airway epithelial cell lines (LA-4) and human cells (A549 cells) were grown in the presence or absence of TNF-α–IL-1β (100 ng/ml) with and without IL-17 at doses ranging from 1 ng/ml to 100 ng/ml. After 24 h, the supernatants were harvested and analyzed for mouse KC (A) or human IL-8 (B). Student's t tests along with nonparametric multivariate analysis to control for the different conditions were done. Black asterisks, comparisons between the different IL-17 concentrations alone; gray asterisks, comparisons between the TNF-α–IL-1β and IL-17 cytokine cocktail. Significance is defined by a P value of ≤0.05 using a two-tailed analysis.
To determine the downstream effects of IL-17 on the regulation of inflammation in CF mice, we evaluated the impact of IL-17 on airway epithelial cells with and without the CF phenotype. In these experiments, we utilized an immortalized cell line created from human airway epithelial cells deficient in CFTR which were derived from matched transformed human airway epithelial cells with a CF genotype (16HBE-AS cells [carrying the CFTR-specific nucleotide sequence in the antisense direction]) or a non-CF genotype (16HBE-S cells [carrying the CFTR-specific nucleotide sequence in the sense direction]). These cells were grown in culture and treated with IL-17 to determine their ability to produce the cytokines IL-8 and IL-6 (Fig. 7). The airway epithelial cells with a CF phenotype secreted significantly greater concentrations of IL-6 at all concentrations of IL-17 tested (Fig. 7A; P ≤ 0.05) than the negative unstimulated control, similar to what was seen with the positive controls (TNF-α–IL-1β-treated cells). As shown in Fig. 7B, IL-17 also induced excessive IL-8 secretion by CF mouse cells relative to the amount secreted by control cells at all concentrations of IL-17 tested, suggesting a dose relationship between IL-17 and the production of IL-8 (P ≤ 0.05) at all concentrations except 1,000 ng/ml, especially in the CF mouse cells. Low concentrations of IL-17 (1 and 10 ng/ml) did not induce IL-6 and IL-8 secretion in healthy epithelium, implying a difference in IL-17 responsiveness from that of CF mouse epithelium.
FIG 7.
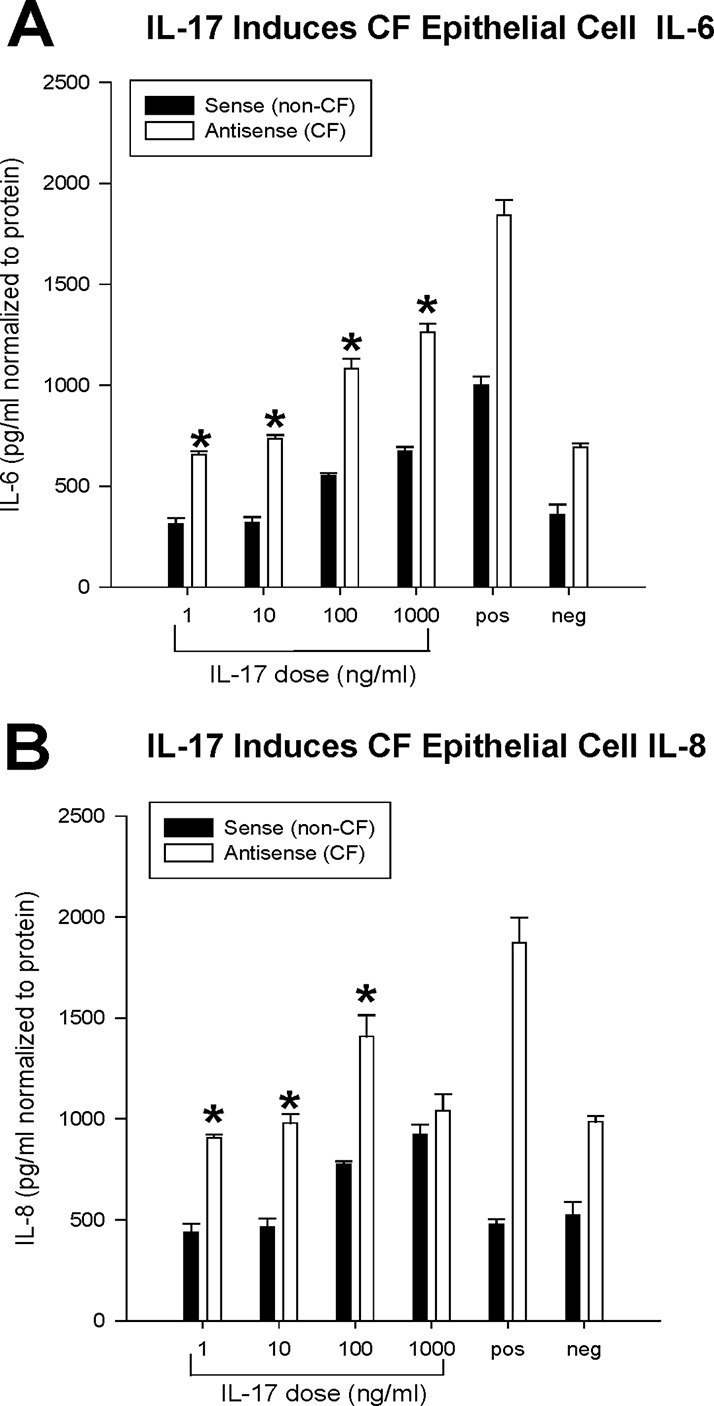
To determine the impact of CFTR on the epithelial cell response to IL-17, cultures of matched CF (antisense) and non-CF (sense) airway epithelial cells were stimulated with increasing IL-17 concentrations (1, 10, 100, and 1,000 ng/ml) for 4 h. Values represent the mean ± SEM concentrations of the cytokines (IL-6 and IL-8). Repeated experiments were performed with each treatment in quadruplicate wells. The results demonstrate a dose-dependent increase in IL-6 and IL-8 concentrations in both CF and non-CF cells. Further, the epithelial cells deficient in Cftr were significantly more sensitive to IL-17 and produced more IL-6 (A) (*, P ≤ 0.05) and IL-8 (B) (*, P ≤ 0.05). The positive control (pos) was TNF-α (100 μg/ml)–IL-1β (100 μg/ml). The negative control (neg) was serum-free medium (DMEM). There was also a dose-dependent increase in IL-6 and IL-8 levels in both cell lines.
CF mouse response to infection and potential pathways for IL-17-mediated responses.
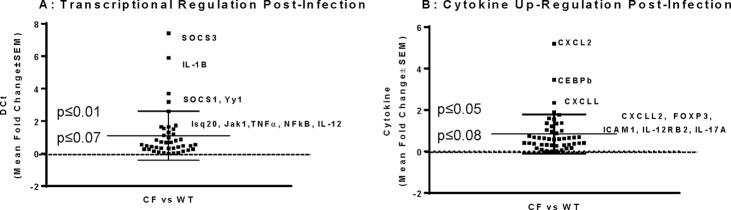
To begin to evaluate the relationship between CFTR function and the IL-17 response to P. aeruginosa infection, CF and WT mice were infected with P. aeruginosa and sacrificed 24 h later. Whole-lung homogenates were made and processed for cDNA and validated for GAPDH and IL-17 expression to ensure the quality and quantity of the RNA preparation as well as the IL-17-specific CF mouse acute response. The amount of IL-17 was significantly increased in the homogenates of the CF animals (the level of IL-17 gene expression was 7.2- ± 2.5-fold higher than the level of expression of the ubiquitous GAPDH gene used as a control), consistent with the increased amount secreted into the BAL fluid (420 ± 57 pg/ml IL-17). The cDNA preparations from the CF KO and WT mice were then evaluated on IL-17 superarrays. To evaluate the large quantity of data generated from the array, the threshold cycle (CT) values were combined and analyzed as the CF KO mouse response relative to the WT mouse response (Fig. 8A and B). These studies showed a significant upregulation of SOCS3, IL-1β, and Yyt (P ≤ 0.05) and modest increases in the levels of expression of Isq20, Jak1, TNF-α, NF-κB, and IL-12 (P ≤ 0.07) (Fig. 8A). Other cytokines and regulatory molecules impacted included CSCL2, CEBPβ, and CXCLL, whose levels of expression changed significantly (P ≤ 0.05), and CXCLL2, FOXP3, ICAM1, IL-12RB2, and IL-17A, for which the changes in the levels of expression showed a trend toward significance (P ≤ 0.07) (Fig. 8B).
FIG 8.
CF and WT animals were infected with a P. aeruginosa suspension, and lungs were obtained at 24 h postinfection. Whole-lung homogenates were processed for mRNA and cDNA, and samples were validated for the presence of GAPDH prior to array analysis. One microgram of cDNA was evaluated in each array. The IL-17 response was validated and found to be associated with the SOCS pathway and chemokines. Significance was defined by the relative difference between the CF and WT mouse responses at 24 h postinfection with P. aeruginosa. Each square is a gene, and the region of significance is defined by a midline change in the threshold cycle value (DCt) of 1. (A) Transcriptional regulation through SOCS and NF-κB; (B) chemokine regulatory pathways.
DISCUSSION
The development of drugs that target the basic defect in CF has generated a great deal of enthusiasm. It is hoped that these drugs will prolong survival by addressing several manifestations of CF lung disease. It is logical to predict that therapies that are directed at the earliest stages of CF lung pathophysiology will improve downstream consequences. However, recent studies have shown that modulators of CFTR function may not drastically decrease the airway inflammatory response (1, 2). We present data indicating that the IL-17 produced in response to P. aeruginosa infection plays a role in initiating the dysregulated inflammatory responses in CF by perpetuating the recruitment of neutrophils into the airway and altering the cytokine response to infection. Specifically, our data demonstrate that (i) in a mouse model of acute infection and inflammation, the concentration of IL-17 present in the BAL fluid from CF mice is greater than the concentration in the BAL fluid from control mice; (ii) when exogenous IL-17 is instilled into the airways, it evokes a neutrophil response which is augmented in CF mice; (iii) pretreatment of CF mice with neutralizing anti-IL-17 antibody is associated with significant reductions in airway neutrophils, bacteria, and inflammation processes; (iv) IL-17 can evoke the production of chemokines from mouse and human airway epithelial cells, which is enhanced in the absence of CFTR activity and which may be induced through chemokine regulation of CSCL2, CEBPβ, and CSCLL; (v) T lymphocytes from both CF and WT mice produce IL-17 in response to a variety of stimuli, especially IL-23, suggesting no defect in the IL-23-induced IL-17 response; and (vi) in studies with Rag1−/− mice, although T cells may participate in the inflammatory response to P. aeruginosa infection, another cell source seems to be the predominant player in the acute response that is unique to the CF environment.
IL-17 is a proinflammatory cytokine that is considered to be produced primarily by Th17 cells after stimulation with cytokines, such as IL-23 (22, 41). Although other cell types, including innate lymphoid cells and neutrophils, also produce IL-17 in individuals with CF, our experiments support the hypothesis that while IL-17 production requires lymphocytes, they do not account for all of the IL-17-induced pathophysiology in CF lung disease. Further, we demonstrated that CF and WT mouse T lymphocytes produce IL-17 in response to IL-23 in a dose-dependent fashion, with CF mouse lymphocytes being particularly sensitive to IL-23 stimulation. This suggests T-cell dysregulation in CF, which is consistent with information published in the literature (6, 42). The studies with Rag1−/− mice demonstrated the presence of IL-17 in response to P. aeruginosa even in the absence of T cells. These data implicate another potential source of IL-17, which seems to be dominant during the acute response to P. aeruginosa in the CF mouse lung, consistent with the findings of other studies (20, 21, 43) and a study from our group recently accepted for publication (32).
While IL-17 has a role in the normal host immune response to Gram-negative bacterial infections (8, 44), it has been associated with a wide spectrum of diseases, such as asthma, rheumatoid arthritis, and inflammatory bowel disease, in which there is a dysregulated inflammatory response typically in the absence of a coexisting bacterial infection (17, 45–48). Further, the IL-17 receptor appears to be ubiquitously expressed throughout the body, implicating IL-17 as an important regulator of the host response during chronic inflammation (49, 50). Within the lung, IL-17 appears to have primary interactions with the airway epithelial cells, smooth muscle cells, and vascular endothelial cells (51). Recent experiments suggest that IL-17 stabilizes IL-6 and IL-8 mRNA in the presence of TNF-α, which may be the etiology of IL-17's proinflammatory effect and potential pathophysiological contribution to disease (52). Our own data suggest that airway epithelial cells respond to IL-17 stimulation with the increased production of IL-6 and IL-8 (KC in the mouse system) in the presence or absence of TNF-α and IL-1β. Additionally, neutralization of IL-17 resulted in decreased amounts of neutrophils, a decreased bacterial burden, and decreased amounts of proinflammatory cytokines in association with the in vivo response to P. aeruginosa concurrently with improved gross lung pathology and clinical scores (43).
In clinical studies analyzing sputum samples induced at the beginning and end of 2 weeks of intravenous antibiotic therapy in patients with CF experiencing a pulmonary exacerbation, the clinical outcome demonstrated only a modest reduction in neutrophils and IL-8, despite a 2- to 3-log reduction in the bacterial count (53). The sustained neutrophil counts and IL-8 concentrations implicate an inflammation stabilization factor, such as IL-17. In fact, the IL-17 level is elevated in the sputum of patients with CF prior to antibiotic treatment for a pulmonary exacerbation (54). Of note, the sputum concentrations of both IL-17A and IL-17F have been measured in these experiments (54), with IL-17F concentrations being higher. If IL-17 stabilizes IL-8 and IL-6, especially in the context of inflammation, the abnormal production of IL-17 in the acute response to P. aeruginosa may initiate the dysregulated inflammatory response in CF by inducing a consistent source of chemokines which are involved in neutrophil recruitment, exaggerating an already augmented inflammatory response in CF. Therefore, inhibition of IL-17 function during acute pulmonary exacerbations might be an alternative route to decreasing the neutrophil chemokine production and subsequent neutrophil influx that occurs during exacerbations (47).
Our data support the hypothesis that IL-17 plays an important pathophysiological role in the CF inflammatory response to P. aeruginosa infection. We suggest that IL-17 production is exaggerated in CF as part of the dysregulated host inflammatory response to infection. The overproduction of IL-17 contributes to the chemokine levels responsible for the massive influx of neutrophils pathological for CF. Further, the exaggerated levels of chemokines in the CF lung are due to the Cftr-deficient epithelium response to both IL-17 and the P. aeruginosa infection (2, 26, 39). In the healthy lung, this exaggerated response does not occur until there are log fold higher levels of pathogen, so the sequences of events are not initiated.
The results of the studies outlined in this report also suggest that the CF pulmonary milieu even in the absence of dysfunctional mucociliary clearance (as seen in the mouse models) has a hypersensitive response to IL-17 as a product of the unique response to P. aeruginosa in individuals with CF. The implication is that the CF lung is hyperresponsive not only to pathogens but also to the cytokines subsequently produced in response to infection, which in the healthy lung would not have as much of an impact during the infection response. Intervening at IL-17 may subdue the initial aberrant cascade of events associated with the dysfunctional response to infection in individuals with CF. From a therapeutic standpoint, the neutralization of IL-17 prior to P. aeruginosa infection has the capacity to decrease the initial aberrant acute response to P. aeruginosa, resulting in a significant improvement in both infection and inflammation. Overall, the findings of our studies support the hypothesis that IL-17 may represent a potential target for anti-inflammatory therapy in individuals with CF with the capacity to modulate the early inflammatory response to infection resulting from CFTR dysfunction.
ACKNOWLEDGMENTS
We thank the CF Animal Core and the CTSC Bioanalyte Core for assistance with these studies.
This work was supported by grants from the National Institutes of Health (P30DK27651, RO1HL73870, R01DK58318), a clinical fellowship award from the Cystic Fibrosis Foundation to D.H., and salary funding from the U.S. Army.
REFERENCES
- 1.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, Ramsey BW. 2014. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols D, Chmiel J, Berger M. 2008. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 34:146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- 3.Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. 2007. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med 175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstan MW, Doring G, Heltshe SL, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton A. 2014. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros 13:148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, Pilewski JM, Kolls JK. 2013. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol 131:1117–1129. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubin PJ, Kolls JK. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 292:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin PJ, McAllister F, Kolls JK. 2007. Is cystic fibrosis a TH17 disease? Inflamm Res 56:221–227. doi: 10.1007/s00011-007-6187-2. [DOI] [PubMed] [Google Scholar]
- 8.Ling Y, Puel A. 2014. IL-17 and infections. Acta Dermosifiliogr 105(Suppl 1):S34–S40. doi: 10.1016/S0001-7310(14)70016-X. [DOI] [PubMed] [Google Scholar]
- 9.Nembrini C, Marsland BJ, Kopf M. 2009. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol 123:986–994. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 10.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol 25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 11.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAleer JP, Kolls JK. 2014. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 260:129–144. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Tan W, Barsoum A, Gu X, Chen K, Huang W, Ramsay A, Kolls JK, Schwarzenberger P. 2010. IL-17 is a potent synergistic factor with GM-CSF in mice in stimulating myelopoiesis, dendritic cell expansion, proliferation, and functional enhancement. Exp Hematol 38:877–884. doi: 10.1016/j.exphem.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Decraene A, Willems-Widyastuti A, Kasran A, De Boeck K, Bullens DM, Dupont LJ. 2010. Elevated expression of both mRNA and protein levels of IL-17A in sputum of stable cystic fibrosis patients. Respir Res 11:177. doi: 10.1186/1465-9921-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LA, van den Berg WB. 2003. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol 170:2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 16.Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol 167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis 12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 18.Afzal N, Zaman S, Asghar A, Javed K, Shahzad F, Zafar A, Nagi AH. 2014. Negative association of serum IL-6 and IL-17 with type-II diabetes retinopathy. Iran J Immunol 11:40–48. [PubMed] [Google Scholar]
- 19.Ankathatti Munegowda M, Deng Y, Chibbar R, Xu Q, Freywald A, Mulligan SJ, van Drunen Littel-van den Hurk S, Sun D, Xiong S, Xiang J. 2011. A distinct role of CD4+ Th17- and Th17-stimulated CD8+ CTL in the pathogenesis of type 1 diabetes and experimental autoimmune encephalomyelitis. J Clin Immunol 31:811–826. doi: 10.1007/s10875-011-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H, Jaffee EM, Drake CG, Housseau F, Maitra A, Kolls JK, Sears CL, Pardoll DM, Leach SD. 2014. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25:621–637. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JE, Sutherland TE, Ruckerl D. 2015. IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr Opin Immunol 34:99–106. doi: 10.1016/j.coi.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhall C, Sjostrand M, Kolls JK, Anderson GP, Linden A. 2007. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am J Respir Cell Mol Biol 36:442–451. doi: 10.1165/rcmb.2006-0020OC. [DOI] [PubMed] [Google Scholar]
- 23.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, Lukacs NW, Kolls JK, Peebles RS Jr. 2012. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol 188:1027–1035. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan HL, Rosenthal M. 2013. IL-17 in lung disease: friend or foe? Thorax 68:788–790. doi: 10.1136/thoraxjnl-2013-203307. [DOI] [PubMed] [Google Scholar]
- 25.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazlett LD. 2005. Inflammatory response to Pseudomonas aeruginosa keratitis. Ocul Surf 3:S139–S141. [DOI] [PubMed] [Google Scholar]
- 27.Li C, McClellan SA, Barrett R, Hazlett LD. 2014. Interleukin 17 regulates Mer tyrosine kinase-positive cells in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 55:6886–6900. doi: 10.1167/iovs.14-14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Qu H, Li Q, Ye L, Ma G, Wan H. 2013. The responses of gammadelta T-cells against acute Pseudomonas aeruginosa pulmonary infection in mice via interleukin-17. Pathog Dis 68:44–51. doi: 10.1111/2049-632X.12043. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, Wan H. 2011. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol 61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Shao B, Wang R, Zhou S, Tang Z, Lu W, Xiong S. 2014. Role of interleukin-17 in defense against Pseudomonas aeruginosa infection in lungs. Int J Clin Exp Med 7:809–816. [PMC free article] [PubMed] [Google Scholar]
- 31.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. 2011. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med 184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PR, Bonfield TL, Chmiel JF, Pearlman E. 4 May 2016. Neutrophils from F508del cystic fibrosis patients produce IL-17A and express IL-23-dependent IL-17C. Clin Immunol doi: 10.1016/j.clim.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 33.van Heeckeren AM, Schluchter MD. 2002. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim 36:291–312. doi: 10.1258/002367702320162405. [DOI] [PubMed] [Google Scholar]
- 34.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. 1997. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Heeckeren AM, Schluchter MD, Xue W, Davis PB. 2006. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med 173:288–296. doi: 10.1164/rccm.200506-917OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. 2012. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, Haxhiu MA, Ferkol TW. 2000. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med 161:271–279. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 38.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. 2004. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 287:L944–L952. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 39.Saadane A, Eastman J, Berger M, Bonfield TL. 2011. Parthenolide inhibits ERK and AP-1 which are dysregulated and contribute to excessive IL-8 expression and secretion in cystic fibrosis cells. J Inflamm (Lond) 8:26. doi: 10.1186/1476-9255-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saadane A, Soltys J, Berger M. 2006. Acute Pseudomonas challenge in cystic fibrosis mice causes prolonged nuclear factor-kappa B activation, cytokine secretion, and persistent lung inflammation. J Allergy Clin Immunol 117:1163–1169. doi: 10.1016/j.jaci.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih VF, Cox J, Kljavin NM, Dengler HS, Reichelt M, Kumar P, Rangell L, Kolls JK, Diehl L, Ouyang W, Ghilardi N. 2014. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc Natl Acad Sci U S A 111:13942–13947. doi: 10.1073/pnas.1323852111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubin PJ, Kolls JK. 2011. IL-17 in cystic fibrosis: more than just Th17 cells. Am J Respir Crit Care Med 184:155–157. doi: 10.1164/rccm.201104-0617ED. [DOI] [PubMed] [Google Scholar]
- 44.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Qian T, Zhang D, Yan H, Hao F. 2015. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy 7:1023–1037. doi: 10.2217/imt.15.50. [DOI] [PubMed] [Google Scholar]
- 46.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. 2005. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol 175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 47.Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, Harintho MT, Lukacs NW, Kolls JK, Peebles RS Jr. 2013. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax 68:717–723. doi: 10.1136/thoraxjnl-2012-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickens SR, Volin MV, Mandelin AM, Kolls JK, Pope RM, Shahrara S. 2010. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol 184:3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolls JK, Linden A. 2004. Interleukin-17 family members and inflammation. Immunity 21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 50.Kolls JK. 2010. Th17 cells in mucosal immunity and tissue inflammation. Semin Immunopathol 32:1–2. doi: 10.1007/s00281-010-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang P, Zhou L, Zhou Y, Kolls JK, Zheng T, Zhu Z. 2014. Immune modulatory effects of IL-22 on allergen-induced pulmonary inflammation. PLoS One 9:e107454. doi: 10.1371/journal.pone.0107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Netea MG, van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. 2005. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother 49:3991–3996. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miossec P, Kolls JK. 2012. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 54.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. 2005. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]