Abstract
Plasmodium falciparum is the most virulent human malaria parasite because of its ability to cytoadhere in the microvasculature. Nonhuman primate studies demonstrated relationships among knob expression, cytoadherence, and infectivity. This has not been examined in humans. Cultured clinical-grade P. falciparum parasites (NF54, 7G8, and 3D7B) and ex vivo-derived cell banks were characterized. Knob and knob-associated histidine-rich protein expression, CD36 adhesion, and antibody recognition of parasitized erythrocytes (PEs) were evaluated. Parasites from the cell banks were administered to malaria-naive human volunteers to explore infectivity. For the NF54 and 3D7B cell banks, blood was collected from the study participants for in vitro characterization. All parasites were infective in vivo. However, infectivity of NF54 was dramatically reduced. In vitro characterization revealed that unlike other cell bank parasites, NF54 PEs lacked knobs and did not cytoadhere. Recognition of NF54 PEs by immune sera was observed, suggesting P. falciparum erythrocyte membrane protein 1 expression. Subsequent recovery of knob expression and CD36-mediated adhesion were observed in PEs derived from participants infected with NF54. Knobless cell bank parasites have a dramatic reduction in infectivity and the ability to adhere to CD36. Subsequent infection of malaria-naive volunteers restored knob expression and CD36-mediated cytoadherence, thereby showing that the human environment can modulate virulence.
INTRODUCTION
Plasmodium falciparum is the most virulent of the six Plasmodium sp. parasites that infect humans. Its ability to cytoadhere and sequester itself in the microvasculature can result in obstruction of blood flow and organ dysfunction, and these are key processes in the development of severe falciparum malaria (1).
Parasite cytoadherence and sequestration are facilitated by parasite-encoded knoblike structures that first appear on early trophozoite-stage parasites and are formed beneath the plasma membrane of parasitized erythrocytes (PEs) (2). Cytoadherence to receptors in the deep vasculature prevents removal and destruction of the parasite by the mononuclear phagocytic system, especially the spleen. Higher knob densities have been reported on PEs collected directly from patients than on cultured PEs infected in vitro (3). Following in vitro cultivation of P. falciparum, knob formation on PEs varies in an isolate-dependent manner (3–5), ranging from a mild reduction in density to complete ablation. The major structural protein in knobs is the knob-associated histidine-rich protein (KAHRP) (2, 6, 7). Deletion of the gene encoding KAHRP results in the loss of knobs (8, 9).
P. falciparum isolates can lose the ability to adhere to tissue receptors in vitro (10). Loss of adherence is isolate dependent and can occur independently of whether the knob phenotype is retained (10). Cytoadherence involves an interaction between parasite ligands and tissue receptors, including CD36, on the vascular endothelium (11, 12). The principal parasite adhesin is P. falciparum erythrocyte membrane protein 1 (PfEMP1) (13), an antigenically variant product of the var gene family that is expressed on the surface of late-stage PEs and is concentrated on knobs (14–16). Knobless parasites continue to express PfEMP1 (17, 18) and have been shown to cytoadhere in static assays (17, 19, 20). However, under physiologic flow conditions, the ability of knob- and KAHRP-negative parasites to bind to tissue receptors is significantly reduced (7). A reduction in the amount of PfEMP1 displayed on the surface of knobless PEs in vitro has been reported (21).
Knobby and knobless parasites have been examined in nonhuman primates (22–24). Knobby clones were more virulent than knobless clones in nonsplenectomized monkeys (22, 23). Knobless P. falciparum parasites were rapidly cleared from the circulation, unlike knob-expressing parasites, an observation attributable to the parasite's ability to sequester itself and replicate without clearance (23). In contrast, in splenectomized monkeys, this knobless clone showed virulence and did not sequester. A knob-positive phenotype was stable in vivo and was not affected by the presence of the spleen (23). Infection of splenectomized monkeys with knobby P. falciparum parasites can result in either loss of knobs (22) or loss of cytoadherence in the presence of knobs (25). Cytoadherence could be restored when parasites from splenectomized animals were subsequently transferred into animals with intact spleens (25). The effect of the spleen on cytoadherence may reflect a fitness cost to the parasite. This is supported by the observation that sequential passage of P. knowlesi in splenectomized monkeys resulted in loss of agglutinability of PEs (26) and a reduction in variant surface antigen expression (24). Together, these simian studies demonstrate that knob expression and the ability to cytoadhere can be variable and affect parasite infectivity.
In humans, knobby and knobless cytoadherent P. falciparum parasites have been derived from splenectomized patients (27, 28). However, the relationship between knob expression, an adherent phenotype, and infectivity of P. falciparum has not been investigated. Controlled human malaria infection (CHMI) of malaria-naive human volunteers provides an opportunity to investigate this. CHMI can be undertaken by three means: allowing laboratory-reared Plasmodium-infected mosquitoes to feed on study participants (29–32), injecting cryopreserved sporozoites (33–36), or injecting PEs (37–40). We have recently undertaken the current good manufacturing practice production of clinical-grade cultured P. falciparum blood-stage cell banks (41). While evaluating the safety and infectivity of these cell banks in malaria-naive volunteers, we demonstrated that although knobless parasites have the ability to grow normally in vitro, they have a dramatic reduction in infectivity in vivo, mirroring a similar reduction in the ability to cytoadhere to CD36 in vitro. Of note, in vivo infection in malaria-naive volunteers restored the expression of knobs and cytoadherence, thus demonstrating a major role for the human environment in the modulation and reprogramming of parasite virulence.
MATERIALS AND METHODS
Clinical studies.
Studies 1 and 2 were conducted at the Gold Coast Hospital, Southport, QLD, Australia; study 3 was conducted at Griffith University, Southport, QLD, Australia; and studies 4 and 5 were conducted at Q-Pharm, Brisbane, QLD, Australia. Study participants were healthy male Caucasians 18 to 45 years old. Key eligibility criteria are listed for each study at the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au; study reference numbers are provided below). Two volunteers were enrolled in each study with a delay of >2 days between the inoculations of participants 1 and 2 in each group.
If clinical or parasitological evidence of malaria (identification of ≥2 malaria parasites on a malaria thick film, a platelet count of <100 × 109/liter, or the onset of clinical features of malaria) was found or the parasitemia threshold defined in the study protocol was reached, antimalaria treatment was initiated. For study 1, antimalaria treatment was initiated as stipulated by the study protocol, despite the lack of parasite growth. Treatment of malaria entailed the administration of artemether-lumefantrine (A/L) according to its approved dosing schedule.
Adverse events (abnormal laboratory values, clinical signs or symptoms) were monitored either via telephone or at the clinical sites and graded in severity by experienced clinicians.
Ethics statement.
Studies 1 to 3 were approved by the Gold Coast Hospital and Health Services District Human Research Ethics Committee (HREC) and/or the Griffith University HREC. Studies 4 and 5 were approved by the Queensland Institute of Medical Research (QIMR) HREC. The studies were registered at the Australian New Zealand Clinical Trials Registry (study 1, ACTRN12612001153808; study 2, ACTRN12613000615785; study 3: ACTRN12613001187730; study 4, ACTRN12613000669796 and study 5, ACTRN12612000824864). Written informed consent was obtained from all of the participants prior to the commencement of this study.
P. falciparum malaria blood-stage cell banks.
For studies 1 to 3, cultured P. falciparum NF54 (studies 1 and 2) and 7G8 (study 3) malaria blood-stage cell banks were manufactured at the Institute for Glycomics, Griffith University, as previously described (41). The P. falciparum 3D7B cultured malaria blood-stage cell bank (study 4) was manufactured at the QIMR Berghofer Medical Research Institute as previously described (41); it was derived from a vial of an ex vivo P. falciparum 3D7 cell bank that has been used in previous clinical studies (37–40, 42).
The ex vivo P. falciparum HMP02 cell bank (study 5) was derived from an Australian resident who contracted malaria in Ghana. Parasitized red blood cells were cryopreserved at the QIMR Berghofer Medical Research Institute as previously described (43).
Using methodology that has been previously described (43), P. falciparum NF54 blood-stage malaria cell banks (NF54-S01 and NF54-S02) were prepared from the peripheral blood of both study participants infected with P. falciparum NF54 (study 2) just prior to the commencement of A/L treatment.
PCR.
Sample preparation, DNA extraction, and parasitemia measurement by quantitative PCR (qPCR) were done as previously described (44), with the following modifications. A standard curve was prepared from a lyophilized WHO P. falciparum international standard (National Institute for Biological Standards and Control code 04/176) (45) that was reconstituted in 500 μl of nuclease-free water and diluted in a 1:1 solution with 1× phosphate-buffered saline (PBS; Gibco). DNA was isolated from 500 μl of this solution at a concentration of 5 × 108 IU/ml. Blood samples from study participants and standards were tested in triplicate. Established modified calculations (46) were used to equate international units per milliliter to parasites per milliliter, with 1 IU/ml equivalent to 0.5 parasite/ml. The number of parasites per milliliter was calculated with the CFX96 Touch Real Time detection system software (Bio-Rad, Australia).
Measurement of antibodies to the surface of P. falciparum PEs by flow cytometry.
Testing for IgG binding to the surface of PEs by flow cytometry was performed as described previously (47), with some minor modifications (see the supplemental material).
Preparation and administration of the parasite inoculum.
Inocula were prepared as previously described, by taking an aliquot of the relevant cell bank and thawing, washing, and diluting it to the appropriate dose and volume with 0.9% saline for injection (42). The number of parasites present in the inoculum was verified retrospectively by qPCR assay of surplus material. The inoculums were dispensed into as many 2-ml syringes as required for administration to the study participants who were inoculated by intravenous injection.
P. falciparum adhesion assays.
Adhesion assays were performed as previously described (48, 49), with P. falciparum trophozoite-stage PEs. Incubation was at 37°C for 30 min, and washing steps were performed with RPMI. Bound cells were fixed in 2% glutaraldehyde in PBS, stained with Giemsa, and counted by microscopy. Adhesion to CD36 at 20 μg/ml (rhCD36/Fc Chimera; R&D Systems) was tested in triplicate.
Scanning electron microscopy.
Scanning electron microscopy was performed with P. falciparum trophozoite-stage PEs as outlined in the supplemental material.
kahrp expression assay.
The level of kahrp expression in the cell bank parasites and participant blood was quantified by reverse transcription-qPCR as outlined in the supplemental material.
Statistical analysis.
The R package was used to calculate the Pearson correlation coefficients for the relative kahrp gene copy number and the average binding data to CD36.
RESULTS
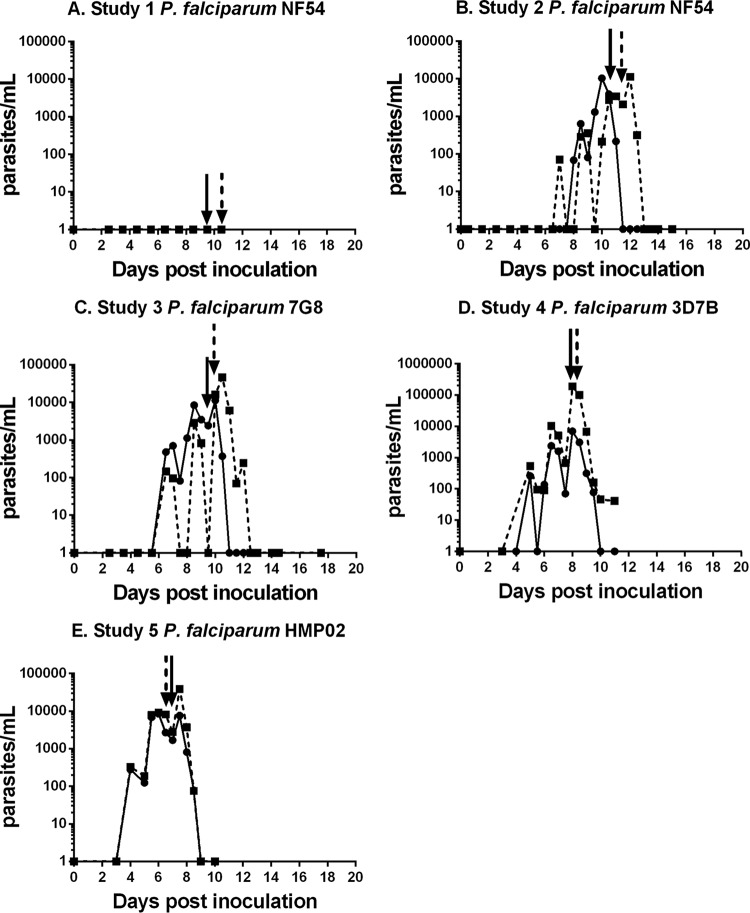
To test the infectivity of the NF54 strain of P. falciparum, two volunteers were inoculated with 1,800 PEs. This number had been previously used for CHMI studies with volunteers infected with an ex vivo bank of the 3D7 clone of NF54 (38). Parasites were not detected by qPCR in the blood of volunteers for up to 10 days (Fig. 1A), at which time participants commenced antimalaria treatment with A/L, in accordance with the study protocol. Six months later, the same volunteers were reinoculated with 30,000 NF54 PEs. We observed parasites in both individuals on day 6 (Fig. 1B). Parasite growth kinetics were similar to those previously reported for ex vivo-initiated infections with 1,800 PEs (38). A/L treatment was initiated on days 10 and 11. We then tested the infectivity of three other parasite lines with a dose of 1,800 PEs: a cultured 7G8 line (41), a 3D7 line referred to as 3D7B (41) that had only recently been cultured from the ex vivo 3D7 bank (43), and a novel ex vivo parasite, HMP02. The growth curves are shown in Fig. 1C to E. The parasite growth kinetics were similar to those of 30,000 NF54 PEs (Fig. 1B). No serious adverse events were observed in any volunteers. Minor adverse events and laboratory abnormalities are listed in Tables S1 and S2 in the supplemental material.
FIG 1.
Course of parasitemia in study participants inoculated with P. falciparum cell banks. Shown are the parasite levels in study participants following inoculation with the different P. falciparum cell bank parasites. (A) Study 1, P. falciparum NF54 (cultured cell bank). (B) Study 2, P. falciparum NF54 (cultured cell bank). (C) Study 3, P. falciparum 7G8 (cultured cell bank). (D) Study 4, P. falciparum 3D7B (cultured cell bank). (E) Study 5, P. falciparum HMP02 (ex vivo cell bank). Arrows indicate the times of administration of drug treatment. Note the different y-axis scale for P. falciparum 3D7B. In each study, n = 2.
During the infectivity studies, new parasite isolates were derived directly ex vivo from the peripheral blood of the volunteers who received 30,000 NF54 PEs (NF54-S01 and NF54-S02) and from one of the volunteers who received 1,800 3D7B PEs (3D7-S102). These were used for further studies (described below) that were performed after six or seven cycles of in vitro culture.
To understand why the infectivity of NF54 was lower, we used scanning electron microscopy to examine the surface composition of the PEs for the expression of knobs. A 3D7 parasite line that was regularly selected by gelatin flotation to maintain knob expression was used as a positive control and displayed numerous prominent knobs on the erythrocyte surface (50) (Fig. 2A). In comparison, the NF54 PEs exhibited a smooth surface, similar to what has been observed in kahrp knockout lines (18) (Fig. 2B). Knobs were present on the surface of the cultured 3D7B PEs (Fig. 2E), on the P. falciparum 7G8 PEs (Fig. 2G), on the ex vivo HMP02 PEs (Fig. 2H), and on PEs from the three ex vivo parasite lines (NF54-S01, NF54-S02, and 3D7-S102) derived from the study participants (Fig. 2C, D, and F). Thus, infection with NF54 PEs resulted in the selection of knob-expressing parasites in the two volunteers.
FIG 2.
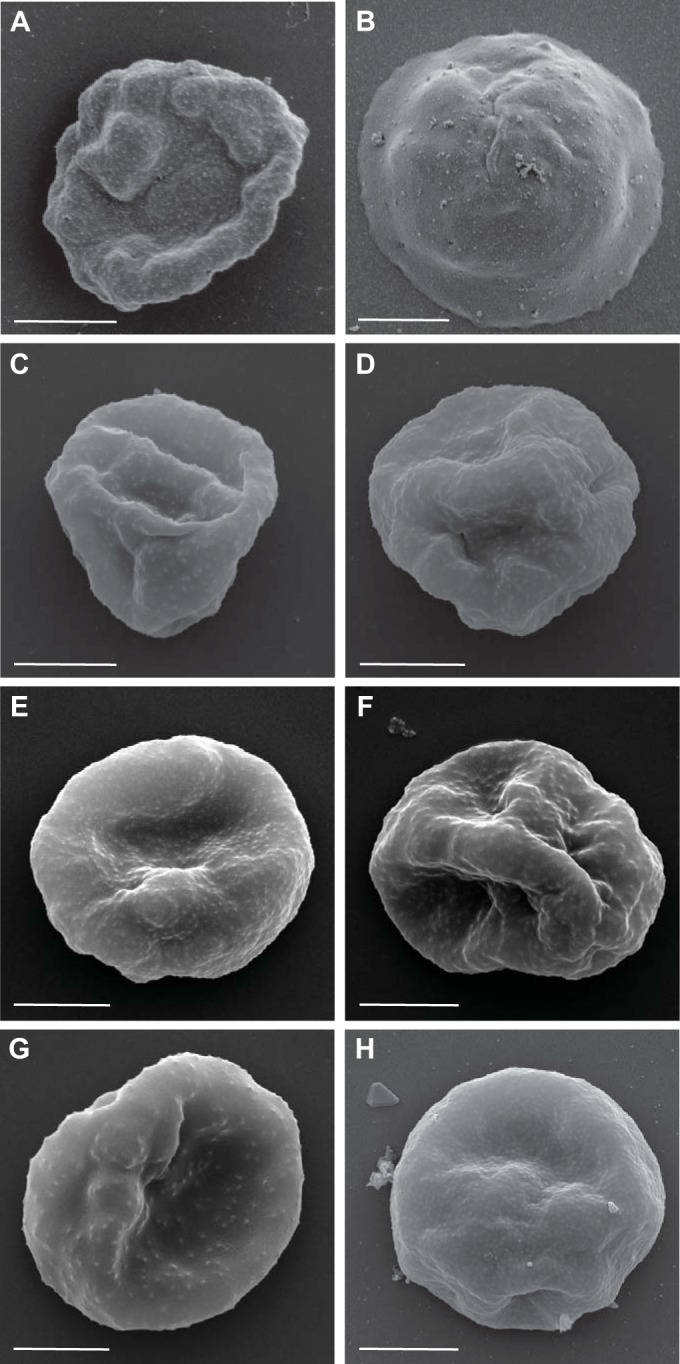
Surface characteristics of PEs from different P. falciparum parasite lines. Shown are scanning electron micrographs of the P. falciparum 3D7 control (A), P. falciparum NF54 (cultured cell bank) (B), P. falciparum NF54-S01 (derived ex vivo from S01 at the time of drug treatment) (C), P. falciparum NF54-S02 (derived ex vivo from S02 at the time of drug treatment) (D), P. falciparum 3D7B (cultured cell bank) (E), P. falciparum 3D7-S102 (derived ex vivo from S102 at the time of drug treatment) (F), P. falciparum 7G8 (cultured cell bank) (G), and P. falciparum HMP02 (ex vivo cell bank) (H). Representative images are shown, and the scale bars all represent 2 μm.
Knobs and PfEMP1 play an important role in parasite adhesion and virulence, with CD36 being an important receptor for PfEMP1. Thus, we performed static binding assays to examine the adhesion of PEs from the different parasite lines to CD36. Adhesion was evaluated relative to that of the same 3D7 control parasite used to characterize knob expression. No adhesion of P. falciparum NF54 PEs to CD36 was observed (Fig. 3), whereas adhesion comparable to that of the 3D7 control was observed in all of the other parasites. This included ex vivo parasite lines NF54-S01 and NF54-S02, indicating that in vivo infection with NF54 also altered the adhesive phenotype of the parasite.
FIG 3.
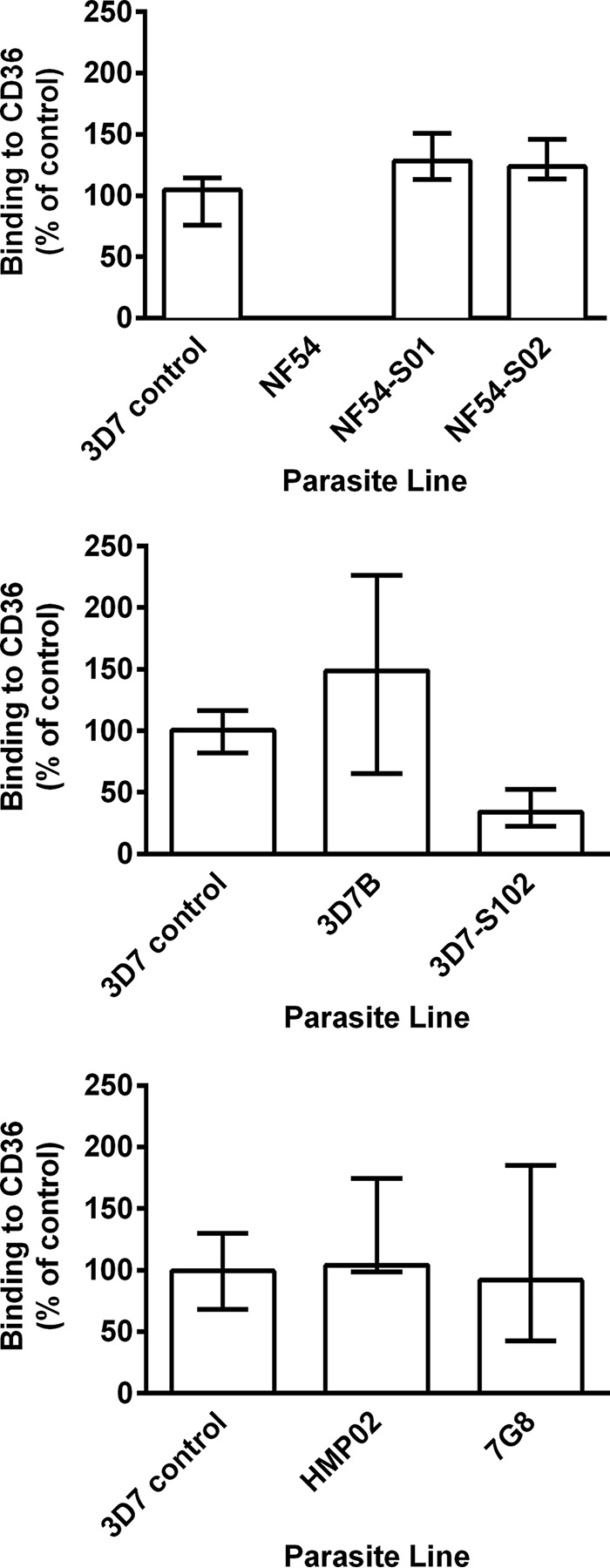
Binding of P. falciparum PEs from different parasite lines to CD36. Shown is the adhesion to recombinant CD36 of P. falciparum NF54 (cultured cell bank) and P. falciparum NF54-S01 and NF54-S02 (derived ex vivo from S01 and S02 at the time of drug treatment) (top), P. falciparum 3D7B (cultured cell bank) and P. falciparum 3D7-S102 (derived ex vivo from S102 at the time of drug treatment) (middle), and P. falciparum HMP02 (ex vivo cell bank) and P. falciparum 7G8 (cultured cell bank) (bottom). Values are expressed as percentages of the P. falciparum 3D7 control parasite binding to CD36. Assays were performed twice independently, and bars represent median values and interquartile ranges of experimental replicates of samples tested in triplicate.
KAHRP is essential for knob formation, and deletion of the kahrp gene from one end of chromosome 2 has been observed in long-term-cultured isolates (8). Thus, we examined the levels of kahrp gene transcription in the parasite lines by qPCR, aiming to determine if the lack of knobs and CD36 receptor binding by NF54 PEs was due to deletion of the kahrp gene or downregulation of gene expression. Expression of kahrp was observed in all of the parasite lines, including P. falciparum NF54, which did not express knobs (Fig. 4). There was no relationship between the relative copy numbers of the kahrp gene present in the parasites and adhesion to CD36 (r = −0.18; P = 0.7).
FIG 4.
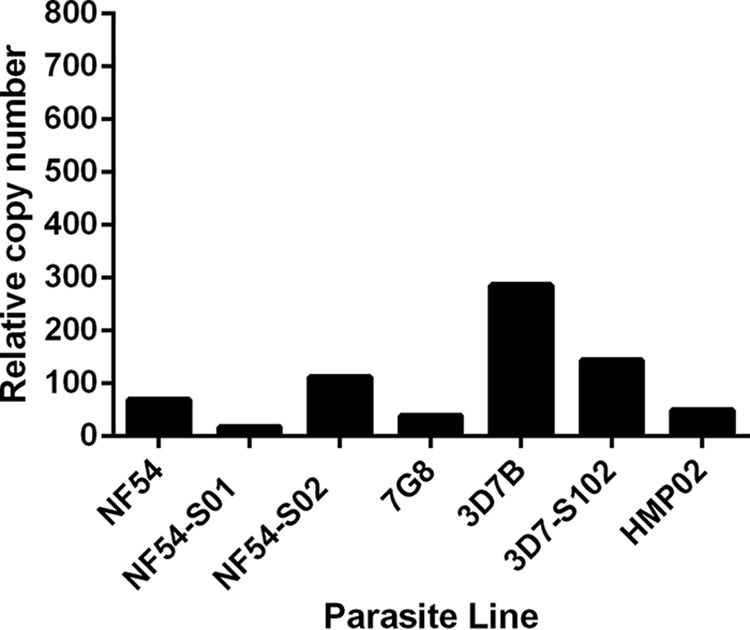
Expression of kahrp in different P. falciparum parasite lines. Shown are kahrp expression levels in P. falciparum cell bank and ex vivo-derived parasites relative to those of the single-copy fructose-bisphosphate aldolase gene.
Using serum samples from adults residing in a region of Papua New Guinea where malaria is endemic, we examined antibody recognition of the surface of PEs from the different parasite lines by flow cytometry. Antibodies to the surface of PEs predominantly recognize PfEMP1 (50). The 3D7 parasite was again used as a control. PEs from all of the parasite lines were recognized by the Papua New Guinea serum samples (Fig. 5; see Fig. S1 in the supplemental material), suggesting that the NF54 PEs continued to express PfEMP1 and other variant surface antigens. It was of interest that PEs from the ex vivo-derived lines (NF54-S01, NF54-S02, and 3D7-S102) were not as well recognized by antibodies in the serum as PEs from the parental parasites with which the volunteers were infected (NF54, 3D7B), perhaps reflecting a switch to the expression of a new PfEMP1-encoding gene during infection.
FIG 5.
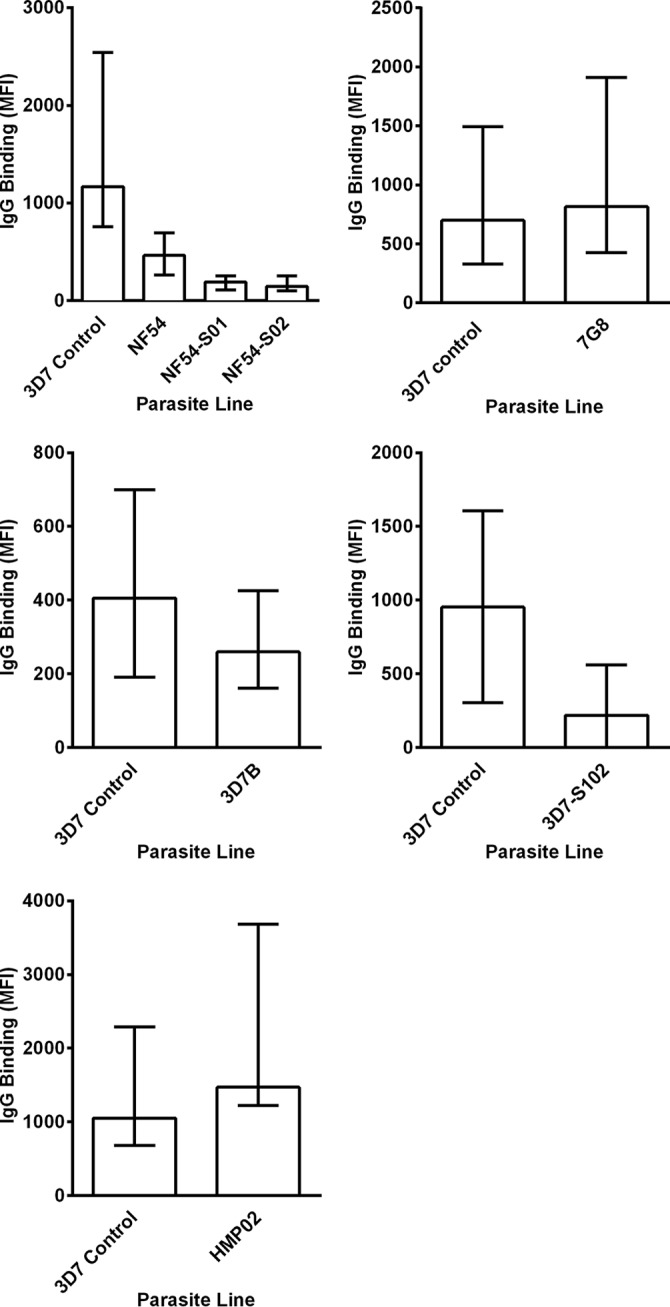
Antibody recognition of the surface of P. falciparum PEs from different parasite lines. Shown is the binding of antibodies in serum samples from Papua New Guinean adults to surface antigens expressed by PEs from P. falciparum NF54 (cultured cell bank), P. falciparum NF54-S01 and NF54-S02 (derived ex vivo from S01 and S02 at the time of drug treatment) (top left), P. falciparum 7G8 (cultured cell bank) (top right), P. falciparum 3D7B (cultured cell bank) (middle left), P. falciparum 3D7-S102 (derived ex vivo from S102 at the time of drug treatment) (middle right), and P. falciparum HMP02 (ex vivo cell bank) (bottom). The IgG binding level is expressed as the geometric mean fluorescence intensity (MFI) in all of the graphs, and the bars represent the median values and interquartile ranges of samples tested in duplicate (n = 10 for all P. falciparum cell banks). Minimal reactivity was observed among serum samples from nonexposed Melbourne controls.
DISCUSSION
Here, using novel P. falciparum cultured and ex vivo-derived blood-stage cell banks (41), we have demonstrated, for the first time in malaria-naive individuals, a relationship between knob expression, cytoadhesion, and the in vivo infectivity of P. falciparum. This is also the first time that blood-stage parasites from clinical-grade P. falciparum cultured cell banks (41) have been administered to human volunteers to evaluate their safety and infectivity.
Following the administration of 1,800 NF54 PEs to two malaria-naive study participants, parasite growth was not detected in their blood up to day 10 postinoculation. We cannot exclude the possibility that if we had continued to monitor these individuals, parasites would have been detected. However, the study protocol required initiation of antimalaria treatment at that time. Limiting-dilution analysis of this cell bank has demonstrated that the viability of these parasites is approximately 50% (41), thereby excluding the possibility that lack of parasite growth was due to unhealthy/dead parasites. We subsequently administered 30,000 NF54 PEs to the same volunteers and observed parasite growth. Infectivity was observed for all other cultured and ex vivo-derived cell banks. In vitro characterization of the NF54 PEs demonstrated that they lacked knobs and were unable to adhere to CD36 but were still recognized by antibodies present in the serum of malaria-exposed individuals, suggesting that PfEMP1 and/or other variant surface antigens were still being expressed. Previous studies have demonstrated that long-term culture can be associated with loss of knobs and loss of CD36-mediated cytoadhesion as measured in vitro (4, 10), as well as in in vivo studies in animal models. Furthermore, this phenotype is associated with attenuation of parasitemia in nonsplenectomized monkeys (23). We do not know when our NF54 cell bank became predominantly knobless PEs. Prior to drug treatment, new parasite isolates (NF54-S01 and NF54-S02) were derived ex vivo from the peripheral blood of the volunteers who received 30,000 NF54 PEs. Knob expression and CD36-mediated adhesion were observed in PEs from these two isolates. Recovery of the knobby, cytoadherent phenotype following administration to the study participants could reflect that the NF54 parasite line is not clonal, with a small proportion of PEs still expressing knobs and able to cytoadhere. With an increased inoculum dose of 30,000 PEs, it is possible that this minor population initiated infection by sequestering in the periphery and evading destruction by macrophages in the spleen. Transcription of the kahrp gene was detected in cell bank parasites, indicating that the lack of knob expression was not due to a gene deletion or transcriptional silencing. We cannot exclude the possibility that epigenetic reprogramming or deletion of other genes important in knob formation contributed. As infectivity was observed with the other cultured banks (7G8 and 3D7B) when 1,800 PEs were administered, and knob expression and adhesion were absent from only NF54, this phenomenon appears to be isolate dependent, as was previously described in vitro (10).
This is the first study to document the administration of blood-stage parasites from cultured P. falciparum malaria cell banks directly to human volunteers. Additionally, it is the first study to evaluate the safety and virulence of well-characterized, clinical-grade P. falciparum lines that may be used in CHMI studies to assess the efficacy of novel antimalaria drugs and vaccines. For all four of the cell banks tested, the inoculum was well tolerated by recipients. Until recently, the ability to conduct CHMI studies has relied on access to clinical-grade sporozoites or P. falciparum-infected erythrocytes derived directly ex vivo from malaria-infected individuals. Our recent production of clinical-grade, cultured P. falciparum blood-stage cell banks (41) offers an additional approach to CHMI. The use of cultured parasites in CHMI studies will accelerate the development of novel antimalaria drugs and malaria vaccine candidates, provided the phenotype and infectivity of the parasites are known. We observed that NF54 infectivity is related to cytoadherence and knob expression. However, loss of these characteristics is isolate dependent.
In conclusion, by using a novel approach to CHMI, we have demonstrated that the human environment can directly modulate the virulence of P. falciparum by altering the surface phenotype of PEs. While we have studied a single virulence phenotype, it seems likely that other virulence phenotypes may also be under selective pressure from the human host. CHMI with cultured malaria parasites provides an exciting opportunity to study malaria pathogenesis in humans.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the study participants. We thank Tanya Forbes for regulatory support and Michael Batzloff and Chris Davis for advice and support throughout this study (Griffith University). We thank Kate Thorpe and Gem Mackenroth of Q-Pharm, Tammy Schmidt and Rachael Dunning of Gold Coast Hospital, and Judy Coote of Griffith University for assistance with project management. We thank Dennis Shanks and Qin Cheng (Australian Army Malaria Institute) for kindly providing the P. falciparum 7G8 parasites from which the P. falciparum 7G8 cell bank was derived. We also thank Dennis Shanks for serving on the safety review team.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00414-16.
REFERENCES
- 1.Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, Hasan MU, Hossain A, Charunwatthana P, Chotivanich K, Maude RJ, Kingston H, Day NP, Mishra S, White NJ, Dondorp AM. 2012. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis 206:571–579. doi: 10.1093/infdis/jis400. [DOI] [PubMed] [Google Scholar]
- 2.Pologe LG, Pavlovec A, Shio H, Ravetch JV. 1987. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc Natl Acad Sci U S A 84:7139–7143. doi: 10.1073/pnas.84.20.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quadt KA, Barfod L, Andersen D, Bruun J, Gyan B, Hassenkam T, Ofori MF, Hviid L. 2012. The density of knobs on Plasmodium falciparum-infected erythrocytes depends on developmental age and varies among isolates. PLoS One 7:e45658. doi: 10.1371/journal.pone.0045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langreth SG, Reese RT, Motyl MR, Trager W. 1979. Plasmodium falciparum: loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol 48:213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- 5.Li A, Mansoor AH, Tan KS, Lim CT. 2006. Observations on the internal and surface morphology of malaria infected blood cells using optical and atomic force microscopy. J Microbiol Methods 66:434–439. doi: 10.1016/j.mimet.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Kilejian A. 1979. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A 76:4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabb B, Cooke B, Reeder J, Waller R, Caruana S, Davern K, Wickham M, Brown G, Coppel R, Cowman A. 1997. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89:287–296. doi: 10.1016/S0092-8674(00)80207-X. [DOI] [PubMed] [Google Scholar]
- 8.Biggs BA, Kemp DJ, Brown GV. 1989. Subtelomeric chromosome deletions in field isolates of Plasmodium falciparum and their relationship to loss of cytoadherence in vitro. Proc Natl Acad Sci U S A 86:2428–2432. doi: 10.1073/pnas.86.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pologe LG, Ravetch JV. 1986. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. Nature 322:474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- 10.Udeinya IJ, Graves PM, Carter R, Aikawa M, Miller LH. 1983. Plasmodium falciparum: effect of time in continuous culture on binding to human endothelial cells and amelanotic melanoma cells. Exp Parasitol 56:207–214. doi: 10.1016/0014-4894(83)90064-4. [DOI] [PubMed] [Google Scholar]
- 11.Maubert B, Guilbert LJ, Deloron P. 1997. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun 65:1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oquendo P, Hundt E, Lawler J, Seed B. 1989. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 13.Leech J, Barnwell J, Miller L, Howard R. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium-infected erythrocytes. J Exp Med 159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 15.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. 1995. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82:101–110. doi: 10.1016/0092-8674(95)90056-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 17.Biggs BA, Gooze L, Wycherley K, Wilkinson D, Boyd AW, Forsyth KP, Edelman L, Brown GV, Leech JH. 1990. Knob-independent cytoadherence of Plasmodium falciparum to the leukocyte differentiation antigen CD36. J Exp Med 171:1883–1892. doi: 10.1084/jem.171.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rug M, Prescott SW, Fernandez KM, Cooke BM, Cowman AF. 2006. The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood 108:370–378. doi: 10.1182/blood-2005-11-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggs BA, Culvenor JG, Ng JS, Kemp DJ, Brown GV. 1989. Plasmodium falciparum: cytoadherence of a knobless clone. Exp Parasitol 69:189–197. doi: 10.1016/0014-4894(89)90187-2. [DOI] [PubMed] [Google Scholar]
- 20.Udomsangpetch R, Aikawa M, Berzins K, Wahlgren M, Perlmann P. 1989. Cytoadherence of knobless Plasmodium falciparum-infected erythrocytes and its inhibition by a human monoclonal antibody. Nature 338:763–765. doi: 10.1038/338763a0. [DOI] [PubMed] [Google Scholar]
- 21.Horrocks P, Pinches R, Chakravorty S, Papakrivos J, Christodoulou Z, Kyes S, Urban B, Ferguson D, Newbold C. 2005. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci 118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 22.Lanners HN, Trager W. 1984. Comparative infectivity of knobless and knobby clones of Plasmodium falciparum in splenectomized and intact Aotus trivirgatus monkeys. Z Parasitenkd 70:739–745. doi: 10.1007/BF00927126. [DOI] [PubMed] [Google Scholar]
- 23.Langreth SG, Peterson E. 1985. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect Immun 47:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnwell JW, Howard RJ, Coon HG, Miller LH. 1983. Splenic requirement for antigenic variation and expression of the variant antigen on the erythrocyte membrane in cloned Plasmodium knowlesi malaria. Infect Immun 40:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David PH, Hommel M, Miller LH, Udeinya IJ, Oligino LD. 1983. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A 80:5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnwell JW, Howard RJ, Miller LH. 1982. Altered expression of Plasmodium knowlesi variant antigen on the erythrocyte membrane in splenectomized rhesus monkeys. J Immunol 128:224–226. [PubMed] [Google Scholar]
- 27.Pongponratn E, Viriyavejakul P, Wilairatana P, Ferguson D, Chaisri U, Turner G, Looareesuwan S. 2000. Absence of knobs on parasitized red blood cells in a splenectomized patient in fatal falciparum malaria. Southeast Asian J Trop Med Public Health 31:829–835. [PubMed] [Google Scholar]
- 28.Ho M, Bannister LH, Looareesuwan S, Suntharasamai P. 1992. Cytoadherence and ultrastructure of Plasmodium falciparum-infected erythrocytes from a splenectomized patient. Infect Immun 60:2225–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL. 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 30.Ballou WR, Sherwood JA, Neva FA, Gordon DM, Wirtz RA, Wasserman GF, Diggs CL, Hoffman SL, Hollingdale MR, Hockmeyer WT, Schneider I, Young JF, Reeve P, Chulay JD. 1987. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet i:1277–1281. [DOI] [PubMed] [Google Scholar]
- 31.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group N Engl J Med 336:86–91. [DOI] [PubMed] [Google Scholar]
- 32.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, Andersen RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. 2006. A DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun 74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roestenberg M, Bijker EM, Sim BK, Billingsley PF, James ER, Bastiaens GJ, Teirlinck AC, Scholzen A, Teelen K, Arens T, van der Ven AJ, Gunasekera A, Chakravarty S, Velmurugan S, Hermsen CC, Sauerwein RW, Hoffman SL. 2013. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 88:5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, Mpina M, Ali Juma O, Schindler T, Huber E, Gunasekera A, Manoj A, Simon B, Saverino E, Church LW, Hermsen CC, Sauerwein RW, Plowe C, Venkatesan M, Sasi P, Lweno O, Mutani P, Hamad A, Mohammed A, Urassa A, Mzee T, Padilla D, Ruben A, Sim BK, Tanner M, Abdulla S, Hoffman SL. 2014. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 91:471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehy SH, Spencer AJ, Douglas AD, Sim BK, Longley RJ, Edwards NJ, Poulton ID, Kimani D, Williams AR, Anagnostou NA, Roberts R, Kerridge S, Voysey M, James ER, Billingsley PF, Gunasekera A, Lawrie AM, Hoffman SL, Hill AV. 2013. Optimising controlled human malaria infection studies using cryopreserved parasites administered by needle and syringe. PLoS One 8:e65960. doi: 10.1371/journal.pone.0065960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodgson SH, Juma E, Salim A, Magiri C, Kimani D, Njenga D, Muia A, Cole AO, Ogwang C, Awuondo K, Lowe B, Munene M, Billingsley PF, James ER, Gunasekera A, Sim BK, Njuguna P, Rampling TW, Richman A, Abebe Y, Kamuyu G, Muthui M, Elias SC, Molyneux S, Gerry S, Macharia A, Williams TN, Bull PC, Hill AV, Osier FH, Draper SJ, Bejon P, Hoffman SL, Ogutu B, Marsh K. 2014. Evaluating controlled human malaria infection in Kenyan adults with various degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 5:686. doi: 10.3389/fmicb.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pombo DJ, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin LB, Wilson D, Elliott S, Eisen DP, Weinberg JB, Saul A, Good MF. 2002. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet 360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy JS, Sekuloski S, Griffin PM, Elliott S, Douglas N, Peatey C, Rockett R, O'Rourke P, Marquart L, Hermsen C, Duparc S, Mohrle J, Trenholme KR, Humberstone AJ. 2011. A pilot randomised trial of induced blood-stage Plasmodium falciparum infections in healthy volunteers for testing efficacy of new antimalarial drugs. PLoS One 6:e21914. doi: 10.1371/journal.pone.0021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence G, Cheng QQ, Reed C, Taylor D, Stowers A, Cloonan N, Rzepczyk C, Smillie A, Anderson K, Pombo D, Allworth A, Eisen D, Anders R, Saul A. 2000. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 18:1925–1931. doi: 10.1016/S0264-410X(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 40.Duncan CJ, Sheehy SH, Ewer KJ, Douglas AD, Collins KA, Halstead FD, Elias SC, Lillie PJ, Rausch K, Aebig J, Miura K, Edwards NJ, Poulton ID, Hunt-Cooke A, Porter DW, Thompson FM, Rowland R, Draper SJ, Gilbert SC, Fay MP, Long CA, Zhu D, Wu Y, Martin LB, Anderson CF, Lawrie AM, Hill AV, Ellis RD. 2011. Impact on malaria parasite multiplication rates in infected volunteers of the protein-in-adjuvant vaccine AMA1-C1/Alhydrogel+CPG 7909. PLoS One 6:e22271. doi: 10.1371/journal.pone.0022271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanisic DI, Liu XQ, De SL, Batzloff MR, Forbes T, Davis CB, Sekuloski S, Chavchich M, Chung W, Trenholme K, McCarthy JS, Li T, Sim BK, Hoffman SL, Good MF. 2015. Development of cultured Plasmodium falciparum blood-stage malaria cell banks for early phase in vivo clinical trial assessment of anti-malaria drugs and vaccines. Malar J 14:143. doi: 10.1186/s12936-015-0663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. 1997. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg 57:495–500. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy JS, Griffin PM, Sekuloski S, Bright AT, Rockett R, Looke D, Elliott S, Whiley D, Sloots T, Winzeler EA, Trenholme KR. 2013. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 208:1688–1694. doi: 10.1093/infdis/jit394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockett RJ, Tozer SJ, Peatey C, Bialasiewicz S, Whiley DM, Nissen MD, Trenholme K, Mc Carthy JS, Sloots TP. 2011. A real-time, quantitative PCR method using hydrolysis probes for the monitoring of Plasmodium falciparum load in experimentally infected human volunteers. Malar J 10:48. doi: 10.1186/1475-2875-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padley DJ, Heath AB, Sutherland C, Chiodini PL, Baylis SA, Collaborative Study Group . 2008. Establishment of the 1st World Health Organization international standard for Plasmodium falciparum DNA for nucleic acid amplification technique (NAT)-based assays. Malar J 7:139. doi: 10.1186/1475-2875-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, Atwal S, Drakeley C, Kibiki G, Bousema T, Chandramohan D, Gosling R. 2013. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J 12:221. doi: 10.1186/1475-2875-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beeson JG, Mann EJ, Elliott SR, Lema VM, Tadesse E, Molyneux ME, Brown GV, Rogerson SJ. 2004. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J Infect Dis 189:540–551. doi: 10.1086/381186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis 180:464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beeson JG, Rogerson SJ, Brown GV. 2002. Evaluating specific adhesion of Plasmodium falciparum-infected erythrocytes to immobilised hyaluronic acid with comparison to binding of mammalian cells. Int J Parasitol 32:1245–1252. doi: 10.1016/S0020-7519(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 50.Chan JA, Howell KB, Reiling L, Ataide R, Mackintosh CL, Fowkes FJ, Petter M, Chesson JM, Langer C, Warimwe GM, Duffy MF, Rogerson SJ, Bull PC, Cowman AF, Marsh K, Beeson JG. 2012. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest 122:3227–3238. doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.